Introduction. Hepatic regenerating nodules (HRN) and focal nodular hyperplasia (FNH) are benign regenerating lesions of the liver that rarely occur in children. An increased incidence of these lesions is reported in children treated for cancer.
Material and methods. Eight children who developed FNH and HRN after treatment for malignancies in the Oncology unit at the “Bambino Gesu” Pediatric Hospital in Rome, were retrospectively analyzed.
Results. The lesions, considered in the differential diagnosis with metastatic relapse of the primitive disease, have been monitored with US or other available imaging techniques. Evolution of the lesions was observed in only 1 patient three years after the initial diagnosis of FNH.
Conclusion. In conclusion serial monitoring with imaging techniques is sufficient to rule out liver metastasis and to monitor the evolution of the lesions. Surgery is suggested only in the case of complications.
Hepatic regenerating nodules (HRN) and focal nodular hyperplasia (FNH) are benign regenerating lesions of the liver that rarely occur in children.1
An increased incidence has been described in patients years after treatment for malignancies.2
Thus, these lesions should be considered in the differential diagnosis with metastatic relapse of the primitive disease. Imaging techniques are usually sufficient to establish a correct diagnosis without histopathologic confirmation and useful for monitoring the evolution of the lesions. Surgery is indicated only in the case of symptomatic lesions.1
In this paper, we report a case series of children who developed FNH and HRN after treatment for malignancies in the Oncology unit at the “Bambino Gesù” Pediatric Hospital in Rome.
Material and MethodsWe retrospectively analyzed the clinical and radiological data of 8 children diagnosed with FNH and HRN at our institution between December 1999 and September 2010 after treatment for cancer. Diagnosis was based on clinical and radiologic imaging, namely color Doppler ultrasonography (US), computed tomographic (CT) scanning and magnetic resonance imaging (MRI). Clinical manifestations and biochemical tests recorded at the time of diagnosis are reported in table 1.
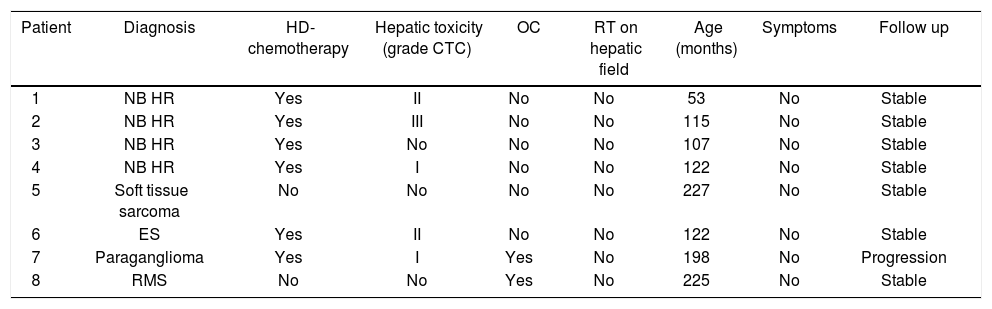
Clinical manifestations and biochemical test.
| Patient | Diagnosis | HD-chemotherapy | Hepatic toxicity (grade CTC) | OC | RT on hepatic field | Age (months) | Symptoms | Follow up |
|---|---|---|---|---|---|---|---|---|
| 1 | NB HR | Yes | II | No | No | 53 | No | Stable |
| 2 | NB HR | Yes | III | No | No | 115 | No | Stable |
| 3 | NB HR | Yes | No | No | No | 107 | No | Stable |
| 4 | NB HR | Yes | I | No | No | 122 | No | Stable |
| 5 | Soft tissue sarcoma | No | No | No | No | 227 | No | Stable |
| 6 | ES | Yes | II | No | No | 122 | No | Stable |
| 7 | Paraganglioma | Yes | I | Yes | No | 198 | No | Progression |
| 8 | RMS | No | No | Yes | No | 225 | No | Stable |
NB HR: high risk neuroblastoma. ES: Ewing sarcoma. RMS: rhabdomyosarcoma. HD: high dose. OC: oral contraception.
Six patients were female and 2 were male. Median age at diagnosis was 122 months (range 53–225). In all cases FNH-HRN was discovered during routine examination with abdominal US or CT in asymptomatic patients. All had normal liver function and negative hepatitis B and hepatitis C virus serology test at diagnosis. All had been previously treated for malignancy: 4 for metastatic neuroblastoma, 1 for Ewing sarcoma, 2 for soft tissue sarcoma and 1 for a paraganglioma.
All patients had received chemotherapy. Two patients, affected by clear cell sarcoma of the right foot and alveolar rhabdomyosarcoma of the gluteus respectively, had been treated according to RMS-96.1 protocol. The patient with rhabdomyosarcoma of the gluteus also received local radiotherapy. The remaining 6 patients (4 affected by high risk neuroblastoma, one with Ewing sarcoma and one with a paraganglioma) received high-dose chemotherapy followed by autologous peripheral stem cell transplantation with 2 also receiving radiotherapy (2100 cGy on primary tumor bed, the field not involving the liver). Conditioning regimen consisted of: Etoposide 200 mg/m2 days 1-2-3, Thiotepa 250 mg/m2 days 1-2-3, and Cyclophosphamide 60 mg/kg days 4–5.
During treatment 5/8 patients had presented hepatic toxicity, grade III CTC in one pt, grade II in 2 pts and grade I in 2 pts. No patient had hepatic veno-occlusive disease (VOD).
FNH-HRN was diagnosed at a median of 71 months from cessation of therapy (range 36–94).
In two patients FNH-HRN was discovered 18 and 36 months after starting hormone replacement therapy for ovarian insufficiency.
Gender, age, previous cancer, treatment and related hepatic toxicity, and use of oral contraception (OC) were analyzed as risk factors for developing FNH and HRN.
ResultsFive patients had multiple focal liver lesions and three patients had one single hepatic lesion.
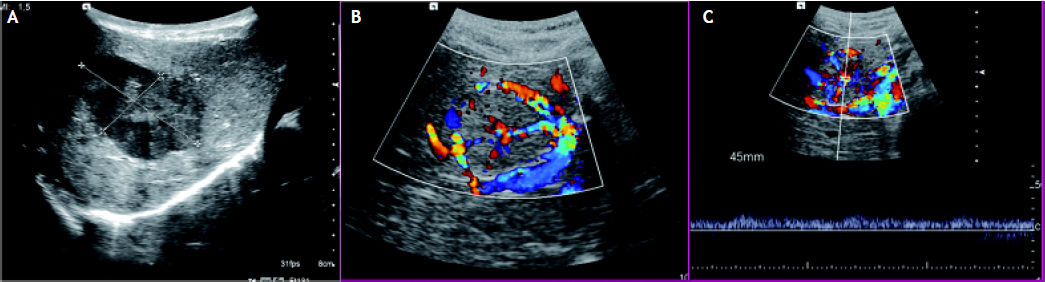
Abdominal Doppler ultrasound (US) was performed in all patients. Different echo patterns were observed in the group of patients with multiple lesions:, hypoechoic, isoechoic and hyperechoic lesions being simultaneously present in three patients. A central stellate area was noted in 3 patients (Figure 1).
US images. A. A hepatic hypoechoic lesion with a lobulated outline, a slightly hypoechoic rim and a central “star like” isoechoic area. B. Color Doppler image shows flow in vessel radiating outward from the central scar. C. On a duplex US image, the Doppler spectrum of the intratumoral vessels shows an arterial waveform.
Liver CT scan was performed in all patients. On CT scan, without contrast medium, the lesions appeared to have dyshomogeneous density. Dynamic computed tomography scanning was performed in all cases (Figure 2). All lesions exhibited strong enhancement in the arterial phase with homogeneous density during the delayed phase. A central stellate area was observed in 3 out of 8 patients (Figure 3).
Overall, 5 patients had multiple liver lesions (median 2.8), 10 being the largest number observed in a single patient.
Mean nodule size was 14.8 mm (range 6-40). Lesions were located in both hepatic lobes.
On unenhanced MRI, performed in 7/8 patients, lesions were predominantly slightly hypointense compared with surrounding parenchyma on fast spin echo T1-weighted images, isointense on gradient echo T1-weighted sequences, and always hyperintense on T2-weighted sequences. In dynamic studies with IV bolus injection of gadolinium chelates, all but one patient had lesions strongly enhanced during the arterial phase and all lesions became isointense to liver in the delayed phase (Figure 4). A central area of high signal intensity on T2-weighted images showed delayed enhancement characteristics of the central scar of FNH in three cases.
Radiological characteristics of the 8 patients are summarized in table 2.
Radiological characteristics.
| Patient | Imaging | Lesions (n) | US features | CT features | MR features |
|---|---|---|---|---|---|
| 1 | US, CT, MR | > 5 | Hypoechoic (with central scar). | Basal: isodense. Arterial phase: hyperdense. Portal phase: isodense. Late phase: isodense (with central scar). Late phase: isointense. | T1 w: hypointense with central hyperintensity. T2 w: hyperintense with central hypointensity. Arterial phase: hyperintense. Portal phase: hyperintense. |
| 2 | US, CT | 1 | Hypoechoic. | Basal: hypodense. Arterial phase: hyperdense. Portal phase: isodense. Late phase: isodense. | |
| 3 | US, CT, MR | 2 | Iso-hyperechoic. | Basal: isodense. Arterial phase: hyperdense. Portal phase: hyperdense. Late phase: isodense. | T1 w: isointense with central hyperintensity. T2 w: slightly hyperintense with central hypointensity. Arterial phase: hyperintense. Portal phase: signal intenisity reduction. Late phase: signal intenisity reduction. |
| 4 | US, CT, MR | 1 | Iso-hypoechoic. | Basal: hypodense. Arterial phase: hyperdense. Portal phase: isodense. Late phase: isodense. | T1 w: hypointense. T2 w: hyperintense. Arterial phase: hyperintense. Portal phase: signal intensity reduction. Late phase: signal intensity reduction. |
| 5 | US, CT, MR | 2 | Isoechoic. | Basal: hypodense. Arterial phase: hyperdense. Portal phase: isodense, with peripheral enhancement. Late phase: isodense, with peripheral enhancement. | T1 w: slightly hypointense. T2 w: slightly hyperintense. Arterial phase: hyperintense. Portal phase: isointense. Late phase: isointense. |
| 6 | US, CT, MR | > 5 | Iso-hypoechoic. | Arterial phase: hyperdense. Portal phase: slightly hyperdense. Late phase: isodense. | T1 w: hypointense. T2 w: slightly hyperintense. Arterial phase: hyperintense. Portal phase: hyperintense. Late phase: isointense. |
| 7 | US, CT, MR | >5 | Hypoechoic. | Arterial phase: hyperdense. Portal phase: hyperdense. Late phase: hyperdense (with central scar). | T1 w: isointense with central hyperintensity. T2 w: isointense with central hypointensity. Arterial phase: hyperintense. Portal phase: hyperintense. Late phase: isointense. |
| 8 | US, CT, MR | 1 | Isoechoic. | Basal: isodense. Arterial phase: hyperdense. Portal phase: isodense. Late phase: isodense. | T1 w: hypointense. T2 w: hyperintense. Arterial phase: hyperintense. Portal phase: hyperintense. Late phase: isointense. |
US: ultrasonography. CT: computed tomography. MR: magnetic resonance.
Two patients underwent fine needle biopsy of one hepatic lesion. In one case, diagnosis of FNH was confirmed, in the other patient hepatic steatosis was detected.
FNH-HRN lesions were monitored by abdominal US for a median of 76 months (range 17–145).
No dimensional and morphological modifications nor alteration of hepatic function were observed during the follow-up period in 7/8 patients. In the girl previously treated for paraganglioma a progression in number and size of the hepatic lesions was recorded nine years after the initial diagnosis.
DiscussionFNH and HRN of the liver are benign and usually asymptomatic proliferations with obscure etiology and a poorly understood pathogenesis. Often detected during routine follow-up examination, FNH represents 2% of all pediatric hepatic tumors and approximately 0.02% of pediatric tumors.3 The annual incidence of this lesion is approximately 2.25 per million children.4
Nodular regenerative hyperplasia (NRH) is a rare hepatic lesion defined as diffuse nodulation of the parenchyma, without annular fibrosis corresponding to alternating atrophic and hyperplastic areas. Conceptually, FNH and NRH are two different entities, but their pathogenesis may be very similar in nature. An increased incidence has been described in patients previously treated for malignancies years after cessation of therapy.5–8 It is hypothesized that focal circulatory disturbance may cause arterial and portal venous thrombosis, and that vascular recanalization and reperfusion of hepatic tissue might lead to hepatocyte proliferation and the development of FNH and HRN. The occurrence of benign hepatic regenerating lesions following treatment with chemotherapy and radiotherapy have been reported in children.8 Polychemotherapy is thought to be a risk factor for vascular occlusion in some cases.9–10
The annual incidence of FNH in a series of children treated for cancer at the Pediatrics Department of the Gustave Roussy Institute was 0.45 %.2 Results from that series emphasized the high prevalence of neuroblastoma and germ cell tumors among patients diagnosed with FNH and the high prevalence of hepatic complications during previous treatment. Ten out of 14 patients in the series suffered from VOD; this condition and treatment with busulfan and melphalan are considered the main risk factors for developing FNH. Half of the patients in our series had been treated for metastatic neuroblastoma and 6/8 received high doses of chemotherapy but none presented VOD during treatment. Only hepatic toxicity of grade III or less had been recorded.
In another report, Towbin et al observed that, in a series of 7 pts with FNH and previously treated for malignancy, none suffered from VOD but 6 of the 7 patients had received RT of the liver. No speculation on the etiology of FNH was proposed by the authors.11
In an Italian series of 18 pts affected by FNH, 6/ 18 had been previously treated for cancer. In these 6 patients no certain risk factors for FNH were identified, nor any association with specific malignancies nor previous VOD, the only correlation being with cytotoxic regimens together with bone marrow transplantation in 4/6 patients.12
In a series of 9 patients with HRN previously treated for malignancies, probable risk factors were RT in the hepatic field in 6 out 9 patients, bone marrow transplantation in 6/9, and VOD in 4/9.5
No clear risk factors for developing FNH and HRN in patients treated for cancer have been definitely established. In our series, neither high prevalence of VOD nor radiotherapy in the hepatic field was recorded, but 6/8 patients received high-dose chemotherapy followed by autologous peripheral stem cell transplantation. In another recent paper, 8 patients were diagnosed with FNH after hematopoietic stem cell transplantation.13 We can speculate that vascular hepatic injury, related to cytotoxic conditioning regimen, may occur also in the absence of clear evidence of hepatic toxicity during treatment.
The diagnosis of FNH is instrumental, lesions are usually slightly hypoechoic or isoechoic with lobulated contours or hypoechoic halo on US. The typical central scar is slightly hyperechoic, but is often difficult to visualize.14 On CT scan, an FNH lesion appears as a homogeneous, hypoattenuated mass. This lesion (except for the central scar) enhances rapidly during the arterial phase of contrast CT.15 On MRI, FNH lesions appear as well-delineated, homogeneous, round lesions, slightly hypointense, or isointense on T1-weighted images, while they are always hyperintense on T2-weighted images. In dynamic studies with IV bolus injection of gadolinium chelates, the lesions are strongly enhanced during the arterial phase, except for the central scar, with complete wash-out in the delayed phase. The central scar is hypointense on T1-weighted and hyperintense on T2-weighted images with decreased signal intensity on the arterial phase and enhancement on the portal venous phase.16 Histological confirmation of FNH and HRN is not mandatory if imaging findings are considered diagnostic. In the paper by Gobbi, et al. the radiological suspicion of FNH is reported to have been confirmed by histological examination in all the 18 cases.12
The possible malignant potential of FNH lesions is still debatable. No cases of malignant evolution of FNH lesions have been reported in literature, but cases of FNH lesions associated with malignancies have been described. Petsas et al reported a case of hepatocellular carcinoma (HCC) arising within a FNH lesion.17 In another paper, in a series of patients operated upon for a preoperative presumptive diagnosis of benign hepatic lesions (FNH and adenoma), diagnosis of HCC was made in 3 cases.18 A case of simultaneous presence of hepatoblastoma and 3 foci of FNH in a patient previously treated for stage IV neuroblastoma has been published.19 In our series diagnosis of FNH and HRN was always incidental. In the paper of Icher-De Bouyn only 3 out of 14 patients presented symptoms at the time of FNH diagnosis. In some papers,1,12,18 in most instances the diagnosis of FNH in previously healthy children was suggested by the presence of clinical symptoms such as abdominal or other pain, probably caused by the space-occupying effect of the tumors.1 Probably, in children treated for cancer, as in our series, incidental diagnosis occurs due to the routine and periodic radiological follow-up performed to avoid relapse of the primitive disease, which renders early discovery of FNH lesions possible. In healthy children, radiological examination is usually performed only after symptoms appear.
As concerns the management and possible surgical treatment for FNH and HRN, the majority of authors suggest a conservative approach based on regular follow-up with US and MRI to monitor the size of the lesions. Progression of the lesions is reported in the minority of cases. Spontaneous regression is also described in some cases20 while in the majority of cases lesions remain stable during prolonged follow-up.12 Surgery is recommended only in the case of complications such as compression of adjacent organs, lesion progression with tumor size > 5 cm and presence of symptoms.1,20–21 In our experience, evolution of the lesions was observed in only 1 patient three years after the initial diagnosis of FNH. This patient, a girl previously treated for paraganglioma, was receiving hormone replacement therapy for ovarian insufficiency. The relation between FNH and the use of the oral contraceptive (OC) pill as hormone replacement is controversial. Some reports have suggested that estrogens may lead to the growth of FNH and vascular changes.22 Being the only patient in our series in whom FNH progressed, we speculate that OC may possibly have played a role. As regards the other patient treated with OC, follow-up is too short (17 months) to evaluate the possible role of hormone therapy in the evolution of the liver lesion.
In conclusion, long-term survivors of cancer are at risk of developing FNH and HRN. These lesions should be considered in the differential diagnosis with metastatic relapse of the primitive disease during follow-up. Usually serial monitoring with US and, if indicated, with other available imaging techniques is sufficient to rule out liver metastasis and to monitor the evolution of the lesions. Surgery is suggested only in the case of complications.
AcknowledgementsThe authors would like to thank Prof. Franco Locatelli for reviewing the manuscript.
The authors declare no conflict of interest.