Metabolic dysfunction-associated steatotic liver disease (MASLD) is one of the leading causes of chronic liver disease globally. Based on the 2023 definition, MASLD is characterized by the presence of metabolic dysfunction and limited alcohol consumption (<140 grams/week for women, <210 grams/week for men). Given the significant burden of MASLD in Latin America, this guidance was developed by the Latin American Association for the Study of the Liver (ALEH) Working Group to address key aspects of its clinical assessment and therapeutic strategies. In Latin America, ultrasonography is recommended as the initial screening tool for hepatic steatosis due to its accessibility, while Fibrosis-4 (FIB-4) is preferred for fibrosis risk stratification, with further evaluation using more specific techniques (i.e., vibration-controlled transient elastography or Enhanced Liver Fibrosis [ELF] test). A Mediterranean diet is advised for all MASLD patients, with a target of 7–10% weight loss for those with excess weight. Complete alcohol abstinence is recommended for patients with significant fibrosis, and smoking cessation is encouraged regardless of fibrosis stage. Pharmacological options should be tailored based on the presence of steatohepatitis, liver fibrosis, excess weight, and diabetes, including resmetirom, incretin-based therapies, pioglitazone, and sodium-glucose cotransporter-2 inhibitors. Bariatric surgery may be considered for MASLD patients with obesity unresponsive to lifestyle and medical interventions. Hepatocellular carcinoma screening is advised for all cirrhotic patients, with consideration given to those with advanced fibrosis based on individual risk. Finally, routine cardiovascular risk assessment and proper diabetes prevention and management remain crucial for all patients with MASLD.
Main Recommendations
• Metabolic dysfunction-associated steatotic liver disease (MASLD) is currently defined by the presence of metabolic dysfunction (1 out of 5 criteria) and alcohol use (< 140 or < 210 grams per week in women and men, respectively).
• Screening of MASLD in the general population is not recommended since isolated steatosis is not associated with a clinically meaningful increase in the risk of liver-related outcomes.
• Screening for MASLD and fibrosis is recommended in individuals with abnormal liver biochemistries and/or radiological signs of hepatic steatosis, as well as those with type 2 diabetes mellitus or excess weight, especially those aged over 50 years old.
• Ultrasonography is the first-line test to screen for hepatic steatosis due to its accessibility and ease of use.
• Magnetic Resonance Imaging (MRI) Proton Density Fat Fraction (PDFF) offers a more accurate quantification of hepatic fat content and a ≥30% relative decline in MRI-PDFF is associated with higher odds of histologic response and MASH resolution.
• Fibrosis-4 (FIB-4) is the biomarker of choice to initially stratify the risk of fibrosis in clinical practice.
• Patients with indeterminate or high risk according to FIB-4 or clinical history should undergo a step 2 analysis using a technique with higher sensitivity and specificity to determine the liver fibrosis stage.
• In Latin America, vibration-controlled transient elastography, Enhanced Liver Fibrosis (ELF) test, and/or the FibroScan®/aspartate aminotransferase (FAST) score are suggested as a secondary technique to stratify the risk of patients with MASLD.
• The Mediterranean diet should be implemented in all patients with MASLD, regardless of fibrosis stage.
• In patients with overweight or obesity (excess body weight), the recommended target weight loss is 7–10% of total body weight (3–5% in lean MASLD), which is associated with improvement in liver histology.
• In adults without cardiovascular or musculoskeletal contraindications, exercise should be recommended, including 150–300 minutes a week of moderate-intensity, 75–150 minutes a week of vigorous-intensity aerobic physical activity, or an equivalent combination of moderate- and vigorous-intensity aerobic activity.
• In individuals with MASLD and significant fibrosis (F2), complete alcohol abstinence should be recommended.
• Patients with MASLD who actively smoke should be advised and assisted to quit.
• Resmetirom in doses of 80 mg or 100mg can be initiated, if available, in individuals with metabolic dysfunction-associated steatohepatitis (MASH) and significant or advanced fibrosis (F2–3).
• The use of Incretin-based therapies could be considered in individuals with MASH and excess weight, based on the local availability and individual risk assessment of potential side effects.
• Other therapies, such as pioglitazone and sodium-glucose cotransporter-2 inhibitors, can be considered on a case-by-case basis.
• Bariatric surgery should be considered in patients with MASLD and obesity class 2 or 3, especially those who have not achieved adequate weight loss or metabolic improvement through lifestyle modifications and anti-obesity medications (medical therapy). However, careful patient selection and comprehensive preoperative assessment are crucial.
• Screening for hepatocellular carcinoma (HCC) should be conducted in all patients with cirrhosis. Patients with advanced fibrosis (F3) could also benefit from screening and hence HCC surveillance could be proposed in this subgroup of patients.
• All patients with MASLD should undergo screening of neoplasia according to current recommendations.
• A comprehensive cardiovascular risk assessment should be routinely performed in patients with MASLD.
Metabolic dysfunction-associated steatotic liver disease (MASLD) is one of the most frequent causes of chronic liver diseases globally [1–3]. Unhealthy dietary patterns, lifestyle habits, and food insecurity have led to an epidemic of obesity and type 2 diabetes mellitus (T2DM) worldwide [4–7]. This, coupled with elevated levels of alcohol consumption in most countries, might explain the high burden of MASLD globally [8,9]. In South America, the current MASLD prevalence is estimated at 35.7% among the general population and could rise up to 68% in high-risk groups (i.e., T2DM or obesity) [2,10]. In consequence, if we do not address MASLD risk factors and access to treatments, there will be a significant increase in MASLD prevalence [11,12], hepatocellular carcinoma (HCC), and other MASLD-related health consequences [13–17]. The natural history of MASLD ranges from isolated steatosis to steatohepatitis, liver fibrosis, and cirrhosis. However, particular interest has been focused on metabolic dysfunction-associated steatohepatitis (MASH) and advanced liver fibrosis, as these have been linked to disease progression [18] and mortality [19], respectively.
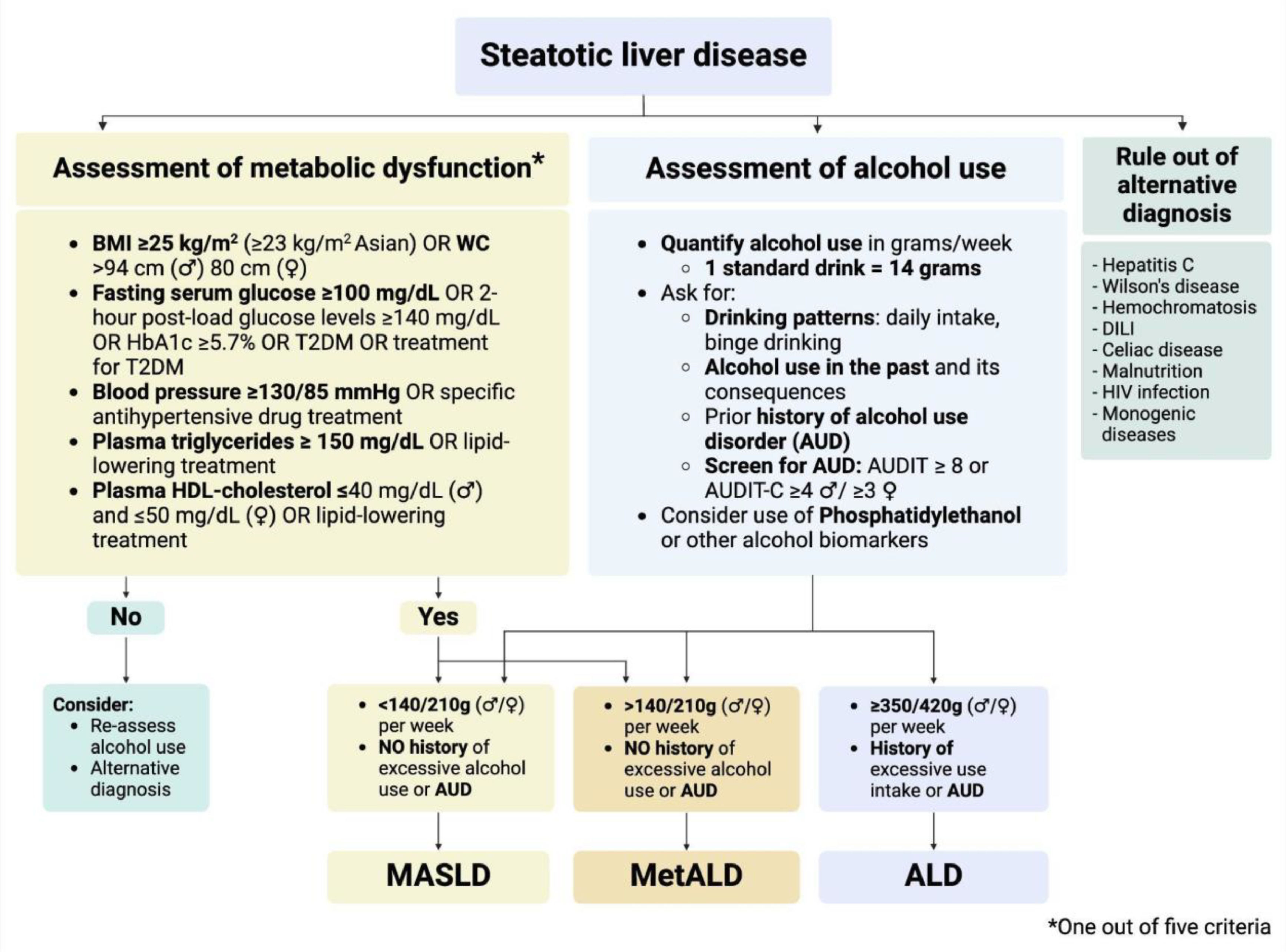
In recent years, alcohol use has been recognized as a significant promoter of hepatic steatosis, even at lower doses than those attributed to alcohol-associated liver disease (ALD) [20,21]. Thus, steatotic liver disease (SLD) is currently defined as a continuum of disease that is predominantly driven by metabolic dysfunction, alcohol use, or both drivers of disease [22]. The 2023 SLD nomenclature addressed the relationship between the most frequent risk factors for SLD establishing objective criteria for metabolic dysfunction (at least 1 of 5 factors) and thresholds for alcohol use in grams per week (Fig. 1) [23]. MASLD replaced the term non-alcoholic fatty liver disease (NAFLD) and is defined by metabolic dysfunction and alcohol use below 140 grams per week in women and 210 grams per week in men. In contrast, ALD was defined by a weekly alcohol intake of more than 350 and 420 grams in women and men, respectively. As a novel feature, the dual etiology due to metabolic dysfunction and alcohol use —termed MetALD— was defined by the presence of metabolic dysfunction and a weekly alcohol intake ranging between 140–350 grams in women and 210–420 grams in men. Recent data suggests that MetALD could resemble clinical features of those with ALD, but precise SLD subtype diagnosis can be affected by alcohol underreporting [24–26].
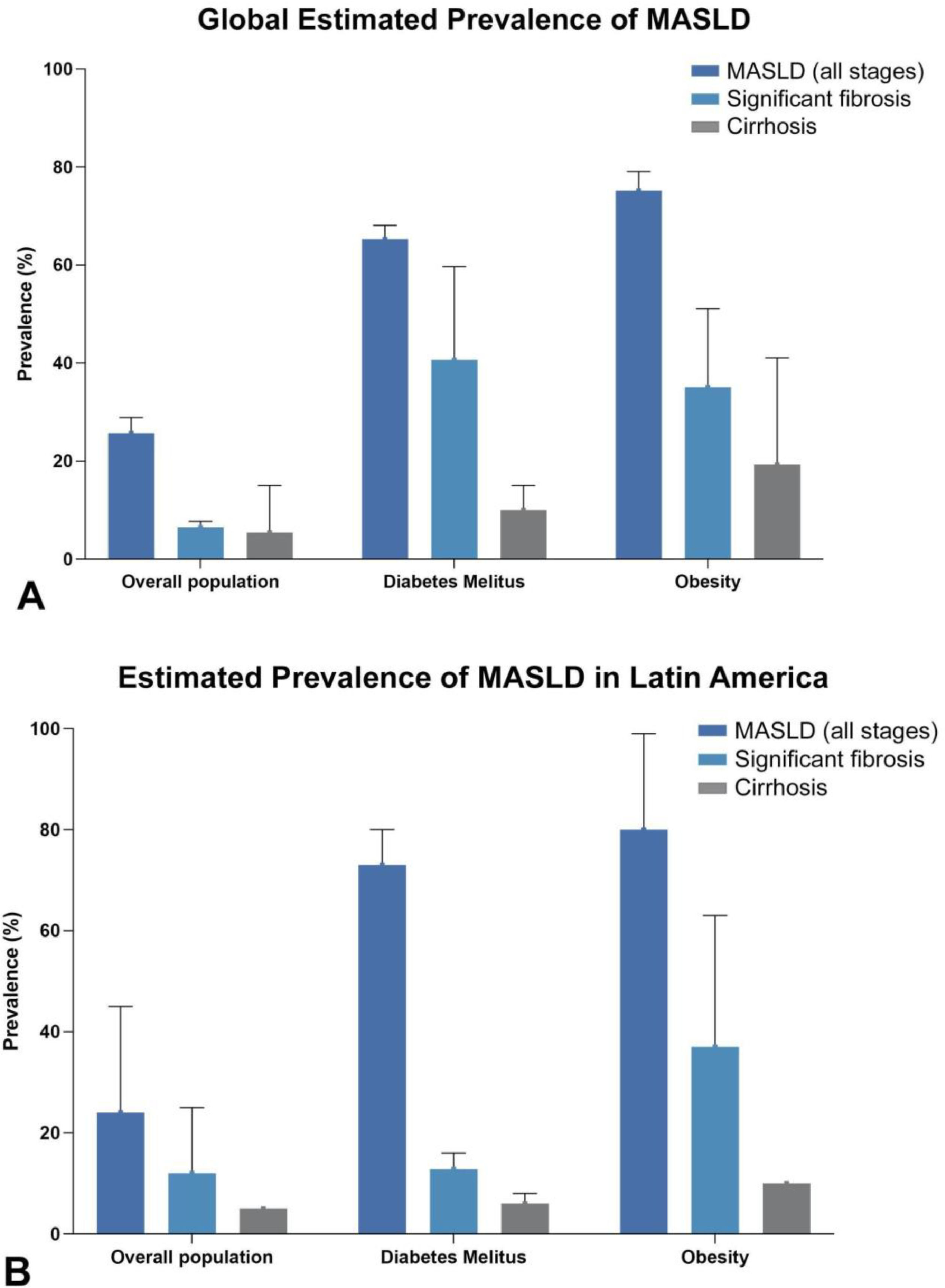
Global prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) according to fibrosis stage in the overall population, type 2 diabetes mellitus (T2DM), and obesity [215–219], and (B) Prevalence of MASLD according to fibrosis stage in the overall population, type 2 diabetes mellitus (T2DM), and obesity in Latin America [10,51,220].
Although MASLD and NAFLD have different diagnostic criteria, they exhibit substantial similarities in clinical characteristics and prognosis, and the terms can be used interchangeably [27]. In addition, this new SLD definition provides an opportunity to understand the role of metabolic dysfunction and alcohol in SLD development and progression. However, this concept also poses challenges to the field related to identifying the leading driver of SLD, and the development of new clinical trials considering individuals with higher levels of alcohol use than those considered in the past. In this guidance, we will discuss relevant aspects related to clinical assessment of patients with suspected MASLD, focusing on updated risk stratification and therapeutic strategies according to baseline risk. The topics and recommendations presented in this article were thoroughly discussed during an in-person meeting held on December 15, 2023, in Santiago, Chile, and were subsequently refined by all authors until August 2024.
2Pathophysiology of MASLDThe pathophysiology of MASLD is complex and multifactorial, involving the metabolic dysregulation itself and related factors (i.e., genetics, epigenetics, lifestyle habits, infections, autoimmunity, and gut microbiota) that lead to disrupted lipid handling and hepatic steatosis [28,29]. Among these factors, insulin resistance has been identified as a key driver of metabolic dysfunction, increasing lipolysis in adipose tissue and resulting in elevated circulating free fatty acids [30]. High-fat diet and adipose tissue dysfunction supply the liver with chylomicron remnants and non-esterified fatty acids, which are stored as lipid droplets of triglycerides within hepatocytes or undergo mitochondrial oxidation [31,32]. The excessive accumulation of lipids in hepatocytes and the disruption in mitochondrial function triggers a cascade of cellular stress responses, including endoplasmic reticulum stress, mitochondrial dysfunction, oxidative stress, and release of extracellular vesicles [22,28]. These mechanisms can also be amplified by changes in the microbiome, circulating pathogen-associated molecular patterns (PAMPs), damage-associated molecular patterns (DAMPs), and recruitment of immune cells to the liver, contributing to activation of hepatic stellate cells (HSCs) and leading to hepatocyte injury and disruption of normal liver architecture. ALD shares some pathogenic mechanisms with MASLD; however, alcohol exerts a direct toxic effect on hepatocytes and, through its byproducts, promotes and amplifies liver damage in individuals with significant alcohol intake [22].
Genetic variants, such as the patatin-like phospholipase domain-containing 3 (PNPLA3) I148M, have been linked to a higher risk of MASLD and HCC [33]. Conversely, other genetic variants, such as the protein-truncating hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) variant, confer a protective effect against the development of MASLD [34]. Sex and hormonal status are other relevant determinants of the development and progression of MASLD, where estrogens exert a protective effect on the liver by regulating lipid metabolism, suppressing inflammation, and promoting hepatocellular regeneration [35]. Moreover, recent data suggest a functional interaction between the estrogen receptor-α and the PNPLA3 I148M variant promoting liver steatosis in women [35,36]. Some endocrinopathies beyond obesity and insulin resistance, including growth hormone deficiency and panhypopituitarism have also been linked to a faster liver disease progression that may be responsive to hormone replacement/agonist therapy [37]. The thyroid axis dysfunction (including thyroid-stimulating hormone, thyroxine, and triiodothyronine) can also influence hepatic lipogenesis and fatty acid oxidation, being a relevant therapeutic target for thyroid hormone receptor-β (THR-β) agonists [38].
Gut microbiome and its produced metabolites could also play a relevant role in the development of MASLD [39]. Patients with MASLD often exhibit an altered ratio of Firmicutes/Bacteroidetes, which correlates with hepatic steatosis and obesity. This dysbiosis leads to the production of metabolites that can disrupt the intestinal barrier, causing portal translocation of bacteria and/or their metabolic products to the liver and triggering sustained inflammation [39,40]. In addition, conditions characterized by an impaired intestinal barrier and significant alterations in microbiome composition—such as inflammatory bowel disease—may be associated with a higher prevalence of MASLD and liver fibrosis [41]. Due to this multifactorial pathogenesis, liver disease development and progression can result from a different hierarchy or pathophysiological mechanism trajectory that determines several disease subphenotypes with distinct natural histories and prognoses and, eventually, different responses to therapy [42,43].
3Screening for MASLD in clinical practice3.1Identification of populations at riskDue to the intricate relationship between MASLD and common cardiometabolic risk factors, hepatic steatosis is frequently identified in individuals with excess weight, T2DM, arterial hypertension, and dyslipidemia (Fig. 1A). In the United States (US), a recent study of prospectively enrolled individuals with overweight or obesity in middle age from primary care and community-based settings showed a prevalence of SLD around 75%, while advanced fibrosis and cirrhosis were estimated at 10.8% and 4.5%, respectively [44]. In particular, 90% of SLD cases were attributed to MASLD, highlighting the high burden of disease among individuals with excess weight. In another prospective study including adults aged ≥50 years with T2DM from outpatient settings in the US, the MASLD prevalence was estimated at 65%, while 14% of T2DM had advanced fibrosis and 6% cirrhosis [45]. European countries and other Western regions face a similar scenario in terms of burden of MASLD, reaching an increase in the MASLD prevalence by +38.7% in the last two decades [46–48].
Although epidemiological data from Latin America is scarce and most comprehensive evidence comes from Hispanic individuals living in the US [49,50], a few local studies have explored the relationship between MASLD and these risk factors. For example, a Chilean study including T2DM patients prospectively recruited estimated a prevalence of hepatic steatosis in 63.9%, advanced fibrosis assessed in 12.8%, and cirrhosis in 6.0% [51]. In Argentina, the estimated MASLD prevalence in overall population was 37.2%; of them, 22.2% had at least significant fibrosis, being higher in excess weight (25%), hypertriglyceridemia (32%), and diabetes or abnormal glucose metabolism (34%) [52]. Finally, a Brazilian study has estimated a prevalence of significant fibrosis in 11.7%, advanced fibrosis in 7.6%, and cirrhosis in 15.4% of biopsy-proven individuals with MASLD [53] (Fig. 1B).
Screening for MASLD in the overall population is not recommended since isolated steatosis in the general population is not associated with a clinically meaningful increase in the risk of liver-related outcomes and is not fully supported by cost-effectiveness studies [54]. However, MASLD and liver fibrosis should be screened in individuals with abnormal liver enzymes, radiological signs of hepatic steatosis, and/or those with metabolic dysfunction. Based on the risk profile in Latin America, it seems appropriate to screen MASLD in patients with T2DM or excess weight, especially those aged over 50 years old [45,51–53,55]. Due to the high prevalence of the PNPLA3 I148M genetic variant in the Latin American population and the high prevalence of advanced fibrosis in first-degree relatives of probands with MASLD and advanced fibrosis (estimated around 14–16%), screening for MASLD could also be done in the first-degree relatives of patients with MASLD and advanced fibrosis [56–58].
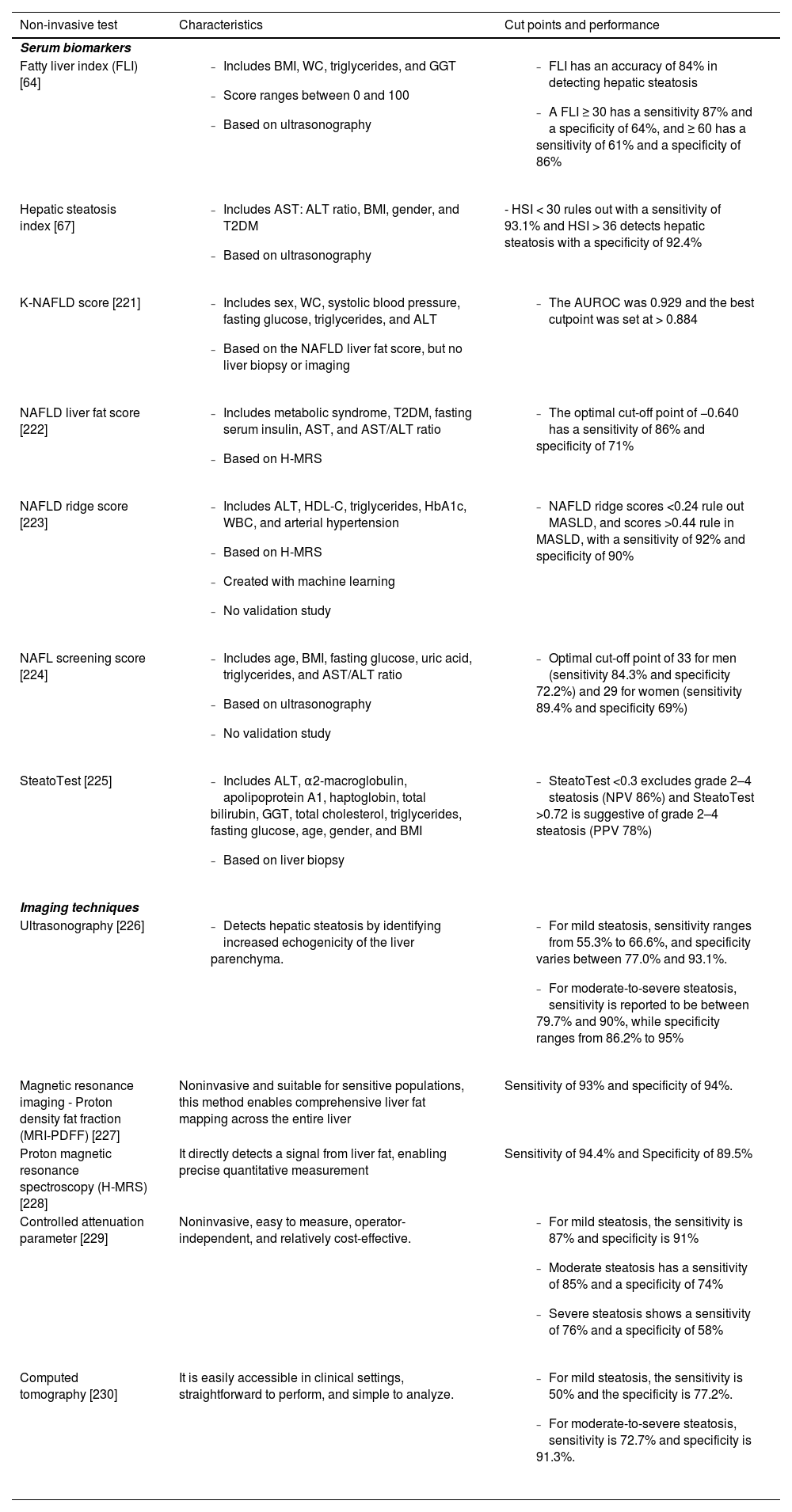
3.2Serum biomarkers for hepatic steatosisThe MASLD diagnosis is based on the detection of hepatic steatosis ≥5% of hepatocytes by histology [59]. While liver biopsy has traditionally been considered the gold standard for diagnosing liver fibrosis, its invasive nature, potential complications, and sampling variability have driven the development and validation of various non-invasive tests (NITs) [60]. Several NITs have been developed to overcome the risks and limitations of liver biopsy in routine practice (Table 1) [61,62]. However, compared to liver fibrosis, the absolute amount of liver fat content is a less robust prognosis marker and can substantially fluctuate over time [63]. In consequence, the main role of NITs in liver steatosis is supporting the diagnosis of SLD. Fatty Liver Index (FLI) is a simple biomarker of hepatic steatosis based on the body mass index (BMI), waist circumference, triglycerides, and gamma-glutamyl transferase (GGT). FLI is especially relevant in primary care settings and can predict liver-related mortality, all-cause mortality, as well as atherosclerosis, diabetes, CKD, and cancer [64–66] (Table 1). However, the most important limitations of FLI include the fact that waist circumference is often disregarded in clinical practice, and up to 27.5% of cases can fall into a grey zone [66]. Hepatic steatosis index (HSI) in another test derived and validated in a large cohort of individuals and has five components: serum aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio, BMI, gender, and T2DM. HSI has also demonstrated a moderate accuracy in detecting hepatic steatosis [67]. Unfortunately, both FLI and HSI have used ultrasonography as a reference standard [59]. Table 1 summarizes the most relevant NITs for hepatic steatosis for clinical practice.
Non-invasive tests to identify hepatic steatosis.
| Non-invasive test | Characteristics | Cut points and performance |
|---|---|---|
| Serum biomarkers | ||
| Fatty liver index (FLI) [64] |
|
|
| Hepatic steatosis index [67] |
| - HSI < 30 rules out with a sensitivity of 93.1% and HSI > 36 detects hepatic steatosis with a specificity of 92.4% |
| K-NAFLD score [221] |
|
|
| NAFLD liver fat score [222] |
|
|
| NAFLD ridge score [223] |
|
|
| NAFL screening score [224] |
|
|
| SteatoTest [225] |
|
|
| Imaging techniques | ||
| Ultrasonography [226] |
|
|
| Magnetic resonance imaging - Proton density fat fraction (MRI-PDFF) [227] | Noninvasive and suitable for sensitive populations, this method enables comprehensive liver fat mapping across the entire liver | Sensitivity of 93% and specificity of 94%. |
| Proton magnetic resonance spectroscopy (H-MRS) [228] | It directly detects a signal from liver fat, enabling precise quantitative measurement | Sensitivity of 94.4% and Specificity of 89.5% |
| Controlled attenuation parameter [229] | Noninvasive, easy to measure, operator-independent, and relatively cost-effective. |
|
| Computed tomography [230] | It is easily accessible in clinical settings, straightforward to perform, and simple to analyze. |
|
There are several imaging techniques to identify hepatic steatosis (Table 1). Among these imaging, ultrasonography is the most commonly used to screen and identify hepatic steatosis in routine practice [68]. Ultrasonography detects hepatic steatosis by identifying increased echogenicity of the liver parenchyma, a hallmark of fat accumulation within hepatocytes. Ultrasonography constitutes the first-line screening tool due to its accessibility and ease of use [69,70]. Some scoring system have been proposed to quantify liver steatosis by using ultrasound. For example, a scoring system ranging from 0 to 6, including bright of the liver and hepatorenal echo contrast, deep attenuation, and vessel blurring, evidenced a high accuracy in diagnosing MASLD [71]. Ultrasonographic Fatty Liver Indicator is another scoring system ranging from 2 to 8 based on the intensity of liver/kidney contrast, THE posterior attenuation of ultrasound beam, vessel blurring, difficult visualization of gallbladder wall, difficult visualization of the diaphragm, and areas of focal sparing [72]. This latter scoring system can predict severity of MASH, highlighting the utility of ultrasonography in clinical practice.
Magnetic Resonance Imaging (MRI), particularly using the Proton Density Fat Fraction (PDFF) technique, offers a more accurate quantification of hepatic fat content and is considered the reference standard in clinical trials, reaching a high correlation with liver biopsy [59]. MRI-PDFF is reproducible and a ≥ 30% relative decline in MRI-PDFF is associated with higher odds of histologic response and MASH resolution, guiding treatment and decision-making, especially in those with more aggressive phenotypes [73]. Controlled attenuation parameter (CAP) is another non-invasive tool to measure ultrasonography attenuation when traveling through fatty liver tissue and is incorporated in the FibroScan® (Echosens, Paris, France) equipment, but the cost of the equipment is higher than the conventional ultrasonography [74]. In MASLD patients using MRI-PDFF as a gold standard, the area under the receiver operating characteristic (AUROC) of CAP for the detection of MRI‐PDFF ≥ 5% was 0.80 with a cut-point ≥ 288 dB/m and 0.87 for the detection of MRI‐PDFF ≥ 10% using a cut‐point ≥ 306 dB/m [75]. Computed tomography (CT) can also detect hepatic steatosis based on the attenuation values of liver tissue. However, the use of CT for this purpose is limited due to radiation exposure and its relatively lower sensitivity for detecting mild steatosis compared to MRI-PDFF [76].
3.4Clinical assessment of metabolic dysfunction and differential diagnosesThe clinical assessment of patients with MASLD is a multifaceted process that requires careful consideration of both metabolic and lifestyle factors to ensure an accurate diagnosis and appropriate management. The initial evaluation should begin with a detailed patient history, focusing on the presence of metabolic risk factors (also known as metabolic dysfunction factors), including 1) waist circumference > 80 cm in women and > 94 cm in men (>90 in Asian); 2) HDL-cholesterol level < 40 mg/dL in men and < 50 mg/dL in women or in treatment; 3) Triglycerides > 150 mg/dL, or in treatment; 4) Fasting blood glucose > 100 mg/dL, or in treatment; and 5) Systolic blood pressure > 130 mm/Hg or diastolic blood pressure > 85 mm/Hg, or in treatment (Fig. 2) [23]. The identification of metabolic dysfunction based on at least 1 of 5 criteria not only supports the diagnosis of SLD, but it can also influence the progression of the disease. For example, glycemic control has been linked to a higher severity of MASLD [77] and poses the need to address and treat these risk factors in patients with MASLD [78]. A thorough understanding of dietary patterns, physical activity, and other lifestyle factors, including smoking, is also essential, as these can influence disease progression and the effectiveness of therapeutic interventions [79,80].
Beyond assessing metabolic and alcohol-related factors, clinicians must also consider potential differential diagnoses that can present with similar clinical features to MASLD. These include viral hepatitis (e.g., hepatitis B and C), autoimmune liver diseases (e.g., autoimmune hepatitis, primary biliary cholangitis), genetic liver disorders (e.g., hemochromatosis, Wilson's disease), and drug-induced liver injury (DILI) [23,54] (Fig. 2). To rule out these conditions, a series of laboratory tests are typically performed, including liver tests (i.e., AST, ALT, alkaline phosphatase, and bilirubin), serological markers for hepatitis B and C, autoantibodies for autoimmune liver diseases. In addition, a detailed medication history is necessary to identify any drugs or supplements that might contribute to liver injury, such as methotrexate, amiodarone, corticosteroids, valproic acid, 5-fluorouracil, irinotecan, among others [81].
4Non-invasive techniques to identify liver fibrosisCurrently, liver fibrosis staging is the most important method to stratify risk, monitor disease progression, and guide therapeutic interventions [82]. In the following paragraphs, we will discuss serum NITs, imaging modalities, and elastography-based methods to stage liver fibrosis, assessing the pros and cons in the routine use.
4.1Serum biomarkersSerum biomarkers have been extensively studied in population-based studies and include the Fibrosis-4 Index (FIB-4), NAFLD fibrosis score (NFS), Hepamet, AST to Platelet Ratio Index (APRI), the Enhanced Liver Fibrosis (ELF) test, among others (Table 2) [83]. The FIB-4 score, which combines age, AST, ALT, and platelet count, is one of the most widely used NITs to estimate liver fibrosis [84]. Its simplicity, low cost, and ability to stratify patients have positioned FIB-4 as the first step triage process to identify MASH patients at risk of fibrosis. In particular, values <1.3 to rule out advanced fibrosis, and >2.67 (or >3.25 in some studies) to suspect a high risk of advanced fibrosis [85]. Other models, such as NFS, Hepamet, and APRI, have demonstrated a reasonable performance in stratifying patients with MASLD. In particular, a Latin American study including biopsy-proven MASLD patients showed that Hepamet and FIB-4 outperformed NFS in detecting significant fibrosis [86]. However, these three scores were not significantly different in predicting advanced fibrosis or cirrhosis.
Non-invasive tests to identify liver fibrosis.
| Non-invasive test | Characteristics | Cut points and performance |
|---|---|---|
| Serum biomarkers | ||
| Fibrosis-4 (FIB-4) [84] |
|
|
| NAFLD fibrosis score (NFS) [231] |
|
|
| Hepamet [232] |
|
|
| AST to Platelet Ratio Index (APRI) [233] | Includes AST level and platelet count |
|
| Enhanced Liver Fibrosis (ELF) [87] |
|
|
| Pro C3 [234] | Marker of type III collagen formation |
|
| Imaging techniques | ||
| Vibration-controlled transient elastography (VCTE) [235] | It measures the speed of a mechanically generated shear wave traveling through the liver to obtain a liver stiffness measurement. | A liver stiffness measurement (LSM) cutoff of 6.5 kPa excluded advanced fibrosis with a negative predictive value of 0.91, while a cutoff of 12.1 kPa excluded cirrhosis with a negative predictive value of 0.99. |
| Magnetic resonance elastography (MRE) [93] | Assesses liver stiffness by evaluating the propagation of mechanical waves, offering detailed imaging of tissue elasticity. | In MASLD, the optimal thresholds for MRE are F1 ≥2.61, F2 ≥2.97, F3 ≥3.62, and F4 ≥4.69 kPa [92] |
| Acoustic radiation force impulse (ARFI) [236] | An ultrasonography-based diagnostic technique that assesses tissue stiffness by evaluating the propagation speed of shear waves. | For staging significant fibrosis (F≥2), ARFI has a sensitivity of 79% and specificity of 81%. For staging severe fibrosis (F≥3), sensitivity is 92% and specificity is 85%. For staging cirrhosis (F=4), ARFI shows both 89% sensitivity and 89% specificity. |
| Two-dimensional shear wave elastography (2D-SWE)[237] | Two-dimensional shear wave elastography (2D-SWE) evaluates liver stiffness in real time, alongside anatomic B-mode ultrasonography imaging, and allows for precise selection of a region of interest within the liver. | The cut-off values for 2D-SWE to rule out liver disease are 6.3 kPa for diagnosing significant fibrosis (≥F2), with a sensitivity of 90%, and 10.5 kPa for diagnosing cirrhosis (F4). |
The ELF test is a more recent advancement that measures serum levels of three direct markers of fibrosis: hyaluronic acid, procollagen III amino-terminal peptide, and tissue inhibitor of metalloproteinases-1 [87]. The ELF test provides a score that correlates with the severity of liver fibrosis and has been validated in various liver diseases, including MASLD. A prior systematic review showed that the ELF > 7.7 had a sensitivity of 90% for excluding fibrosis [88]. However, to achieve a specificity of 90% for advanced and significant fibrosis, ELF thresholds >10.18 (sensitivity 57%) and > 9.86 (sensitivity 55%) are required [88]. Other serum biomarkers, such as FibroTest, FIBC3, ADAPT, and PRO-C3 are available in practice. However, most current direct NITs only marginally outperform FIB-4 when targeting the presence of advanced fibrosis, while limited availability and cost may also limit their wider adoption [83].
4.2Imaging modalitiesFor the assessment of liver fibrosis, elastography-based techniques have become the cornerstone of non-invasive evaluation [59]. Liver stiffness measurement (LSM) by vibration-controlled transient elastography (VCTE) assesses the velocity of a shear wave generated by a mechanical pulse [89]. LSM is directly correlated with the degree of fibrosis, and VCTE has been extensively validated across different liver diseases, including MASLD [90]. VCTE is non-invasive, quick, and can be performed at the point of care. Although VCTE may be less accurate in patients with high BMI, an XL probe can be used in those with no valid shot or unreliable measurement [91]. In MASLD, the cut-points for VCTE are ≥ 6.2, ≥ 7.6, ≥ 8.8, and ≥ 11.8 kPa, for F1, F2, F3 and F4, respectively [92]. However, these cut-points may differ according to the clinical characteristics of the studied population.
Magnetic resonance elastography (MRE) is an advanced imaging technique that combines the principles of MRI and elastography to assess liver stiffness [93]. MRE has shown superior accuracy compared to VCTE in detecting advanced fibrosis, particularly in patients with obesity or other conditions that may interfere with VCTE measurements [92]. MRE provides a comprehensive assessment of liver fibrosis with high sensitivity and specificity, making it a valuable tool in clinical practice and research. However, like other MRI-based techniques, MRE is costly and less available in routine practice. The optimal thresholds for MRE are F1 ≥ 2.65, F2 ≥ 3.14, F3 ≥ 3.53, and F4 ≥ 4.45 kPa for MASLD patients [94].
Other techniques, such as two-dimensional shear wave elastography (2D-SWE) [95], have evidenced a comparable performance to VCTE in predicting liver fibrosis. The advantage of 2D-SWE is its integration with standard ultrasonography machines, allowing simultaneous assessment of hepatic steatosis and fibrosis during a single examination.
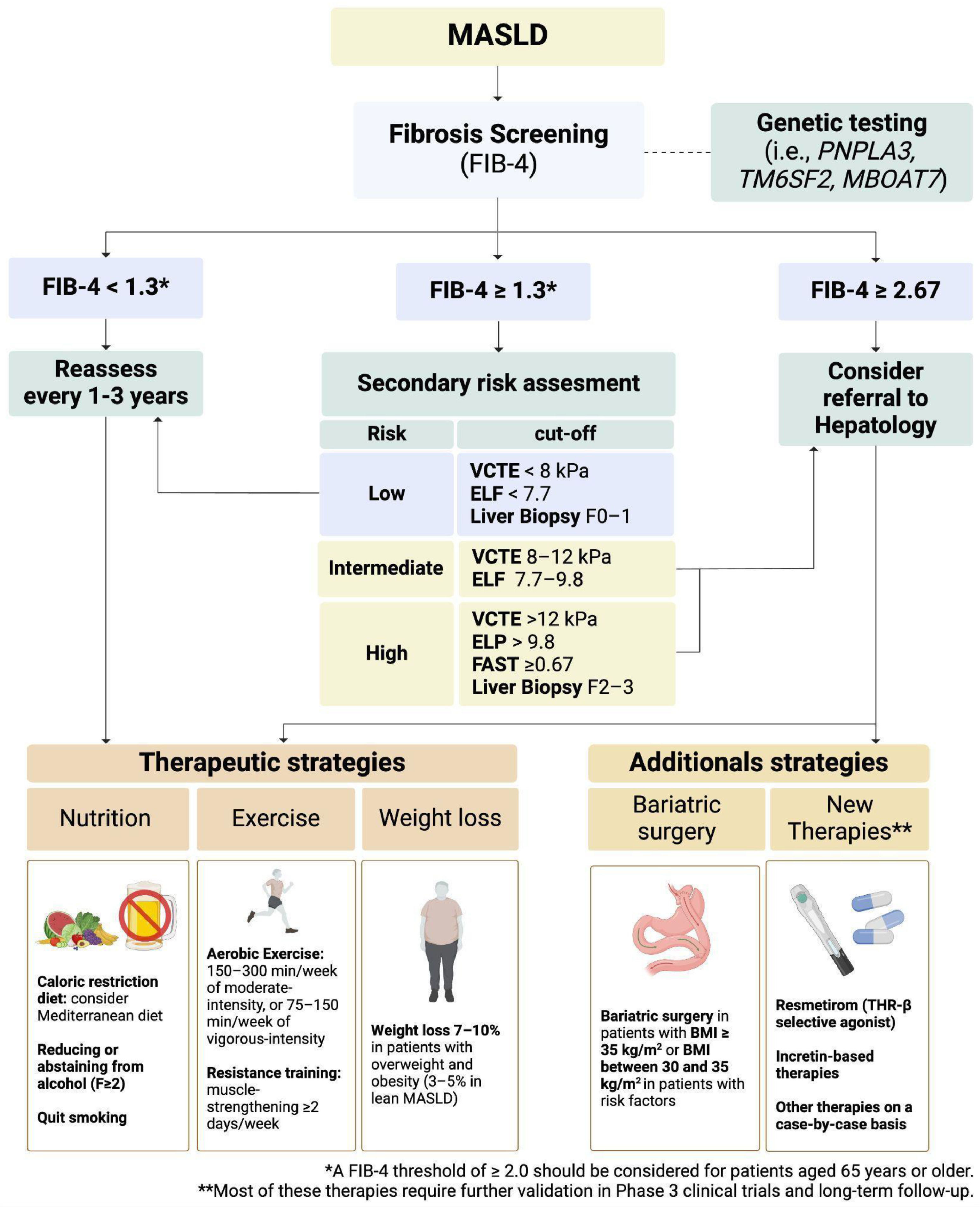
5Risk stratification in routine practice5.1Sequential algorithms combining NITs of liver fibrosisGiven the limitations of individual NITs, combining different modalities can improve diagnostic accuracy. For example, a combination of FIB-4 and VCTE can enhance the identification of advanced fibrosis in patients with MASLD [82]. Similarly, integrating serum biomarkers with imaging findings can provide a more comprehensive assessment of liver disease severity and guide clinical decision-making. Thus, most accepted algorithms to identify liver fibrosis include the first stratification with FIB-4 in low-risk (FIB-4 < 1.3 or < 2.0 for the age > 65 years), indeterminate risk (FIB-4 1.3–2.67 or 2.0–2.67 for the age > 65 years), and high-risk (FIB-4 > 2.67). Posteriorly, those with indeterminate and high risk should undergo a step 2 analysis using a technique with higher sensitivity and specificity to determine risk and liver fibrosis stage [82] (Fig. 3). Therefore, patients with indeterminate risk according to an elastography method or FIB-4 > 2.67 should be referred to the gastroenterologist or hepatologist for further assessment of liver fibrosis and management. Finally, a liver biopsy should be considered in case of indeterminate-risk or discordant results, conflict with other radiologic findings, or when alternative etiologies for liver disease are suspected [82]. Although this algorithm seems the most appropriate to be to stratify liver fibrosis in Latin America due to its simplicity and accessibility, recent evidence suggests that it could underestimate the fibrosis risk in the Hispanic population [96]. In addition, other alternative NITs can be tailored according to the resources and local availability. Further studies should explore the best combination of strategies and thresholds to stratify liver fibrosis risk in Latin America.
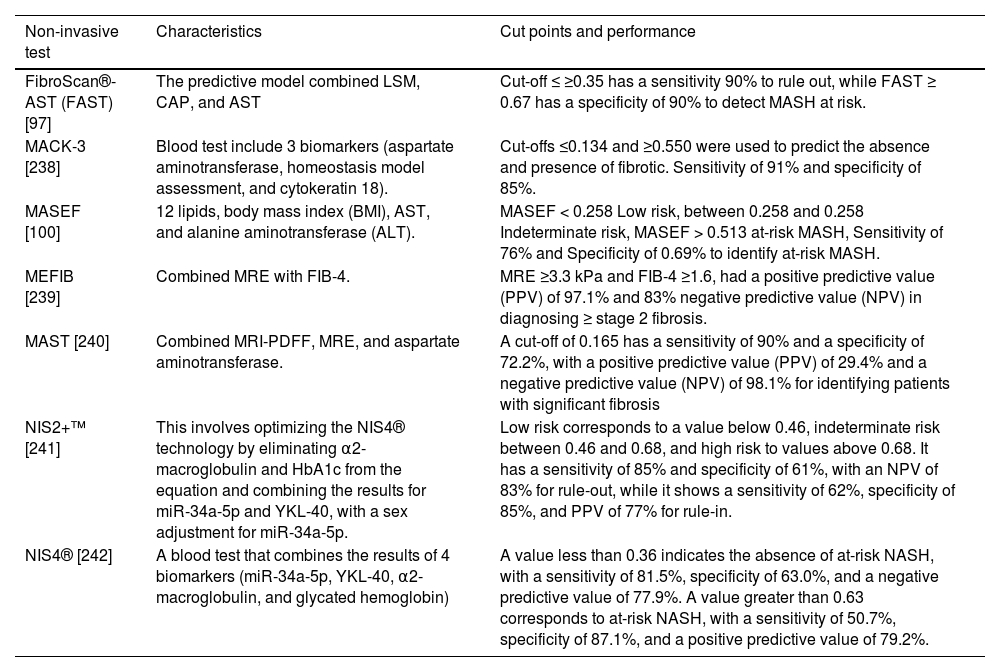
5.2Identification of at-risk MASHAs mentioned before, patients with active MASH and significant fibrosis are at higher risk of liver fibrosis progression [97]. This particular population has been named at-risk MASH and is defined by a significant liver fibrosis (≥F2), and elevated NAFLD activity score (NAS≥4). The identification of patients with at-risk MASH is key for decision-making, being the population that could benefit most from pharmacological therapies and participate in clinical trials [98]. Since most patients would not undergo a liver biopsy, several NITs can facilitate the identification of at-risk MASH, including, FibroScan®-AST (FAST), MAST, MEFIB, MASEF, MACK-3, NIS2+™, NIS4®, among others (Table 3) [99,100]. Among these NITs, FAST includes LSM from VCTE, CAP, and AST, and the cut-off ≤ 0.35 has a sensitivity ≥90% to rule out, while FAST ≥ 0.67 has a specificity ≥ 90% to detect at-risk MASH [97]. Despite some limitations, FAST seems a reasonable method to identify at-risk MASH in Latin America due to the wide availability of VCTE in our region.
Non-invasive tests to identify patients with metabolic dysfunction associated steatohepatitis (MASH) at-risk (significant liver fibrosis [≥F2] and elevated NAFLD activity score [NAS≥4]).
| Non-invasive test | Characteristics | Cut points and performance |
|---|---|---|
| FibroScan®-AST (FAST) [97] | The predictive model combined LSM, CAP, and AST | Cut-off ≤ ≥0.35 has a sensitivity 90% to rule out, while FAST ≥ 0.67 has a specificity of 90% to detect MASH at risk. |
| MACK-3 [238] | Blood test include 3 biomarkers (aspartate aminotransferase, homeostasis model assessment, and cytokeratin 18). | Cut-offs ≤0.134 and ≥0.550 were used to predict the absence and presence of fibrotic. Sensitivity of 91% and specificity of 85%. |
| MASEF [100] | 12 lipids, body mass index (BMI), AST, and alanine aminotransferase (ALT). | MASEF < 0.258 Low risk, between 0.258 and 0.258 Indeterminate risk, MASEF > 0.513 at-risk MASH, Sensitivity of 76% and Specificity of 0.69% to identify at-risk MASH. |
| MEFIB [239] | Combined MRE with FIB-4. | MRE ≥3.3 kPa and FIB-4 ≥1.6, had a positive predictive value (PPV) of 97.1% and 83% negative predictive value (NPV) in diagnosing ≥ stage 2 fibrosis. |
| MAST [240] | Combined MRI-PDFF, MRE, and aspartate aminotransferase. | A cut-off of 0.165 has a sensitivity of 90% and a specificity of 72.2%, with a positive predictive value (PPV) of 29.4% and a negative predictive value (NPV) of 98.1% for identifying patients with significant fibrosis |
| NIS2+™ [241] | This involves optimizing the NIS4® technology by eliminating α2-macroglobulin and HbA1c from the equation and combining the results for miR-34a-5p and YKL-40, with a sex adjustment for miR-34a-5p. | Low risk corresponds to a value below 0.46, indeterminate risk between 0.46 and 0.68, and high risk to values above 0.68. It has a sensitivity of 85% and specificity of 61%, with an NPV of 83% for rule-out, while it shows a sensitivity of 62%, specificity of 85%, and PPV of 77% for rule-in. |
| NIS4® [242] | A blood test that combines the results of 4 biomarkers (miR-34a-5p, YKL-40, α2-macroglobulin, and glycated hemoglobin) | A value less than 0.36 indicates the absence of at-risk NASH, with a sensitivity of 81.5%, specificity of 63.0%, and a negative predictive value of 77.9%. A value greater than 0.63 corresponds to at-risk NASH, with a sensitivity of 50.7%, specificity of 87.1%, and a positive predictive value of 79.2%. |
Genotyping in patients with MASLD has gained increasing attention as a tool for understanding the genetic predispositions that contribute to disease risk and progression. The PNPLA3 I148M variant, which is highly prevalent in Latin American populations, has been strongly associated with increased risk of MASLD, progression of liver disease, and HCC [101]. Some studies assessing polygenic risk scores (including PNPLA3) have demonstrated an adequate stratification risk and the reduction of misclassification of traditional algorithms to identify liver fibrosis [102–104]. However, the implementation of widespread genotyping in Latin America faces challenges, including limited access to genetic testing, cost considerations, and the need for robust infrastructure to integrate genetic data into clinical workflows [105]. Although genotyping holds promise for improving the diagnosis and management of MASLD in midlife [105], it is necessary to have validated criteria to apply data from genetic testing in clinical practice.
6Non-pharmacological treatments in MASLDThe management of MASLD primarily focuses on non-pharmacological interventions, which are considered the cornerstone of treatment [106]. These interventions aim to address the underlying metabolic dysfunctions and lifestyle factors contributing to the disease, reduce hepatic steatosis, prevent disease progression, and improve overall health outcomes.
6.1Dietary interventions and weight lossDietary modification is a key component of the non-pharmacological management of MASLD. Several dietary patterns have been studied for their impact on liver health, with the Mediterranean diet emerging as one of the most beneficial [107,108]. The Mediterranean diet is rich in fruits, vegetables, whole grains, lean proteins (particularly fish), and healthy fats such as olive oil [108]. The Mediterranean diet has a high content of monounsaturated fats and omega-3 fatty acids, which help reduce liver fat accumulation, improve insulin sensitivity, and reduce hepatic steatosis even in the absence of significant weight loss [109]. Additionally, the Mediterranean diet may impact gut microbiota, which can reduce hepatic inflammation and improve metabolic parameters [110]. This diet is not only effective in reducing hepatic steatosis but also can reduce major cardiovascular events, which are particularly important given the high prevalence of cardiovascular disease in patients with MASLD [111]. Another dietary approach is the low-carbohydrate diet, which restricts the intake of refined carbohydrates and sugars, particularly fructose, which is strongly associated with liver fat accumulation [112]. Reducing the intake of sugary beverages, processed foods, and high-fructose corn syrup is recommended to decrease hepatic fat content [113]. Additionally, reducing overall caloric intake and avoiding foods high in saturated fats and trans fats can further support weight loss and liver health. In absence of other medical contraindications, coffee intake is safe and evidence suggests that could reduce the development of MASLD [114].
Weight loss is the most effective non-pharmacological approach for managing MASLD, producing a significant decrease in liver fat content (LFC), steatohepatitis, and liver fibrosis in a dose-dependent fashion [106]. The recommended target weight loss is 7-10% of total body weight (10% in at-risk MASH), as this degree of weight reduction is associated with a significant improvement in liver histology [115]. In addition, those with lean MASLD could benefit from a weight loss of 3–5% of total body weight. Unfortunately, achieving and sustaining weight loss is not easy in the overall population, and only a fraction of MASLD patients achieve these goals and maintain adequate weight in the long term [116].
6.2Physical activity and exerciseRegular physical activity is another critical component of the non-pharmacological treatment of MASLD. Particularly, non-occupational physical activity can reduce MASLD prevalence and reduce all-cause mortality [117]. In the case of structured and planned physical activity, exercise improves insulin sensitivity, promotes weight loss, and reduces hepatic fat accumulation [118]. Both aerobic exercise (i.e., walking, running, cycling, or swimming) and resistance training (i.e., weightlifting or bodyweight exercises) have been shown to be beneficial for patients with MASLD [119]. Adults without cardiovascular or musculoskeletal contraindications should do 150–300 minutes a week of moderate-intensity, or 75–150 minutes a week of vigorous-intensity aerobic physical activity or an equivalent combination of moderate- and vigorous-intensity aerobic activity [120]. Also, they should do muscle-strengthening activities on 2 or more days a week [121].
6.3Alcohol minimization or abstinence and cigarette smokingFor patients who consume alcohol, clinicians should assess the level of intake and provide counseling on the benefits of reducing or abstaining from alcohol [122]. Alcohol use evidenced an increase in the risk of liver fibrosis in a dose-dependent fashion, exhibiting a supra-additive interaction with cardiometabolic risk factors [123]. Given the potential role of alcohol in exacerbating MASH, contributing to fibrosis progression, and the increase in the risk of HCC [22], complete alcohol abstinence should be recommended for all patients with MASLD and at least significant fibrosis (F2) [81]. Cigarette smoking negatively affects the progression of MASLD, and increases risk of HCC, and major cardiovascular events [79]. In consequence, all patients with MASLD who are active cigarette smokers should be advised and assisted to quit smoking.
7Pharmacologic therapies for MASH: progress in Phase 3 and 2 clinical trialsTo date, only resmetirom has been approved by the Food and Drug Administration (FDA) in MASLD, highlighting the scarce therapeutic options and urgent need for the development of new drugs [124]. Recent advancements in pharmacotherapy targeting various aspects of MASH pathogenesis have shown promise in Phase 3 and 2 clinical trials. The drugs discussed here are categorized by their phase of clinical testing and target mechanisms within MASH pathophysiology (Table 4).
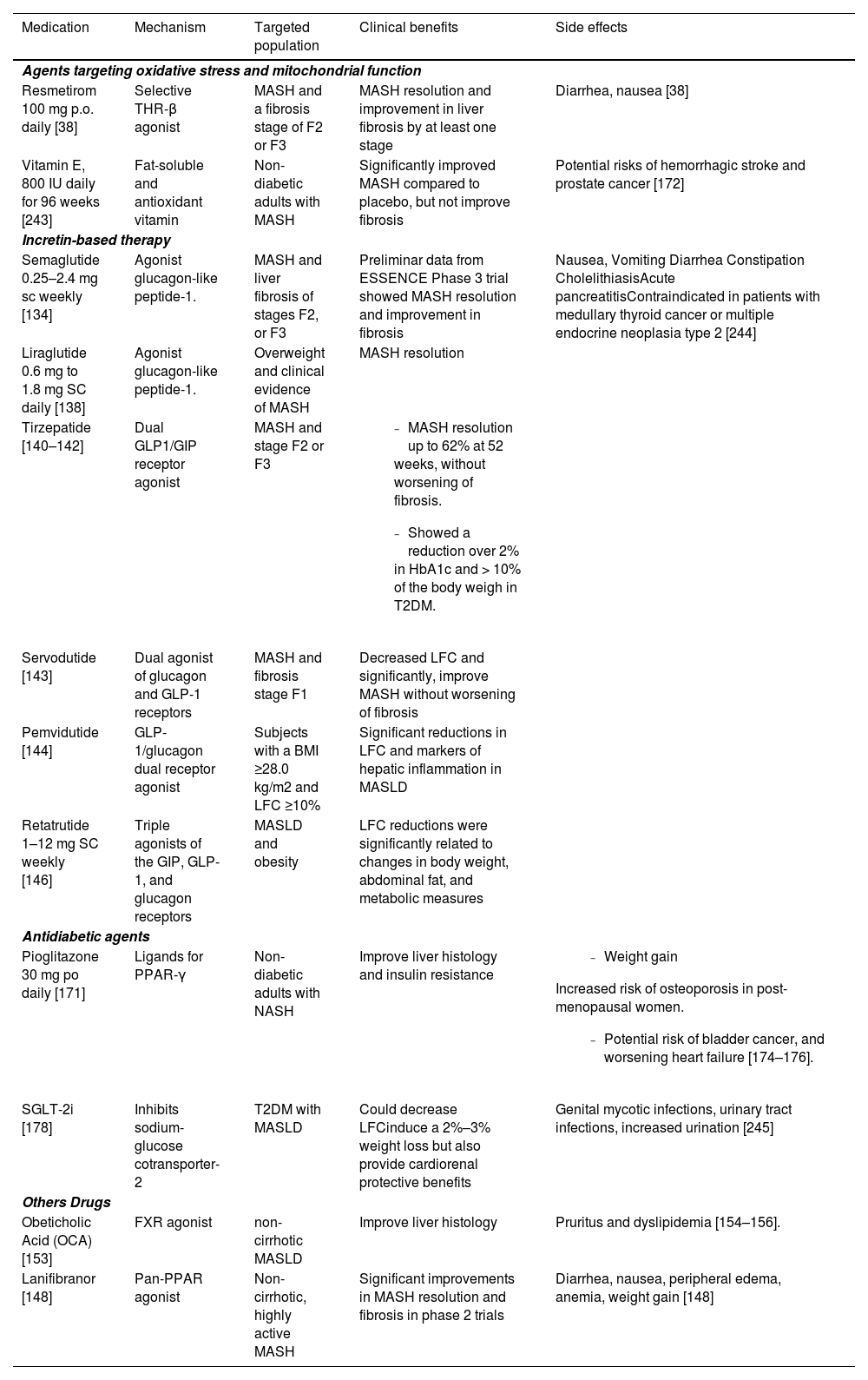
Pharmacological therapies in metabolic dysfunction-associated steatotic liver disease (MASLD). All medications listed have demonstrated benefit in patients with MASLD based on histological endpoints and/or magnetic resonance imaging. Although only resmetirom have been approved for treatment of MASH, other therapies can be used in carefully selected individuals with MASH and comorbid conditions such as diabetes and obesity or for off-label use on a case-by-case basis.
| Medication | Mechanism | Targeted population | Clinical benefits | Side effects |
|---|---|---|---|---|
| Agents targeting oxidative stress and mitochondrial function | ||||
| Resmetirom 100 mg p.o. daily [38] | Selective THR-β agonist | MASH and a fibrosis stage of F2 or F3 | MASH resolution and improvement in liver fibrosis by at least one stage | Diarrhea, nausea [38] |
| Vitamin E, 800 IU daily for 96 weeks [243] | Fat-soluble and antioxidant vitamin | Non-diabetic adults with MASH | Significantly improved MASH compared to placebo, but not improve fibrosis | Potential risks of hemorrhagic stroke and prostate cancer [172] |
| Incretin-based therapy | ||||
| Semaglutide 0.25–2.4 mg sc weekly [134] | Agonist glucagon-like peptide-1. | MASH and liver fibrosis of stages F2, or F3 | Preliminar data from ESSENCE Phase 3 trial showed MASH resolution and improvement in fibrosis | Nausea, Vomiting Diarrhea Constipation CholelithiasisAcute pancreatitisContraindicated in patients with medullary thyroid cancer or multiple endocrine neoplasia type 2 [244] |
| Liraglutide 0.6 mg to 1.8 mg SC daily [138] | Agonist glucagon-like peptide-1. | Overweight and clinical evidence of MASH | MASH resolution | |
| Tirzepatide [140–142] | Dual GLP1/GIP receptor agonist | MASH and stage F2 or F3 |
| |
| Servodutide [143] | Dual agonist of glucagon and GLP-1 receptors | MASH and fibrosis stage F1 | Decreased LFC and significantly, improve MASH without worsening of fibrosis | |
| Pemvidutide [144] | GLP-1/glucagon dual receptor agonist | Subjects with a BMI ≥28.0 kg/m2 and LFC ≥10% | Significant reductions in LFC and markers of hepatic inflammation in MASLD | |
| Retatrutide 1–12 mg SC weekly [146] | Triple agonists of the GIP, GLP-1, and glucagon receptors | MASLD and obesity | LFC reductions were significantly related to changes in body weight, abdominal fat, and metabolic measures | |
| Antidiabetic agents | ||||
| Pioglitazone 30 mg po daily [171] | Ligands for PPAR-γ | Non-diabetic adults with NASH | Improve liver histology and insulin resistance |
|
| SGLT-2i [178] | Inhibits sodium-glucose cotransporter-2 | T2DM with MASLD | Could decrease LFCinduce a 2%–3% weight loss but also provide cardiorenal protective benefits | Genital mycotic infections, urinary tract infections, increased urination [245] |
| Others Drugs | ||||
| Obeticholic Acid (OCA) [153] | FXR agonist | non-cirrhotic MASLD | Improve liver histology | Pruritus and dyslipidemia [154–156]. |
| Lanifibranor [148] | Pan-PPAR agonist | Non-cirrhotic, highly active MASH | Significant improvements in MASH resolution and fibrosis in phase 2 trials | Diarrhea, nausea, peripheral edema, anemia, weight gain [148] |
THR-β: Selective thyroid hormone receptor-β; LFC: Liver Fat Content; GLP-1: glucagon-like peptide-1; FXR: Farnesoid X receptor; PPAR: peroxisome proliferator-activated receptor; p.o., by mouth; s.c., subcutaneous; SGLT-2i, sodium glucose cotransporter-2 inhibitor; T2DM, type 2 diabetes mellitus.
Resmetirom is a selective THR-β agonist that reduces hepatic de novo lipogenesis and improves mitochondrial function, thereby reducing the detrimental effects of liver metabolic reprogramming in MASH [125]. Resmetirom has been associated with a significant reduction in hepatic fat content and liver stiffness [126–128]. In addition, a recent Phase 3 trial (MAESTRO-NASH) including 966 patients with MASH demonstrated a MASH resolution with no worsening of fibrosis in 25.9% of the patients in the 80-mg resmetirom group and 29.9% of those in the 100-mg resmetirom group, as compared with 9.7% of the placebo group [38]. Fibrosis improvement by at least one stage with no worsening of the NAFLD activity score was achieved in 24.2% of the patients in the 80-mg resmetirom group and 25.9% of those in the 100-mg resmetirom group, as compared with 14.2% of those in the placebo group. In addition, resmetirom also induces the low-density lipoprotein (LDL) receptor and increases LDL-cholesterol uptake by the liver [129], decreasing up to -16.3% of LDL cholesterol at 24 weeks [38]. Resmetirom has an adequate safety profile and is approved in doses of 80 or 100 mg daily for patients with MASH and significant or advanced fibrosis (F2–3). Other THR-β agonists like VK2809 and TERNS-501 are also under investigation for their metabolic benefits in the liver [130,131].
7.2Incretin-based therapiesGLP-1 (glucagon-like peptide-1) and glucose-dependent insulinotropic polypeptide (GIP; gastric inhibitory polypeptide) are gastrointestinal hormones with receptors mainly in the pancreas, but also in other organs such as the stomach, heart, and central nervous system [132,133]. There are several GLP-1 receptor agonists (RAs), which vary in their frequency of administration, potency in metabolic control, weight control, and reducing cardiovascular events. Semaglutide and liraglutide are two GLP-1-RAs with more evidence in MASH. In particular, semaglutide showed promising results in Phase 2 trials for MASH resolution in doses of 0.4 mg subcutaneously (SC) daily [134]. A phase 3 trial (ESSENCE) investigating semaglutide in patients with MASH and fibrosis stage F2–3 is currently ongoing. This trial randomized participants in a 2:1 ratio to receive once-weekly subcutaneous semaglutide 2.4 mg or placebo for 240 weeks [135]. Preliminary results from this trial at 72 weeks, which included 800 participants, showed that 62.9% of those receiving semaglutide achieved resolution of steatohepatitis compared to 34.1% in the placebo group (p<0.0001) [136]. Additionally, improvement in liver fibrosis without worsening of steatohepatitis was observed in 37.0% of participants receiving semaglutide versus 22.5% in the placebo group (p<0.0001). Moreover, 32.8% of participants in the semaglutide group achieved both resolution of steatohepatitis and improvement in liver fibrosis, compared to 16.2% in the placebo group (p<0.0001). Of note, in a trial including MASLD and compensated cirrhosis, semaglutide did not significantly improve fibrosis or achievement of NASH resolution versus placebo [137], suggesting that most benefit from semaglutide could be restricted for those with MASH and significant or advanced fibrosis. Liraglutide has also evidenced the benefit in MASH resolution in a small phase 2 trial (N=52), and usual doses range from 0.6 mg to 1.8 mg SC daily [138].
Dual incretin mimetics are also being evaluated for their potential to reduce LFC and improve metabolic outcomes in MASH patients [139]. Tirzepatide is a dual GLP-1/GIP receptor agonist molecule with synergistic activity that has exhibited a greater reduction in body weight and blood glucose than other molecules [140]. In patients with T2DM, tirzepatide has shown a reduction of over 2% in HbA1c and > 10% of the body weight [141]. In people living with obesity who do not have diabetes, tirzepatide also demonstrated a weight reduction of at least 15%, even at low doses [140]. In MASH, a phase 2 trial including patients with moderate or severe fibrosis evidenced that tirzepatide achieved a MASH resolution of up to 62% at 52 weeks, without worsening of fibrosis [142]. Survodutide is another dual agonist of glucagon and GLP-1 receptors with potential benefits in MASH. In particular, a phase 2 trial showed that survodutide can decrease LFC and significantly improve MASH without worsening of fibrosis [143]. Pemvidutide is another dual agonist of glucagon and GLP-1 receptor agonists that has shown significant reductions in LFC and markers of hepatic inflammation in MASLD [144].
Retatrutide is a triple agonist of the GIP, GLP-1, and glucagon receptors that has demonstrated a decrease of up to 24.2% in body weight [145]. In patients with MASLD and obesity, retatrutide has shown a decrease in LFC in up to 86% of patients in a dose of 12 mg SC weekly [146]. Some GLP-1-RAs have also evidenced a potential effect of decreasing alcohol use, being a promising therapeutic agent in individuals with SLD and significant levels of alcohol use [147]. Further phase 3 as well as head-to-head trials will provide more insights into the benefit from incretin-based therapies and criteria for routine use in clinical practice.
Incretin mimetics are safe drugs to be used in routine practice; however, the most common adverse effects include gastrointestinal events (nausea, vomiting, diarrhea, and constipation) and more events of cholelithiasis. Although a link with pancreatic adverse events is difficult to rule out, no signals of acute pancreatitis were present in longer-term cardiovascular outcomes trials. Also, they are contraindicated in patients with medullary thyroid cancer or multiple endocrine neoplasia type 2 (MEN 2).
7.3Lanifibranor and other peroxisome proliferator-activated receptor (PPAR) agonistsLanifibranor, a pan-peroxisome proliferator-activated receptor (PPAR) agonist, exhibited significant improvements in MASH resolution and fibrosis in phase 2 trials [148]. The ongoing phase 3 trial employs the SAF score for histological endpoints and has stringent dual primary endpoints [148]. In addition, saroglitazar and elafibranor represent dual PPAR agonists with differing fates in clinical development; the former showed positive results in phase 2 trials [149–151], while the latter was discontinued after phase 3 trials [152].
7.4Farnesoid X receptor (FXR) agonistsObeticholic Acid (OCA) is an FXR agonist that was initially shown to improve liver histology in the FLINT study [153]. However, the subsequent REGENERATE trial revealed concerns about pruritus and dyslipidemia [154–156]. Thus, despite benefits in the improvement of liver fibrosis, the FDA has denied approval, citing modest efficacy and significant risks, leading to the cessation of its MASH program by Intercept Pharmaceuticals [156]. Other FXR agonists are in development; however, the FDA's decision on OCA has set a challenging precedent for this class of drugs, potentially affecting their future in MASH therapeutics [157,158].
7.5Other novel therapies in the pipelineFibroblast growth factor (FGF) analogs, including aldafermin, pegbelfermin, efruxifermin, and pegozafermin, are being investigated for their effects on glucose and lipid metabolism. While aldafermin development was halted [159–161], other FGF-21 analogs are showing favorable results and are moving toward phase 3 trials [162–166]. Also, agents such as fircostat and denifanstat targeting de novo lipogenesis pathways have demonstrated reductions in hepatic steatosis, with ongoing studies to further elucidate their therapeutic value [167–169]. Therapies focusing on fibrosis and mitochondrial function, as well as combinatorial approaches, are also under investigation [170]. These approaches are tailored to the multifaceted nature of MASH, aiming to concurrently target diverse pathological pathways.
8Medications approved for other indications with benefits for MASH8.1Vitamin EVitamin E has shown efficacy in MASH, including the PIVENS trial that compared Pioglitazone and vitamin E [171]. In PIVENS, vitamin E at a dose of 800 IU daily for 96 weeks significantly improved MASH compared to placebo. However, the benefits in the reduction of fibrosis are unclear and there are several concerns regarding the potential risks of vitamin E, particularly related to hemorrhagic stroke and prostate cancer [172]. Thus, vitamin E should not be used as a first-line therapy and patients should be informed of these potential risks before initiating long-term high-dose vitamin E therapy (e.g., 800 IU daily).
8.2PioglitazoneThiazolidinediones are ligands for PPAR-γ and are approved for treating T2DM. In MASLD, pioglitazone treatment has been shown to improve liver histology and insulin resistance [171]. Additionally, pioglitazone has been found to enhance lipid profiles [173]. However, potential side effects of pioglitazone include weight gain, an increased risk of osteoporosis in postmenopausal women, a debated risk of bladder cancer, and the potential for worsening heart failure in patients with preexisting cardiac conditions [174–176]. These adverse effects must be weighed against the reduced risk of stroke, non-fatal myocardial infarction, and all-cause mortality associated with pioglitazone. Therefore, pioglitazone could be considered as an alternative option in MASLD, especially in scenarios where more expensive agents are not accessible.
8.3Sodium-glucose cotransporter-2 inhibitors (SGLT-2i)SGLT-2i targets renal glucose reabsorption from the glomerular filtrate and are approved for the treatment of T2DM [177]. These inhibitors not only induce a 2%–3% weight loss but also provide cardiorenal protective benefits [177]. Only a few studies have investigated the role of SGLT-2i in MASLD and MASH, suggesting that the use of SGLT-2i could decrease LFC [178].
8.4MetforminMetformin is primarily used for its insulin-sensitizing effects, which help address the underlying insulin resistance commonly seen in MASLD patients. Although small and uncontrolled initial trials of metformin have shown an ALT reduction and an insulin-sensitising effect [179], there is no evidence that metformin alone can improve histology in MASH. Metformin is safe in most patients with MASLD, but should be discontinued in those individuals with decompensated cirrhosis or renal failure as this could increase mortality [180].
8.5StatinsStatins are lipid-lowering agents for the management of dyslipidemia, reducing cardiovascular risk [181]. Additionally, statins have been shown to have pleiotropic effects, including anti-inflammatory and antifibrotic properties, which can be beneficial in MASLD. For example, a recent cohort study showed that statin usage was associated with a lower risk of all-cause mortality, liver-related clinical events, and liver stiffness progression in patients with MASLD [182]. Although these findings require validation in large cohorts and clinical trials, the use of statins in MASLD is safe, with no increased risk of serious liver injury [183].
8.6AspirinAspirin has a key role in the management of cardiovascular risk. However, aspirin could also have a role in the decrease of hepatic inflammation, oxidative stress, and insulin resistance, potentially preventing the progression of liver fibrosis in MASLD [184]. A recent trial evidenced that low-dose aspirin (81 mg daily) significantly reduced hepatic fat content in patients with MASLD without cirrhosis over a 6-month period compared to placebo [185]. However, further studies with larger sample sizes and longer follow-up periods are necessary to confirm the long-term efficacy and safety of aspirin in MASLD beyond the management of cardiovascular risk.
9Bariatric surgery in MASLDBariatric surgery has emerged as a highly effective intervention for managing MASLD in patients with severe obesity, particularly those who have not achieved adequate weight loss or metabolic improvement through lifestyle modifications and medical therapy [186]. The profound weight loss induced by bariatric surgery leads to significant reductions in hepatic steatosis, inflammation, fibrosis, and cardiovascular events [187,188]. In contrast, bariatric surgery has been linked to complications, such as gastric leaks, stenosis, marginal ulcer, choledocholithiasis, short bowel syndrome, dumping syndrome, hernia, electrolyte imbalance, nutritional deficiency, or even rarely, acute liver failure [189]. Careful patient selection and comprehensive preoperative assessment are crucial, as individuals with advanced liver disease, particularly those with cirrhosis, may require specialized management or alternative treatment approaches [186]. In consequence, bariatric surgery should be considered as part of a multidisciplinary approach to MASLD and obesity, offering a powerful tool to not only address obesity but also to mitigate the risk of progression to more severe liver disease and its associated complications.
10Hepatocellular carcinoma and other malignancies in patients with MASLDHCC is a significant concern in patients with MASLD, as the risk of developing HCC is elevated even in the absence of cirrhosis, which traditionally has been considered a prerequisite for liver cancer [190]. In fact, 20-30% of HCC in MASLD occurs in individuals without cirrhosis [191]. The pathogenesis of HCC in MASLD is multifactorial, involving chronic hepatic inflammation, oxidative stress, and the accumulation of genetic and epigenetic alterations that promote carcinogenesis [192]. The metabolic abnormalities associated with MASLD, such as insulin resistance, obesity, and hyperlipidemia, further contribute to the oncogenic process by creating a pro-inflammatory and pro-fibrogenic hepatic environment [192]. In terms of chemoprevention, a prior systematic review evidenced that aspirin and statins could decrease HCC risk over time, making them attractive therapies to be tested in future clinical trials involving patients with MASLD [193].
Besides HCC, patients with MASLD are also at increased risk for other malignancies, particularly colorectal, pancreatic, and breast cancers [14,194]. Current evidence only supports the screening for HCC in all patients with cirrhosis, while some patients with advanced fibrosis (F3) could also benefit from a screening according to individual risk assessment [54]. Finally, clinicians must ensure that all patients with MASLD have all the screenings that correspond by age up to date.
11Management of relevant comorbidities in MASLD11.1Cardiovascular risk assessmentCardiovascular disease (CVD) is a leading cause of morbidity and mortality in patients with MASLD, making cardiovascular risk assessment a critical component of clinical management [195]. The presence of MASLD is closely associated with an increased risk of atherosclerosis, coronary artery disease, and other cardiovascular conditions, largely due to shared metabolic risk factors such as obesity, T2DM, dyslipidemia, and arterial hypertension [196]. Patients with MASLD often exhibit a pro-inflammatory and prothrombotic state, which further amplifies cardiovascular risk. Therefore, comprehensive cardiovascular risk assessment should be routinely performed in patients with MASLD, incorporating traditional risk factors like blood pressure, lipid profile, and glycemic control, as well as specific markers of subclinical atherosclerosis, such as carotid intima-media thickness or coronary artery calcium scoring [197]. Additionally, non-invasive imaging techniques, including echocardiography and stress testing, may be warranted in patients with higher cardiovascular risk profiles or symptoms suggesting coronary heart disease. Optimal care should also include an early referral of patients with high cardiovascular risk and a strong collaborative work with other related healthcare professionals. Thus, early identification and aggressive management of cardiovascular risk are crucial in improving long-term outcomes in patients with MASLD [196].
11.2Chronic kidney disease (CKD)MASLD has been linked to an increased risk of CKD, especially in those with liver fibrosis. For example, a systematic review evidenced a risk of incident CKD stage ≥3 1.45-fold higher in MASLD [198]. However, there is no consensus if there are direct pathophysiological mechanisms or if both diseases share similar risk factors [54]. Management of MASLD with CKD should involve a multidisciplinary approach, including hepatologists, nephrologists, endocrinologists, and primary care physicians. Due to the potential renal effects, patients with MASLD and T2DM could benefit from GLP-1-RAs and SGLT-2i [199].
11.3MASLD in people living with Human immunodeficiency virus (HIV)People living with HIV are at an increased risk of developing MASLD due to a combination of factors including metabolic syndrome, systemic inflammation, and the effects of antiretroviral therapy [200–202]. For instance, systematic review including European patients showed a MASLD prevalence of 42% in people living with HIV [203]. Studies have shown that MASLD in HIV is associated with higher fibrosis stages despite lower disease activity compared to those without HIV. This suggests that HIV-specific factors, beyond hepatic necroinflammation, contribute to fibrosis progression in MASLD with HIV [204]. Management of MASLD in people with HIV should involve a multidisciplinary approach and may require regular monitoring of liver function and liver fibrosis.
12Conclusions and perspectivesMASLD is extremely prevalent in the adult population globally, requiring NITs to stratify the risk of advanced liver disease or progression. A stepwise approach using a FIB-4 and posteriorly a VCTE including FAST estimation could facilitate the estimation of liver fibrosis stage and MASH at-risk. The landscape of MASH pharmacotherapy is rapidly evolving, with several drugs in advanced stages of clinical trials. While these therapies offer hope, the complexity of MASH pathophysiology and patient heterogeneity pose significant challenges. Resmetirom is the first FDA-approved therapy for MASLD with significant or advanced fibrosis, but several other therapies, including incretin-based therapies and SGLT-2i, can be considered according to the presence of comorbidities such as excess weight or T2DM. Multifaceted approaches and personalized medicine remain crucial in the quest to address this complex disease effectively. In particular, aspects such as genetic variants, body weight composition, and coexistence of alcohol use can facilitate the stratification of different trajectories of liver fibrosis progression and development of HCC [205–207].
Future drug approvals are anticipated, but there is a critical need for novel clinical trials. Further studies should focus on refining trial endpoints, optimizing patient selection, and developing personalized therapeutic strategies. Other key challenges in the MASLD research agenda include assessing the human and economic burden, identifying better models of care, understanding the needs and experiences of MASLD patients and communities, and determining the most effective educational and awareness strategies for healthcare providers and the general public [3,208]. Finally, public health policies also play a critical role in the prevention and management of MASLD. Regions with limited resources, such as parts of Latin America, lack comprehensive public health strategies on MASLD, and inadequate healthcare infrastructure may exacerbate the MASLD epidemic [209–213]. Unfortunately, MASLD has not been included in the World Health Organization (WHO) Global Non-communicable Diseases Action Plan [214]. Therefore, strengthening public health initiatives and ensuring their equitable distribution across populations are crucial steps in mitigating the impact of MASLD on a global scale.
FundingM.A. receives support from the Chilean government through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1241450). We acknowledge Novo Nordisk for supporting the Summit Task Force on Steatotic Liver Disease through the Latin American Association for the Study of the Liver (ALEH).
Author contributionsLuis Antonio Diaz: Conceptualization, Writing - Original Draft, Writing - Review and Editing, Juan Pablo Arab: Writing - Original Draft, Writing - Review and Editing; Francisco Idalsoaga: Writing - Original Draft, Writing - Review and Editing; Javiera Perelli: Writing - Review and Editing; Javier Vega: Review and Editing; Melisa Dirchwolf: Review and Editing, Javiera Carreño: Review and Editing, Bárbara Samith: Review and Editing, Cynthia Valério: Review and Editing, Rodrigo Oliveira Moreira: Review and Editing, Mónica Acevedo: Review and Editing, Javier Brahm: Review and Editing, Nelia Hernández: Review and Editing, Adrian Gadano: Review and Editing, Claudia P. Oliveira: Review and Editing, Marco Arrese: Review and Editing, Graciela Castro-Narro: Conceptualization, Review and Editing, Mario G. Pessoa: Conceptualization, Review and Editing.






![Global prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) according to fibrosis stage in the overall population, type 2 diabetes mellitus (T2DM), and obesity [215–219], and (B) Prevalence of MASLD according to fibrosis stage in the overall population, type 2 diabetes mellitus (T2DM), and obesity in Latin America [10,51,220]. Global prevalence of metabolic dysfunction-associated steatotic liver disease (MASLD) according to fibrosis stage in the overall population, type 2 diabetes mellitus (T2DM), and obesity [215–219], and (B) Prevalence of MASLD according to fibrosis stage in the overall population, type 2 diabetes mellitus (T2DM), and obesity in Latin America [10,51,220].](https://static.elsevier.es/multimedia/16652681/unassign/S1665268125001279/v1_202503201443/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)






