MASLD is frequent and significant in primary care. Abbreviations: FIB-4: fibrosis-4; FLI score: fatty liver index; HbA1c: glycated hemoglobin; MASLD: metabolic dysfuntion-associated steatotic liver disease; T2DM: type 2 diabetes mellitus;
Non-alcoholic fatty liver disease (NAFLD), recently renamed as metabolic dysfunction-associated fatty liver disease (MASLD), is the most prevalent liver disease worldwide, affecting up to 25 % of the global population and emerging as a leading cause of liver transplantation [1-3]. It is strongly associated with obesity and metabolic syndrome (MS), especially type 2 diabetes mellitus (T2DM). Despite its significant burden and link to non-communicable diseases (NCD), MASLD has received limited attention, and there are no strong public policies to defeat it [4]. Its natural history comprises isolated steatosis, steatohepatitis with or without fibrosis, cirrhosis, and hepatocellular carcinoma [2]. Early detection plays a crucial role in preventing disease progression, highlighting the need for increased awareness, especially given that most patients are under general practitioner care [5].
Fibrosis is the most important risk factor related to liver and cardiovascular mortality in MASLD patients [6,7]. Recently, non-invasive markers such as biochemical scores and elastography have gained prominence in assessing fibrosis presence, reducing the reliance on liver biopsy, and enabling the identification of patients at risk of fibrosis at an earlier stage [8,9]. However, there is a lack of robust epidemiological studies, including initiatives within primary health care (PHC) to assess the presence of MASLD in this setting [4]. A recent study in 29 European countries showed that major gaps in disease confrontation include strategies, clinical guidelines, awareness, and education [10]. In Latin America, as the incidence of MS, T2DM and obesity is increasing, further regional epidemiological studies are required [11]. Indeed, in Brazil, a collaborative evidenced-based guideline from the hepatology and endocrinology national societies, published in 2023, suggested screening patients with body mass index > 25 kg/m2 for the presence of MASLD [12].
Globally, the prevalence and severity of MASLD in PHC remain largely unknown, including in high-risk regions like Latin America [13,14]. Also, there is no clear recommendation for MASLD screening in PHC, but attention is suggested, especially in cases of T2DM, given the accelerated progression observed in this population [11,15,16]. This study aimed to assess the prevalence of MASLD, estimating liver fibrosis through non-invasive markers in PHC patients in Brazil.
2Patients and MethodsA two-phase study was conducted: a retrospective analysis (RETR) of all electronic records of patients seen in PHC in a microregion in the south of Brazil and a prospective (PROS) study in patients from the same unit. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. The study was approved by the Ethics committee of Hospital de Clinicas de Porto Alegre (HCPA).
2.1Retrospective studyIn RETR, demographic, clinical-pharmacological, laboratory and imaging data were collected to evaluate metabolic and hepatic profiles. Liver fibrosis was estimated by Fibrosis-4 (FIB-4). All the adult patients (>18 years) being followed up at the HCPA PHC Unit were included. The following information was evaluated as described in the medical records: a) age, sex, schooling and ethnicity; b) height, weight and body mass index (BMI); c) glucose, platelets, triglycerides, total cholesterol, HDL-cholesterol, LDL-cholesterol, glycated hemoglobin (HbA1c), alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyl transferase (GGT); d) medications currently in use (hypoglycemic drugs, insulin, anti-hypertensives, hypolipidemic drugs); e) diagnosis of medical conditions: T2DM, hypertension, obesity, malnutrition, metabolic syndrome [based on Diabetes Federation (IDF), National Cholesterol Education Program's Adult Treatment Panel III (NCEP-ATP III) and European Group for the Study of Insulin Resistance (EGIR)]; hypertension (information on the use of anti-hypertensives and /or recorded diagnosis); obesity (BMI >30 kg/m2); dyslipidemia (triglycerides > 150 mg/dL; HDL-cholesterol < 40 mg/dL; previously diagnosed T2DM and/or HbA1c > 6.5; pre-diabetes (fasting glucose >100 mg/dL; HbA1c > 5.7); f) FIB-4 calculation — [age (years) × AST (U/L) / platelets (109/L) x √ALT (u/L)], graduated as follows: low-risk of fibrosis (≤ 1.3), undetermined risk (> 1.3), risk of advanced fibrosis (2.67); g) liver image (abdominal ultrasonography, computed tomography and/or magnetic resonance); h) liver biopsy.
2.2Prospective studyThis cross-sectional study involved a random sample of 350 adult (> 18 years) patients drawn from the medical records of 7,519 who were attended over the course of one year at a PHC unit. They were invited to participate via phone call and were asked to attend a specialized consultation at the institution. Enrollment in the study occurred after patients signed an informed consent form. For the study purposes, patients were excluded in the presence of hepatitis B or C, human immunodeficiency virus, steatogenic drugs such as steroids, chemotherapy, immunosuppressants, valproic acid, and amiodarone, occupational exposure. and inflammatory bowel disease. Patients reporting alcohol consumption >20 g/day (women) or >30 g/day (men) were excluded as well.
2.2.1Clinical assessmentThe clinical assessment consisted of anthropometry, food frequency questionnaire (FFQ), and HBV/HCV rapid testing. Blood samples were collected to evaluate glucose and lipid profile, AST, ALT, GGT, albumin, and platelet counts. Nutritional advice was offered to all patients.
2.2.2Acquisition and definition of variablesWeight and height were evaluated through a P-200 C high-precision scale, with a 200 kg capacity and 1.5 kg accuracy, combined with a stadiometer accurate to 1 mm and measuring up to 200 cm. Body mass index (BMI) was calculated and classified according to the World Health Organization's guidelines for non-elderly adults, and Lipschitz's criteria for older adults [17]. Waist circumference (WC) was measured at the midpoint between the lower edge of the rib cage and the iliac crest, and compared to the standards set by the Brazilian Association for the Study of Obesity and Metabolic Syndrome [18].
Metabolic syndrome (MS) was diagnosed primarily based on a WC of ≥94 cm (men) and ≥80 cm (women), along with the presence of two or more of the following criteria: fasting glucose (IFG) ≥100 mg/dL or a history of diabetes mellitus (DM), triglycerides ≥150 mg/dL or undergoing treatment, systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg or being treated for arterial hypertension (AH), and HDL-cholesterol levels <40 mg/dL for men and <50 mg/dL for women [19].
Hepatic steatosis was identified using the Fatty Liver Index (FLI), which incorporates WC, BMI, triglycerides, and GGT levels, and interpretated as <30 (no steatosis), ≥30 and <60 (inconclusive or suggestive of steatosis), and ≥60 (probable steatosis). MASLD was diagnosed in patients with an FLI ≥30, in addition to a cardiometabolic risk factor, as recently recommended [3].
The risk of liver fibrosis was evaluated using either a FIB-4 score >1.30 or the NAFLD Fibrosis Score (NFS), which considers variables such as age, hyperglycemia, BMI, platelet count, albumin, AST, and ALT levels. An NFS ≤ -1.455 suggests no advanced fibrosis, while a score >0.675 indicates the presence of advanced fibrosis [20,21].
The 10-year risk of cardiovascular disease was estimated using the Atherosclerotic Cardiovascular Disease (ASCVD) risk score, based on guidelines from the American Heart Association and the American College of Cardiology. This score includes demographic, clinical and laboratory ASCVD <7.5 % means low risk, while scores ≥10 %, high cardiovascular risk [22].
2.2.3Eating habitsA FFQ based on standard portion sizes was used [23]. The daily intake in grams was calculated by multiplying the portion size by the portion weight by the frequency of consumption (e.g., 3 for "more than three times per day," 2.5 for "2-3 times per day," 1 for "once a day," 0.8 for "5-6 times per week," 0.4 for "2-4 times per week," 0.1 for "once a week," 0.07 for "1-3 times per month," and 0 for "never or almost never"). For each item, consumption values exceeding the 99th percentile were replaced with the corresponding 99th percentile value. Macronutrient intake was then determined by multiplying the food consumption in grams by the nutrient composition per 100 grams as specified in the Brazilian Food Composition Table (TACO) [24]. If an item was not listed in TACO, data from the US Department of Agriculture (USDA) Nutrient Database was used. Data analysis was performed using SAS software, version 9.4. Afterwards, all participants received dietary guidance focused on preventing liver steatosis and promoting healthy lifestyle choices.
2.3Statistical analysisQuantitative variables were presented as either mean with standard deviation or median with interquartile range, depending on the data distribution. Qualitative variables were summarized using absolute and relative frequencies. To compare mean values, the Student's t-test was employed. For variables with non-normal distribution, the Mann-Whitney U test was used. Proportional comparisons were conducted using Pearson's chi-square test or Fisher's exact test when appropriate. Adjusted residuals were analyzed to supplement the comparisons. A significance level of 5 % (p<0.05) was applied, and all analyses were conducted using SPSS software, version 21.0.
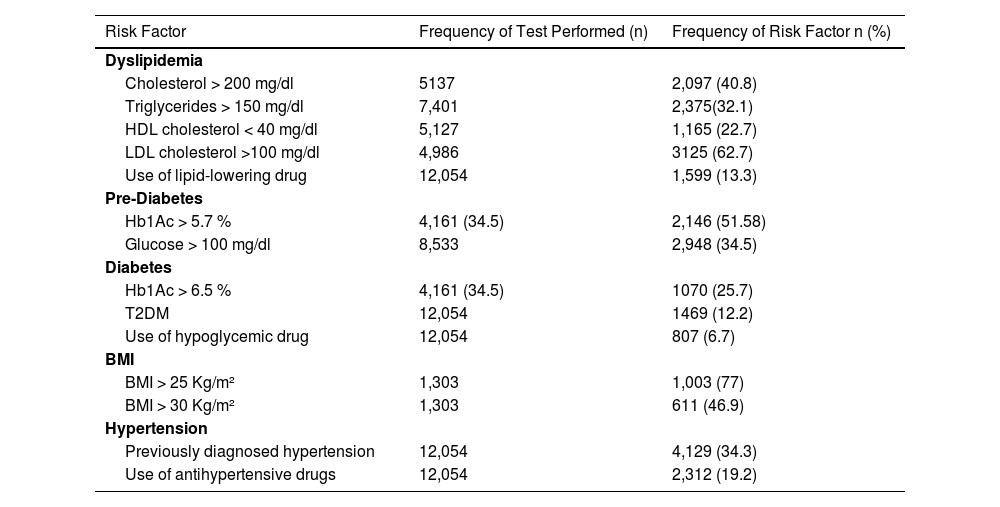
3Results3.1Retrospective studyA total of 12,054 patients were included in the study and their records were meticulously evaluated. Most of them were female, with a mean age of 55.3. Table 1 summarizes the demographic characteristics. Only 10.8 % of patients had BMI described at the medical records. From them, 30.1 had overweight, 26.8 % obesity grade I, 13 % grade 2, and 7.1 % grade III. The prevalence of MS features was high, as described in Table 2. Imaging tests such as ultrasound, computed tomography, or magnetic resonance were performed in only 12.6 % of the study population. Of these, the detection of steatosis occurred in 216 patients, which represents 1.8 % of the total. However, the term NAFLD was not found in the medical records, nor was it in its newer denominations, metabolic associated fatty liver diseases (MAFLD) or MASLD. About 1 % of patients had a liver biopsy, but none of them had a histological diagnosis of NAFLD/NASH (non-alcoholic steatohepatitis), as they were patients with hepatitis C virus. No patient was referred to a specialized service due to MASLD.
General characteristics of the population – retrospective cohort (n = 12,054.)
| General Characteristics of the Population | |
|---|---|
| Sex (n, %)FemaleAge (±SD)Age, meanEthnicity (self-declared) (n, %)WhiteBlackBrownUnknownSchooling (n, %)Complete high schoolIncomplete higher educationHigher EducationUnknown | 7,882 (65.4)55.3 (19.0)10,862 (90.1)860 (7.1)291 (2.5) 41 (0.3)3,927 (32.6)960 (8.0)2,023 (16.8)1,045 (8.7) |
SD: standard deviation; BMI: body mass index.
Prevalence of MASLD Risk Factors in the studied patients – retrospective cohort (n = 12,054).
| Risk Factor | Frequency of Test Performed (n) | Frequency of Risk Factor n (%) |
|---|---|---|
| Dyslipidemia | ||
| Cholesterol > 200 mg/dl | 5137 | 2,097 (40.8) |
| Triglycerides > 150 mg/dl | 7,401 | 2,375(32.1) |
| HDL cholesterol < 40 mg/dl | 5,127 | 1,165 (22.7) |
| LDL cholesterol >100 mg/dl | 4,986 | 3125 (62.7) |
| Use of lipid-lowering drug | 12,054 | 1,599 (13.3) |
| Pre-Diabetes | ||
| Hb1Ac > 5.7 % | 4,161 (34.5) | 2,146 (51.58) |
| Glucose > 100 mg/dl | 8,533 | 2,948 (34.5) |
| Diabetes | ||
| Hb1Ac > 6.5 % | 4,161 (34.5) | 1070 (25.7) |
| T2DM | 12,054 | 1469 (12.2) |
| Use of hypoglycemic drug | 12,054 | 807 (6.7) |
| BMI | ||
| BMI > 25 Kg/m² | 1,303 | 1,003 (77) |
| BMI > 30 Kg/m² | 1,303 | 611 (46.9) |
| Hypertension | ||
| Previously diagnosed hypertension | 12,054 | 4,129 (34.3) |
| Use of antihypertensive drugs | 12,054 | 2,312 (19.2) |
T2DM: Diabetes Mellitus; Hb1Ac: Glycated Hemoglobin; BMI: Body Mass Index.
From the 350 participants selected, 20 were excluded due to high alcohol consumption. Table 3 shows the demographic and biochemical data of the 330 included patients. Only eight patients (2.4 %) reported previously knowing that they had hepatic steatosis. MASLD was present in 205 (62.1 %) patients. FLI score was ≥ 60 in 130 (39.4 %) of the sample. In patients with steatosis, age over 60 years and non-white skin color were more common. Table 3 also compares patients with and without steatosis through FLI in terms of some clinical aspects.
Demographic characteristics of the included patients – cross-sectional study.
| Variables | Total sample (n = 330) | Steatosis (n = 130) | No steatosis (n = 200) | p |
|---|---|---|---|---|
| Sex – n (%) | 0.086 | |||
| Male | 93 (28.2) | 44 (47.3) | 49 (52.7) | |
| Female | 237 (71.8) | 86 (36.3) | 151 (63.7) | |
| Age (years) – average ± SD | 58.0 ± 13.5 | 60.8 ± 10.9 | 56.1 ± 14.6 | 0.002 |
| Age range – n (%) | 18-82 | 0.150 | ||
| <50 years | 81 (24.5) | 24 (29.6) | 57 (70.4) | |
| 50 to 59 years | 84 (25.5) | 34 (40.5) | 50 (59.5) | |
| 60 to 69 years | 98 (29.7) | 40 (40.8) | 58 (59.2) | |
| ≥ 70 years | 67 (20.3) | 32 (47.8) | 35 (52.2) | |
| Ethnicity – n (%) | 0.003 | |||
| White | 300 (90.9) | 110 (36.6) | 190 (63.4) | |
| Non-white | 30 (9.1) | 20 (66.6) | 10 (33.4) | |
| BMI ≥ 30 kg/m2 – n (%) | 105 (31.8) | 89 (84.8) | 16 (15.2) | <0.001 |
| High WC – n (%) | 249 (75.5) | 124 (49.8) | 125 (50.2) | <0.001 |
| Glycemia – n (%) | <0.001 | |||
| IFG ⁎⁎⁎ | 80 (21.2) | 48 (60.0)* | 32 (40.0) | |
| T2DM – n (%) | 36 (10.9) | 27 (75.0)* | 9 (25.0) | <0.001 |
| Elevated tryglycerides – n (%) | 97 (29.4) | 67 (69.0) | 30 (31.0) | <0.001 |
| AH – n (%) | 172 (52.1) | 92 (53.4) | 80 (46.6) | <0.001 |
| MS – n (%) | 157 (47.6) | 96 (63.7) | 61 (36.3) | <0.001 |
| Laboratory - average ± SD | ||||
| Total cholesterol | 200.2 ± 43.2 | 201.3 ± 41.9 | 199.5 ± 44.3 | 0.202 |
| HDL-cholesterol | 51.2 ± 13.6 | 46.2 ± 11.0 | 54.9 ± 14.0 | 0.038 |
| LDL-cholesterol | 122.4 ± 38.7 | 121.6 ± 35.6 | 122.9 ± 40.7 | 0.042 |
| Platelets | 248.1 ± 57.7 | 249.5 ± 59.5 | 247.1 ± 56.6 | 0.438 |
| Albumin | 4.3 ± 0.2 | 4.2 ± 0.2 | 4.3 ± 0.2 | 0.725 |
| AST | 19.4 ± 6.3 | 21.3 ± 7.6 | 18.2 ± 4.5 | <0.001 |
| ALT⁎⁎ | 17 (13 – 23) | 20 (15 – 30) | 15 (12 – 20) | <0.001 |
| GGT⁎⁎ | 23 (16 – 33) | 30 (24 – 40) | 19 (14 – 27) | <0.001 |
| Smokers – n (%) | 33 (10) | 12 (36.3) | 21 (63.7) | 0.851 |
| Physically Active – n (%)⁎⁎⁎⁎ | 85 (25.8) | 32 (24.6) | 53 (26.5) | 0.800 |
Data expressed as mean ± standard deviation or median (25th - 75th percentiles), compared by Student's t-test or Mann-Whitney test, respectively, or N (%) with Pearson's chi-square test or Fisher's exact test.
In Table 4, patients were divided by BMI and MS features were compared. Steatosis was more prevalent in patients with BMI > 25 kg/m2. NFS calculation demonstrated that 50 % had an intermediate risk, and that 9.2 % of patients had a high risk of fibrosis or cirrhosis. Mean FIB-4 was 1.14 (min 0.18; max 3.39). FIB-4 > 1.3 in 30.9 % of patients, and > 2.67 in 0.9 % of them. Cardiovascular risk was higher in patients with steatosis (Table 5). There were no differences in total energy value (2072.8 ± 716.3 vs. 2031.5 ± 618.1 Kcal – p = 0.578), carbohydrates (236.0 ± 82.2 vs. 233.8 ± 80.8 g – p = 0.807), proteins (114.5 ± 49.7 vs. 109.4 ± 41.1 g – p = 0.308), and fat intake (74.7 ± 29.1 vs. 73.2 ± 25.8 – p = 0.631) in patients with or without steatosis, respectively.
Presence of hepatic steatosis and metabolic risk factors, according to the body mass index values in the studied population – cross-sectional study.
| Variables | TotalSample(n = 330) | BMI≤25 kg/m²(n = 115) | BMI>25 kg/m²(n = 215) | p |
|---|---|---|---|---|
| Steatosis n(%) | 130 (41.5) | 9 (7.8) | 121 (56.3) | <0.001 |
| IFG * (%) | 119 (36.0) | 28 (24.3) | 91 (42.3) | 0.002 |
| Metabolic syndrome – n(%) | 157 (47.5) | 30 (26.0) | 127 (59.0) | <0.001 |
| Arterial hypertension- n(%) | 173 (52.4) | 46 (40) | 127 (59.0) | <0.001 |
Cardiovascular risk over next 10 years based on the presence of hepatic steatosis – cross-sectional study.
| Cardiovascular risk (ASCVD) | Total sample(n = 255) | Steatosis positive (n = 110) | Steatosis negative (n = 145) | p |
|---|---|---|---|---|
| Low risk | 125 (49.0) | 37 (33.7) | 88 (60.8)* | |
| Moderate risk | 30 (11.7) | 13 (11.8) | 17 (11.7) | |
| High risk | 100 (39.3) | 60 (54.5)* | 40 (27.5) | <0.001 |
Data expressed as N (%), compared by Pearson's chi-square test.
In this two-phase study conducted in PHC, which involved a large and unselected patient population, it was found that the prevalence of MASLD is significant, with a notable risk of hepatic steatosis and fibrosis. These findings highlight the importance of focusing on education and raising awareness about the disease in this context. In the RETR study, in patients who had laboratory, more than 40 % had evidence of dyslipidemia and more than 50 %, HbA1c > 5.7. Moreover, median FIB-4 above 1.3 attested to the risk of fibrosis. Indeed, 5 % of the patients had FIB-4 suggesting advanced fibrosis. The PROS study corroborated these findings, showing that MS was present in almost 50 % of the patients, and steatosis was present in approximately 40 % of the sample. MS was more frequent in those with steatosis and with a BMI above 25. The risk of liver fibrosis, assessed by the NFS score, was high, with more than 50 % of patients at intermediate risk and almost 10 % at risk of advanced fibrosis. In addition, patients with steatosis had a significantly higher cardiovascular risk. Moreover, MASLD diagnosis, assuming FLI as a surrogate marker of steatosis, was higher than 60 %. These findings, taken together, point to a high risk of MASLD in PHC in Brazil and highlight the need to act in the early diagnosis in this scenario.
The high prevalence of MASLD in all continents is related to the development of obesity and T2DM. In this study, liver steatosis estimation by FLI ≥ 60 was around 40 %. The result is higher than reported in South America (30.5 %), but it is probably the result of different methods of steatosis assessment [25]. A limitation of this study was documenting steatosis only based on FLI. However, it would have been very helpful in this study if included a more reliable estimate of steatosis, such as continuous attenuation parameter (CAP) or magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF). Nevertheless, FLI is quite appropriate for use in PHC due to its availability. The fact that patients with high FLI (or steatosis) had more MS and cardiovascular risk suggests that, even if it was overestimated, the result does not seem to be far from the real one.
The variations in the prevalence of MASLD and non-alcoholic steatohepatitis (NASH) may be related to the differences in genetic and environmental risk factors [26]. Race and ethnicity may also be considered a risk for the development of MASLD. Recently, assessing NAFLD global burden, it was demonstrated a higher prevalence in Hispanics, followed by non-Hispanic white individuals, with a lower prevalence in African Americans [27]. Even in national strategies and clinical guidelines for related conditions, such as obesity or T2DM, NAFLD is rarely mentioned [5]. These discoveries highlight the need for a joint effort to shape and supply a robust response to public health.
A very significant finding was the little attention paid to liver disease: only 10 % of patients in the RETR study had calculated BMI and less than 50 % had tests to assess for hypercholesterolemia or hyperglycemia, as well as aminotransferases. As the percentage of obese adults in Brazil has more than doubled in 17 years, from 12.2 % (2002-2003) to 26.8 % (2019), it is impressive that BMI was not available in medical consultations in such a risky population [28]. Therefore, in this study, we demonstrate that the absence of these data in the RETR patient cohort indicates neglect of the disease in this population, making our findings even more valid. Additionally, it is worth noting that the findings in this study do not represent the general population but rather the population of patients seeking PHC. This introduces a selection bias, but aligns with the study's objective of illustrating the prevalence of MASLD in PHC.
The best way to identify the patients at early risk of liver fibrosis in PHC is still a challenge but also an opportunity since most patients are at a potentially reversible disease stage [29]. Given the high world prevalence of individuals with MASLD, it is not feasible to screen the populations at risk by means of liver biopsy and histological classification, which is currently the gold standard in clinical studies [30]. Non-invasive scores, such as FIB-4 and/or NFS, can be important tools for PHC providers to identify individuals at risk of developing fibrosis and decrease unnecessary referrals [11,31,32]. Recently, a Danish study with 3,378 individuals (1,973 general population) has demonstrated that enhanced liver fibrosis (ELF) test alone or combined with FIB-4 is useful for screening of either alcoholic or non-alcoholic fatty liver [33]. Among the hepatic abnormalities found in the studied population, ALT elevation was significant. Nevertheless, even when AST and ALT are normal, it does not rule out the presence of chronic liver disease. This concept must be disseminated in PHC, since both in fatty liver disease and in NASH the patients appear not to be aware of the impact of metabolism on the health of their liver [34]. Although not yet established, a two-way step in evaluation seems to be useful, as suggested by Alkhouri and colleagues [35]. FIB-4 would be the first step: if higher than 1.30, it is advisable to obtain a FibroScan-AST (FAST) score; if it is higher than 0.35, patients should be referred to the specialist.
It is interesting to note that the terms MAFLD or MASLD were not found in the medical records in this study, perhaps because they are relatively new terms, but NAFLD was not either. A global study of 102 countries presented a similar picture, highlighting the lack of attention to MASLD in health agendas [4]. A recent American study conducted a survey of 115 primary care providers with an 80 % response rate [36]. Over 40 % were unsure of which diagnostic tests to order and which data constituted a diagnosis of NAFLD. Moreover, few knew the components of FIB-4, few used FIB-4 in practice, and yet the most common reason for referral was to obtain fibrosis staging. Hepatologists need to teach non-specialists to recognize MASLD, but it is necessary first to create a consensus on how to assess it, as suggested by an article signed by the Britain Specialist Interest Group in the Early Detection of Liver Disease [37]. Moreover, the dissociation between PHC and specialized care causes a delay in the diagnosis of liver diseases, as recently shown [38]. The COVID-19 pandemic will probably make the situation even worse since it was related to weight gain, poor T2DM control, and increased alcohol-drinking behavior, all of them related to MASLD progression [37]. Nevertheless, PHC usually follows some guidelines for investigation of NCD, especially in asymptomatic patients, like US Preventive Services Task Force (USPSTF), which does not suggest universal liver disease screening in general population or among obese people. It would be recommended that liver medical societies alert these sectors to the potential risks of hepatic steatosis [39].
5ConclusionsIn summary, this two-phase study showed that there is a high prevalence of MASLD in PHC, and a significant number of patients are at risk of developing liver fibrosis and deserve specialized attention. These findings reinforce it is necessary to train health professionals and develop clinical protocols for these services. Although expected, results like these have rarely been demonstrated with such a scientific basis, and they can serve to guide changes in public policies.
FundingThis study was supported by the following Brazilian funding agencies: Financiamento e Incentivo à Pesquisa - Hospital de Clínicas de Porto Alegre (FIPE/HCPA) - MRAS, grant 2019-0140; National Council for Scientific and Technological Development - Brazil (CNPq) - MRAS, grant 310791/2021-9; and Coordination for the Improvement of Higher Education Personnel (CAPES/PNPD) - LL. No funding sources were involved in the study design or in data collection, analysis, interpretation, writing of the report and in the decision to submit it for publication.
Authors contributionsMRAS (concept, design, analysis, supervision, funding, writing of the article); MSV, SMSR (procedures, data bank, analysis, writing of article draft); GJ, PGL (procedures, data bank); LL (experiments); MRG, VCL (design, analysis); DJ (concept, design, analysis, supervision, funding); All authors approved the final version of the manuscript.