Introduction. Female patients exhibit better survival and less hepatic damage from ischemia reperfusion (IR) injury following surgery. However, the effects of sex and estrogens on liver function in the acute phase of IR are not well understood.
Objective. The aim was to investigate this question.
Material and methods. A rat model of segmental hepatic ischemia was employed. Rats were pre-treated with the estrogen receptor antagonist ICI182,780 and/or the estrogen receptor agonist 17β-estradiol. Bile flow, blood concentrations of bilirubin and liver enzymes were measured, and liver histology was assessed.
Results. Bile flow recovery immediately after the initiation of reperfusion was faster in females than in males. ICI182,780 reduced the rate of bile flow recovery in females but this reduction was not reversed by co-administration of 17 β-estradiol. In males, 17 β-estradiol alone did not enhance bile flow recovery. The changes in bile flow recovery observed under a given condition were correlated with small changes in blood liver enzymes and liver histology.
Conclusions. Sex has a significant influence on the early recovery of liver function in the acute phase of IR injury. However, in female rats estrogen receptors play only a limited role in mediating enhanced recovery of liver function.
Hepatic ischemia-reperfusion (IR) injury is a major problem in liver surgery and can lead to liver dysfunction and liver failure. Several protective strategies have been proposed to reduce hepatic IR injury and its related hepatic complications.1,2 One of these strategies, which involves pharmaceutical interventions targetted to estrogen receptors' is based on results which indicate that females are better protected than males against IR injury and trauma-haemorrhage.3–6 Experiments with rodent models of IR injury have shown that females exhibit better survival and less hepatic damage than males following IR.4 Analysis of clinical data has provided evidence that female patients survive better after resection for hepatocellular carcinoma compared to their male counterparts.7,8 There is also evidence which suggests that female recipients of livers from female donors exhibit more favourable graft survival than do male recipients of livers from female donors in orthotopic liver transplantation.9–12 However, interpretation of results for the effect of donor and recipient sex on the outcome of liver transplants is complex. A more recent analysis of the United Network for Organ Sharing database, while finding that survival was higher in women than in men following liver transplantation, also found that female sex was not protective when the analysis took into account the nature of the underlying disease, severity of illness and surgical and clinical variables.13 Surgical and clinical variables include donor liver size14 and times of cold and warm ischemia for the donor liver. An apparently more favourable outcome for female compared to male recipients may be due to several factors of which a better recovery from IR injury is just one.10,13
The mechanisms responsible for the observed differences in the severity of hepatic IR injury between males and females are not yet clear. Hormonal factors leading to an increased sensitivity to IR injury in males have been postulated to play a key role.15 Moreover, it has been suggested that the estrogen could act as a ‘survival factor’ for hepatic endothelial cells.16,17 Thus, male mice pre-treated with estrogen exhibit improved outcomes following IR and, conversely, ovariectomised mice or female mice pre-treated with an estrogen receptor antagonist exhibit poorer outcomes.4,18–22 Other studies suggest that estrogen is cytoprotective in hepatic IR injury.19,22 While the results of all these studies suggest a role for estrogens in reducing the severity of IR injury, that role is incompletely understood. Furthermore, studies assessing the influence of sex and estrogen on hepatic IR injury have principally assessed liver recovery following IR by monitoring liver damage and survival in the late stages of IR injury. The effects of sex and estrogen on the early (acute) stage of recovery after hepatic IR injury are not well understood. The aim of the present study was to assess the effects of sex and estrogen receptors on liver function, monitored using bile flow, during the acute phase of IR injury. Bile flow recovery after IR has previously been shown to provide a dynamic assessment of liver injury and of the functional capacity of the liver.23–28
Material and MethodsAnimals and surgical procedureMale and female Sprague Dawley rats (8–12 weeks, 170–465 g) were bred and housed in the Flinders Medical Centre Animal House at 22 °C, 60% humidity, with a 12-hour light/dark cycle and free access to food and water. Animals received humane care, and the experimental protocols were conducted according to the criteria outlined in the “Australian Code of Practice for the Care and Use of Animals for Scientific Purposes” (National Health and Medical Research Council of Australia). A rat model of segmental (60–70%) hepatic ischemia in which the bilateral median and left lateral liver lobes are made ischemic,27,29 and bile flow from the ischemic liver lobes was measured by cannulation of the common bile duct, was employed as described previously.27 At the end of the protocol the rat was euthanased and tissue samples from the ischemic and non-ischemic lobes collected in paraformaldehyde for histological analysis. Blood samples (heparin tubes) were taken from the inferior caval vein after laparotomy at the beginning of the experiment, and at the end of the protocol before the removal of liver tissue.
Rats were randomly allocated to five experimental groups: male pre-treated for 24 h with vehicle (n = 5), male pre-treated for 24 h with the estrogen agonist 17β-estradiol (n = 6), female pre-treated for 24 h with vehicle (n = 6), female pre-treated for 2 4 h with the estrogen receptor antagonist ICI182,78030 (n = 6), and female pre-treated for 24 h with ICI182,780 plus 17β-estradiol (n = 9). 17β-estradiol (Sigma-Aldrich Inc, St Louis, MO, USA) (4 mg per kg body wt) and ICI182,780 (Tocris Tookson Ltd, Bristol, UK) (3 mg per kg body wt) were each dissolved in a vehicle of ethanol and canola oil, and were administered by subcutaneous injection 24 h before anaesthesia, laparotomy and IR. Animals underwent common bile duct cannulation and bile collection for 170 min; 20 min baseline, 60 min of ischemia induced by clamping the blood supply to the medial and left lateral lobes of the liver, and 90 min of reperfusion.
Measurement of bile flowBile flow was measured gravimetrically as described previously.31,32 The rate of bile flow recovery during the first 30 min of reperfusion was determined by linear regression. Total bile flow during the reperfusion period was analysed by calculating the area under the plot of bile flow as a function of time.
Blood concentrations of bilirubin, liver enzymes, and estrogenConcentrations of the liver enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH), and total and conjugated bilirubin in blood plasma were measured spectrophotometrically using a Roche Modular Automatic Analyser and standard Roche-Hitachi methodology in the SA Pathology laboratories (Flinders Medical Centre, Bedford Park SA). Plasma estrogen concentrations were measured using a competitive binding assay33 employing the Elecsys Estradiol II chemiluminescence system and a Roche Modular E170 analyser.
Assessment of liver histology
Liver tissue fixed in paraformaldehyde was embedded in paraffin, sectioned (3 µm thick), stained with haematoxylin and eosin, and analysed histologically using light microscopy in a blinded fashion. The severity of hepatic cell injury in the ischemic and non-ischemic stained liver sections was analysed using a point-counting method.34 Five features in the liver sections were evaluated: cytoplasmic vacuolization, cytoplasmic hypereosinophilia, nuclear pyknosis, inflammation, and haemorrhage into the space of Disse. These different features were graded in a scale of 0–10 and a total score of the four features was calculated for each liver section.
Statistical analysisStatistical analysis was performed using SPSS 16.0 (SPSS Inc, Chicago, I1, USA) and GraphPad Prism 5.0. Results are expressed as means ± SEM. Continuous variables between the groups were compared with one-way analysis of variance (ANOVA Brown Forsythe) with post-hoc contrast Bonferroni. Histopathology scores for ischemic and non-ischemic lobes within a given experimental group and “Pre” and “Post” values of blood parameters were compared using a paired Student’s t-test. Statistical significance was accepted when P values were less than 0.05.
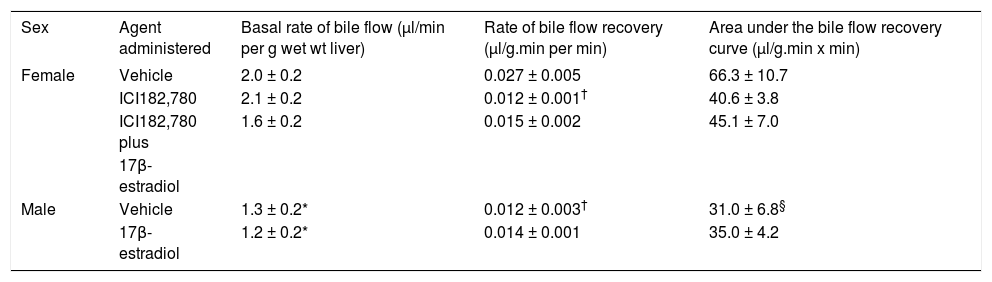
ResultsIn order to assess the role of estrogens in the acute phase of liver IR injury, the effects of the estrogen receptor antagonist ICI182,78030 administered to female rats, and of the estrogen 17β-estradiol administered to male rats, on the recovery of bile flow following IR were investigated. The basal rate of bile flow (expressed per g wet wt of liver) in male rats treated with vehicle was about 65% of that in female rats treated with vehicle (Table 1). When liver lobes were subjected to ischemia for 60 min followed by reperfusion, the rate and extent of bile flow recovery during reperfusion in male rats was about 50% of that in female rats (Figure 1B c.f. 1A and Table 1). Pre-treatment of female rats with ICI182,780 did not alter the basal rate of bile flow (expressed in units of µ1/min per g body wt), but inhibited by about 50% the rate of bile flow recovery during the reperfusion period (Figure 1A and Table 1). Pre-treatment of female rats with 17β-estradiol in addition to ICI182,780 reduced the basal rate of bile flow and caused a small increase in the rate of bile flow recovery following ischemia, compared with the values for pre-treatment with ICI182,780 alone (Figure 1A and Table 1). Pre-treatment of male rats with 17β-estradiol did not alter the basal rate of bile flow or the rate of bile flow recovery following ischemia (Figure 1B and Table 1).
Effects of sex, the estrogen receptor antagonist ICI182,780 and the estrogen receptor agonist 17β-estradiol on the basal rate of bile flow and the rate of bile flow recovery during reperfusion following ischemia.
| Sex | Agent administered | Basal rate of bile flow (µl/min per g wet wt liver) | Rate of bile flow recovery (µl/g.min per min) | Area under the bile flow recovery curve (µl/g.min x min) |
|---|---|---|---|---|
| Female | Vehicle | 2.0 ± 0.2 | 0.027 ± 0.005 | 66.3 ± 10.7 |
| ICI182,780 | 2.1 ± 0.2 | 0.012 ± 0.001† | 40.6 ± 3.8 | |
| ICI182,780 plus | 1.6 ± 0.2 | 0.015 ± 0.002 | 45.1 ± 7.0 | |
| 17β-estradiol | ||||
| Male | Vehicle | 1.3 ± 0.2* | 0.012 ± 0.003† | 31.0 ± 6.8§ |
| 17β-estradiol | 1.2 ± 0.2* | 0.014 ± 0.001 | 35.0 ± 4.2 |
The basal rate of bile flow, the rate of bile flow recovery, and the area under the bile flow recovery curve were determined as described in Methods. The results are means ± SEM n= 5 (male vehicle), 6 (male 17 β-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus 17β-estradiol). The degree of significance for comparison of the basal rate of bile flow of female vehicle with each of male vehicle and male 17ß-estradiol (*) and for comparison of female ICI182,780 with male vehicle and with male 17β-estradiol (*) was P < 0.05 (ANOVA Brown Forsythe with post hoc contrast Bonferroni). The degree of significance for comparison of the rate of bile flow recovery of female vehicle with each of male vehicle and female ICI182,780 (†), and for comparison of area under the bile flow recovery curve for female vehicle compared with male vehicle (§) was P < 0.05 (ANOVA Brown Forsythe with post hoc contrast Bonferroni)
Effects of sex, the estrogen receptor antagonist ICI182,780, and the estrogen receptor agonist 17β-estradiol, on bile flow recovery during reperfusion following ischemia. A. Bile flow recovery in female rats pre-treated with vehicle, ICI182,780, or ICI182,780 plus 17β-estradiol. B. Bile flow recovery in male rats pre-treated with vehicle or 17β-estradiol. Blood vessels to the bilateral median and left lateral liver lobes were clamped for 60 min, unclamped, and reperfusion continued for 90 min. The results, expressed as μl/min per g wet liver, are means ± SEM [n= 5 (male vehicle), 6 (male 17 β-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus 17β-estradiol)].
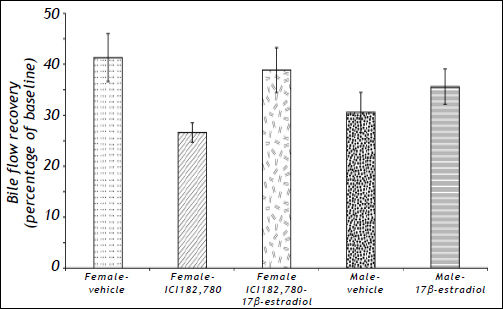
Results for bile flow recovery expressed as a percentage of the value of the baseline rate of bile flow for each of the conditions tested are summarised in figure 2. In female rats ICI182,780 reduced bile flow recovery (expressed as a percentage of baseline). While co-administration of 17β-estradiol with ICI182,780 to female rats increased bile flow recovery compared to administration of ICI182,780 alone, and the administration of 17ß-estradiol to male rats caused a slight increase in bile flow recovery, these increases were not significant.
Effects of sex, the estrogen receptor antagonist ICI182,780, and the estrogen receptor agonist 17β-estradiol, on bile flow recovery measured at 90 min after the initiation of reperfusion, and expressed as a percentage of the initial baseline rate of bile flow. The results, which are derived from the data shown in figure 1, are the means ± SEM of n= 5 (male vehicle), 6 (male 17 ß-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus 17ß-estradiol) rats.
Following 17β-estradiol pre-treatment, the concentration of estrogen in the blood (plasma) of female rats increased from about 150 to about 5,000 pmol/L (Figure 3) while that in the blood of male rats increased from about 80 to about 10,000 pmol/L (Figure 3B). In female rats pre-treated with ICI182,780 (in the absence of administration of 17β-estradiol), the blood concentration of estrogen following IR was slightly higher than that measured before IR (Figure 3A, inset). In male rats subject to IR (in the absence of pre-treatment with 17β-estradiol), the blood concentration of estrogen following IR was higher than that measured before IR (Figure 3B, inset).
Blood estrogen concentrations in female (A) and male (B) rats pre-treated with vehicle, the estrogen receptor antagonist ICI182,780, or the estrogen receptor agonist 17β-estradiol. Blood vessels to the bilateral median and left lateral liver lobes were clamped for 60 min, unclamped, and reperfusion continued for 90 min. Blood estrogen concentrations were measured at the beginning of the experiment (Pre) and at the end of the 90 min reperfusion period (Post). The insets show, using a larger scale, the blood estrogen concentrations in the rats pre-treated with vehicle or ICI182,780 in the absence of 17β-estradiol administration. The values are the means ± SEM [n= 5 (male vehicle), 6 (male 17 β-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus17β-estradiol)]. The degree of significance for a comparison of the blood estrogen concentration in female rats pre-treated with ICI182,780 plus 17β-estradiol compared with those pre-treated with vehicle or ICI182,780 alone, and for male rats treated with 17β-estradiol compared with vehicle was *P < 0.01 (ANOVA Brown Forsythe with post hoc Bonferroni). The degree of significance for comparison of the Post and Pre blood estrogen concentrations for the female ICI182,780 group and for the male vehicle group is †P < 0.05 (paired Student’s t-test).
For each of the conditions tested, the blood concentration of total bilirubin was substantially higher at the end of the 90 min reperfusion than the value before beginning ischemia (Figure 4A). The increase in female rats pre-treated with ICI182,780 was slightly higher than that in female rats pretreated with vehicle.
Effects of sex, the estrogen receptor antagonist ICI182,780, and the estrogen receptor agonist 17β-estradiol, on the blood concentrations of total bilirubin (A) and alanine aminotransferase (ALT) (B) following ischemia and reperfusion. Blood vessels to the bilateral median and left lateral liver lobes were clamped for 60 min, unclamped, and reperfusion was contained for 90 min. Blood total bilirubin and ALT concentrations were measured at the beginning of the experiment (Pre) and at the end of the 90 min reperfusion period (Post). The values are means ± SEM [n= 5 (male vehicle), 6 (male 17 β-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus 17β-estradiol)]. The degree of significance for comparison of the Post blood total bilirubin and ALT value with the Pre blood value for each condition was *P<0.05 (paired Student’s t-test). The degree of significance for comparison of the Post blood ALT value of the male vehicle with each of the Post values of the female vehicle, female ICI182,780 and female ICI182,780 plus 17β-estradiol, and for comparison of the Post blood ALT value of the male 17β-estradiol with the female ICI182,780 plus 17β-estradiol was †P<0.05 (paired Student’s t-test).
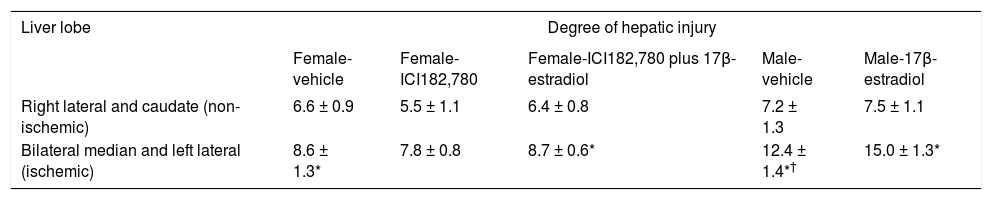
Liver damage following IR was assessed by measuring the blood concentrations of liver enzymes and by changes in liver histology at the end of the 90 min reperfusion period. Male rats subject to IR exhibited a much greater release of alanine amino transferase (ALT) after ischemia when compared with ALT release in female rats (Figure 4B). Similar results were obtained for the measurement of aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) (results not shown). Histological examination of sections of the bilateral median and left lateral liver (ischemic) lobes of female rats stained with hematoxylin and eosin showed a mild degree of injury (principally cytoplasmic vacuolization with some nuclear pyknosis) compared to sections of the right lateral and caudate (non-ischemic) lobes for all three conditions tested (Table 2). In male rats, the degree of injury in the ischemic lobes was considerably greater than that in the non-ischemic lobes (Table 2).
Effect of sex, the estrogen receptor antagonist ICI182,780, and the estrogen receptor agonist 17β-estradiol, on histological damage to the ischemic lobes following ischemia and reperfusion.
| Liver lobe | Degree of hepatic injury | ||||
|---|---|---|---|---|---|
| Female-vehicle | Female-ICI182,780 | Female-ICI182,780 plus 17β-estradiol | Male-vehicle | Male-17β-estradiol | |
| Right lateral and caudate (non-ischemic) | 6.6 ± 0.9 | 5.5 ± 1.1 | 6.4 ± 0.8 | 7.2 ± 1.3 | 7.5 ± 1.1 |
| Bilateral median and left lateral (ischemic) | 8.6 ± 1.3* | 7.8 ± 0.8 | 8.7 ± 0.6* | 12.4 ± 1.4*† | 15.0 ± 1.3* |
The degree of hepatic injury observed in liver sections stained with hematoxylin and eosin was quantitated using the grading scale described in Material and methods. The results are means ± SEM [n= 5 (male vehicle), 6 (male 17 β-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus 17β-estradiol)]. The degree of significance (paired Student’s t-test) for comparison of cell injury in the ischemic lobes compared with the non-ischemic lobes was
The results show that female rats exhibit a faster rate of bile flow recovery than male rats following hepatic IR injury. This indicates that in the acute phase of IR injury, liver function in female rats is recovered more quickly. To our knowledge, this is the first time the effect of sex on liver function during the acute phase of IR has been reported. This observation indicates that sex affects the initial mechanisms, likely to involve an increase in cytoplasmic free Ca2+ and the generation of reactive oxygen species 35, responsible for hepatocyte injury following IR. Other cell types, in addition to hepatocytes, might also be involved. Thus the slower rate of bile flow recovery following IR in male rats may be due, in part, to a more pronounced reduction in the hepatic microcirculation following IR.18 The difference in bile flow recovery between male and female during the first 90 min of reperfusion was associated with small differences in liver injury, assessed by the release of liver enzymes and histological damage measured at 90 min after commencement of reperfusion. These observations indicate that sex affects the initiation of longer term mechanisms of hepatocyte damage at an early stage after IR.
The results obtained using an estrogen receptor antagonist and agonist suggest that, although estrogens are likely involved in the mechanism by which female rats exhibit improved bile flow recovery following IR, estrogen action cannot completely explain the sex difference. Thus the observation that ICI182,780, which has been shown to be an effective antagonist of estrogen receptors,30 reduces bile flow recovery in female rats suggests that estrogen receptors in hepatocytes and/or cholangiocytes are involved in the mechanism by which improved bile flow recovery is observed in female rats. However, co-administration of the estrogen agonist 17β-estradiol only partially reversed the effect of ICI182,780 in female rats, and 17β-estradiol did not greatly enhance bile flow recovery in male rats.
The possibility that in female rats, the increase in the blood concentration of estrogen was not sufficient to compete with ICI182,780 for the binding sites on estrogen receptors is considered unlikely. Measurement of the blood concentration of estrogen in female rats treated with 17β-estradiol showed an elevation of at least 20-fold, to 5,000 pmol/L. Moreover, 17β-estradiol increased the blood estrogen concentration in male rats more than 100-fold, to 10,000 pmol/L. It is expected that these concentrations would activate estrogen receptors. A potentially interesting observation is the small increase in the blood concentration of endogenous estrogen following IR in both male and female rats. The reason for this is not known but it may involve a change in the blood volume and/or the extracellular space available to 17β-estradiol, or enhanced estrogen secretion in response to IR.
The presence of different types of estrogen receptor may provide an explanation of the results. Cholangiocytes express the α- and β-isoforms of the nuclear estrogen receptors. Hepatocytes predominantly express the α-isoform with some ß-isoform. In addition, the G-protein coupled estrogen receptor GPR30 is also expressed in the liver.36–39 ICI182,780 is a high affinity antagonist of the nuclear estrogen receptors30 but a high affinity agonist of the GPR30 estrogen receptor.40 Thus the actions of ICI182,780 on hepatocytes are likely complex since activation of the estrogen receptor GPR30 may also alter intracellular Ca2+ concentrations37 which can, in turn, modulate bile flow.41 It is also possible that higher concentrations of testosterone in male rats affect the rate of bile flow recovery following IR since there is evidence that testosterone can affect cholangiocyte function.42
The observation that female rats exhibit a faster rate of bile flow recovery than do male rats following IR may be relevant to outcomes for patients transplanted with livers donated after cardiac death (DCD). While the use of DCD livers increases the available pool of donor livers, there is a significant risk of increased graft failure following transplantation with DCD livers.43–47 Biliary complications are responsible for a substantial proportion of graft failures in DCD liver transplantation.44,45,47 A recent analysis of available data has provided evidence that female recipients have a better outcome for DCD liver transplantation than male recipients.46 In view of the results reported here for a rat model of bile flow recovery following IR injury, consideration might be given to further assessing whether female recipients of male or female DCD livers may have more favourable outcomes compared to those for male recipients, and whether this is associated with reduced biliary complications.
It is concluded that (i) sex has a substantial influence on the recovery of liver function in the acute phase of IR, (ii) the effect of sex is exerted on the early intracellular events which initiate IR injury to hepatocytes, cholangiocytes, endothelial cells and other cell types in the liver and (iii) estrogens and estrogen receptors play only a limited role in these sex effects. This knowledge is of value in the development of potential pharmaceutical interventions targetting estrogen receptors which could be used in treatment to improve the outcome of patients undergoing liver surgery or liver transplantation.
Abbreviations- •
IR: Ischemia and reperfusion.
- •
ALT: Alanine aminotransferase.
- •
AST: Aspartate aminotransferase.
- •
LDH: Lactate dehydrogenase.
- •
DCD: Donation after cardiac death.
The authors gratefully acknowledge Brad Rumbelow and SA Pathology (Flinders Medical Centre) for measurement of blood proteins and metabolites; Yabin Zhou for extensive advice; Professor Robert J. Porte, Department of Surgery, University Medical Centre, Groningen, The Netherlands for suggestions and advice; and Diana Kassos and Eric Lum for preparation of the manuscript. This research was supported by grants from the Flinders Medical Centre Foundation and Flinders University of South Australia.
Disclosures, Conflicts of InterestNone.








![Effects of sex, the estrogen receptor antagonist ICI182,780, and the estrogen receptor agonist 17β-estradiol, on bile flow recovery during reperfusion following ischemia. A. Bile flow recovery in female rats pre-treated with vehicle, ICI182,780, or ICI182,780 plus 17β-estradiol. B. Bile flow recovery in male rats pre-treated with vehicle or 17β-estradiol. Blood vessels to the bilateral median and left lateral liver lobes were clamped for 60 min, unclamped, and reperfusion continued for 90 min. The results, expressed as μl/min per g wet liver, are means ± SEM [n= 5 (male vehicle), 6 (male 17 β-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus 17β-estradiol)]. Effects of sex, the estrogen receptor antagonist ICI182,780, and the estrogen receptor agonist 17β-estradiol, on bile flow recovery during reperfusion following ischemia. A. Bile flow recovery in female rats pre-treated with vehicle, ICI182,780, or ICI182,780 plus 17β-estradiol. B. Bile flow recovery in male rats pre-treated with vehicle or 17β-estradiol. Blood vessels to the bilateral median and left lateral liver lobes were clamped for 60 min, unclamped, and reperfusion continued for 90 min. The results, expressed as μl/min per g wet liver, are means ± SEM [n= 5 (male vehicle), 6 (male 17 β-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus 17β-estradiol)].](https://static.elsevier.es/multimedia/16652681/0000001200000001/v1_201906141005/S166526811931395X/v1_201906141005/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

![Blood estrogen concentrations in female (A) and male (B) rats pre-treated with vehicle, the estrogen receptor antagonist ICI182,780, or the estrogen receptor agonist 17β-estradiol. Blood vessels to the bilateral median and left lateral liver lobes were clamped for 60 min, unclamped, and reperfusion continued for 90 min. Blood estrogen concentrations were measured at the beginning of the experiment (Pre) and at the end of the 90 min reperfusion period (Post). The insets show, using a larger scale, the blood estrogen concentrations in the rats pre-treated with vehicle or ICI182,780 in the absence of 17β-estradiol administration. The values are the means ± SEM [n= 5 (male vehicle), 6 (male 17 β-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus17β-estradiol)]. The degree of significance for a comparison of the blood estrogen concentration in female rats pre-treated with ICI182,780 plus 17β-estradiol compared with those pre-treated with vehicle or ICI182,780 alone, and for male rats treated with 17β-estradiol compared with vehicle was *P < 0.01 (ANOVA Brown Forsythe with post hoc Bonferroni). The degree of significance for comparison of the Post and Pre blood estrogen concentrations for the female ICI182,780 group and for the male vehicle group is †P < 0.05 (paired Student’s t-test). Blood estrogen concentrations in female (A) and male (B) rats pre-treated with vehicle, the estrogen receptor antagonist ICI182,780, or the estrogen receptor agonist 17β-estradiol. Blood vessels to the bilateral median and left lateral liver lobes were clamped for 60 min, unclamped, and reperfusion continued for 90 min. Blood estrogen concentrations were measured at the beginning of the experiment (Pre) and at the end of the 90 min reperfusion period (Post). The insets show, using a larger scale, the blood estrogen concentrations in the rats pre-treated with vehicle or ICI182,780 in the absence of 17β-estradiol administration. The values are the means ± SEM [n= 5 (male vehicle), 6 (male 17 β-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus17β-estradiol)]. The degree of significance for a comparison of the blood estrogen concentration in female rats pre-treated with ICI182,780 plus 17β-estradiol compared with those pre-treated with vehicle or ICI182,780 alone, and for male rats treated with 17β-estradiol compared with vehicle was *P < 0.01 (ANOVA Brown Forsythe with post hoc Bonferroni). The degree of significance for comparison of the Post and Pre blood estrogen concentrations for the female ICI182,780 group and for the male vehicle group is †P < 0.05 (paired Student’s t-test).](https://static.elsevier.es/multimedia/16652681/0000001200000001/v1_201906141005/S166526811931395X/v1_201906141005/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Effects of sex, the estrogen receptor antagonist ICI182,780, and the estrogen receptor agonist 17β-estradiol, on the blood concentrations of total bilirubin (A) and alanine aminotransferase (ALT) (B) following ischemia and reperfusion. Blood vessels to the bilateral median and left lateral liver lobes were clamped for 60 min, unclamped, and reperfusion was contained for 90 min. Blood total bilirubin and ALT concentrations were measured at the beginning of the experiment (Pre) and at the end of the 90 min reperfusion period (Post). The values are means ± SEM [n= 5 (male vehicle), 6 (male 17 β-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus 17β-estradiol)]. The degree of significance for comparison of the Post blood total bilirubin and ALT value with the Pre blood value for each condition was *P<0.05 (paired Student’s t-test). The degree of significance for comparison of the Post blood ALT value of the male vehicle with each of the Post values of the female vehicle, female ICI182,780 and female ICI182,780 plus 17β-estradiol, and for comparison of the Post blood ALT value of the male 17β-estradiol with the female ICI182,780 plus 17β-estradiol was †P<0.05 (paired Student’s t-test). Effects of sex, the estrogen receptor antagonist ICI182,780, and the estrogen receptor agonist 17β-estradiol, on the blood concentrations of total bilirubin (A) and alanine aminotransferase (ALT) (B) following ischemia and reperfusion. Blood vessels to the bilateral median and left lateral liver lobes were clamped for 60 min, unclamped, and reperfusion was contained for 90 min. Blood total bilirubin and ALT concentrations were measured at the beginning of the experiment (Pre) and at the end of the 90 min reperfusion period (Post). The values are means ± SEM [n= 5 (male vehicle), 6 (male 17 β-estradiol, female vehicle, female ICI182,780), and 9 (female ICI182,780 plus 17β-estradiol)]. The degree of significance for comparison of the Post blood total bilirubin and ALT value with the Pre blood value for each condition was *P<0.05 (paired Student’s t-test). The degree of significance for comparison of the Post blood ALT value of the male vehicle with each of the Post values of the female vehicle, female ICI182,780 and female ICI182,780 plus 17β-estradiol, and for comparison of the Post blood ALT value of the male 17β-estradiol with the female ICI182,780 plus 17β-estradiol was †P<0.05 (paired Student’s t-test).](https://static.elsevier.es/multimedia/16652681/0000001200000001/v1_201906141005/S166526811931395X/v1_201906141005/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)




