Frailty and sarcopenia are well-recognized factors related to worse outcomes in patients with cirrhosis, including liver transplant (LT) candidates. Implications of pre-LT functional and muscle deterioration also affect post-LT outcomes. Patients with cirrhosis and acute-on-chronic liver failure (ACLF) have a lower survival rate, both before and after LT. There is a need to better identify those patients with ACLF who would benefit from LT. This review aims to present the available data about frailty and sarcopenia in patients with ACLF in the LT setting. An exhaustive review of the published literature was conducted. Data regarding frailty and sarcopenia in LT candidates with ACLF are scarce and heterogeneous. Studies evaluating frailty and sarcopenia in critically ill patients outside the liver literature are also presented in this review to enrich the knowledge of this field in expansion. Frailty and sarcopenia seem to contribute to worse outcomes in LT candidates with ACLF, both before and after LT. Sarcopenia evaluation may be the most prudent approach for those very sick patients. Skeletal muscle index assessed by computed tomography is recommended to evaluate sarcopenia. The role of muscle ultrasound and bioelectrical impedance analysis is to be determined. Frailty and sarcopenia are crucial factors to consider on a case-by-case basis in LT candidates with ACLF to improve patient outcomes.
Frailty is a multidimensional syndrome related to worse outcomes in patients with liver cirrhosis, with a negative impact both before and after liver transplantation (LT) [1-7].
Similarly, sarcopenia, one major component of the frailty construct, is a dominant predictor of outcomes in patients with cirrhosis, both before and after LT [8-14].
Definitions of frailty and sarcopenia share common aspects, and usually, both are identified in a single patient. In patients with cirrhosis, the concept of physical frailty has been chosen over the more holistic definition from the geriatric field, in which cognitive, social, and emotional aspects are also included. The consensus definition by the American Association for the Study of Liver Diseases (AASLD) for patients with cirrhosis has established that physical frailty “represents clinical manifestations of impaired muscle contractile function such as decreased physical function, decreased functional performance, and disabilit” and sarcopenia is the “phenotypic manifestation of loss of muscle mass” [15].
Acute-on-chronic liver failure (ACLF) is a distinct syndrome characterized by a high mortality rate related to acute decompensation and organ failure (OF) in patients with cirrhosis, where systemic inflammation is the primary driver of ACLF in patients with cirrhosis [16]. The number of organs failing defines the severity and grade of ACLF, according to the chronic liver failure consortium (CLIF) definition [17,18]. Twenty-eight-day mortality of patients with ACLF grade 3 (ACLF-3) is about 80 %, and LT is currently the only available treatment [18,19].
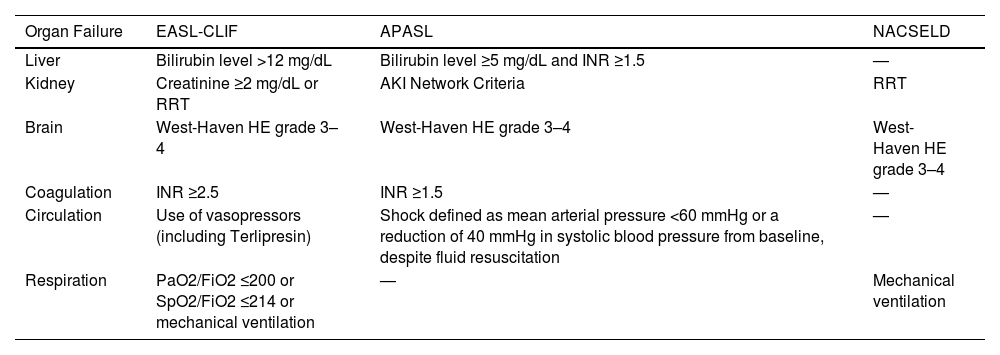
There is not a single definition of ACLF, and three major definitions coexist. The European Association for the Study of the Liver-CLIF (EASL-CLIF), the Asian Pacific Association for the Study of the Liver (APASL), and the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) definitions. The definitions of organ failure and the organs considered are summarized in Table 1. In summary, the EASL-CLIF definition combines hepatic and extrahepatic organ failure variables, the APASL definition considers mainly hepatic failure variables, and the NACSELD definition considers principally extrahepatic organ failure variables.
ACLF Definition of organ failure according to the different scientific societies.
ACLF, acute on-chronic liver failure; AKI, acute kidney injury; ALI, acute lung injury; APASL, Asian Pacific Association for the Study of the Liver; EASL-CLIF, European Association for the Study of Liver-Chronic Liver Failure; Fi02, fraction of inspired oxygen; HE, hepatic encephalopathy; INR, international normalized ratio; NACSELD, North American Consortium for the Study of End-Stage Liver Disease; PaO2, partial pressure of arterial oxygen; RRT, renal replacement therapy; SpO2, pulse oximetry saturation.
EASL and AASLD have both recently published ACLF guidelines summarizing current knowledge and providing clinical recommendations [20,21]. Throughout this review, the term ACLF will refer to the EASL-CLIF definition.
Since the first descriptions of ACLF in patients with cirrhosis, it has been clear that this syndrome is common (about 30 % prevalence in hospitalized patients with cirrhosis) and adds a significant increase in mortality (28-day mortality rate 33.8 %−52.0 %; 90-day mortality rate 48.4 %−62.7 %). Also, the CLIF Consortium ACLF score (CLIF-C ACLFs) has been shown to be better at predicting mortality among patients with ACLF than the Model for End-stage Liver Disease (MELD), MELD incorporating sodium (MELDNa) or Child-Pugh scores [17,22]. These patients with ACLF-3 on the LT waitlist (WL) have greater 14-day mortality than those patients listed as status 1a [23].
The negative impact of ACLF development on the outcomes of patients on the WL has been described in several publications, and different pre-LT factors have been identified as related to greater mortality: incidental ACLF after listing, patient age greater than 60 years, the number of organs failing and multidrug-resistant organism infections before the LT as well as the grade of ACLF [24,25].
The highest mortality rate has been reported among patients with ACLF-3, independently of the MELD score. Importantly, those patients with lower MELDNa scores present a greater risk of death, suggesting that MELD score alone is inadequate to predict WL mortality in this setting and that other factors might have to be taken into account when evaluating the prognosis of these patients [25,26].
A higher post-LT mortality has been associated with several pre-LT factors; a recent infection from multidrug resistant organisms, arterial lactate levels greater than 4, and renal replacement therapy [24]. The Sundaram ACLF-LT-Mortality (SALT-M) score has identified older age, body mass index (BMI), diabetes, and respiratory and circulatory failure as factors independently associated with 1-year post-LT mortality, while age, respiratory failure, BMI, and infection were associated with length of stay (LOS) after LT [27]. The transplantation for ACLF-3 model (TAM) score has identified age >53 years, arterial lactate ≥4 mmol/L, mechanical ventilation with PaO2/FiO2 ratio ≤200, and leukocyte count ≤10 G/L as factors related to a higher post-LT mortality. Those patients with two or more than two of these factors had a significantly lower 1-year post-LT survival (10.0% vs. 71.9 %, p = 0.001) [28].
Another important concept is that ACLF grade is dynamic and pre-LT improvement is related to better post-LT survival. Those patients with ACLF-3 at listing, who were transplanted after improvement, with ACLF-0–2 had a better 1-year post-LT survival (88.2 %) than those transplanted with ACLF-3 (82.0 %); p < 0.001 [29].
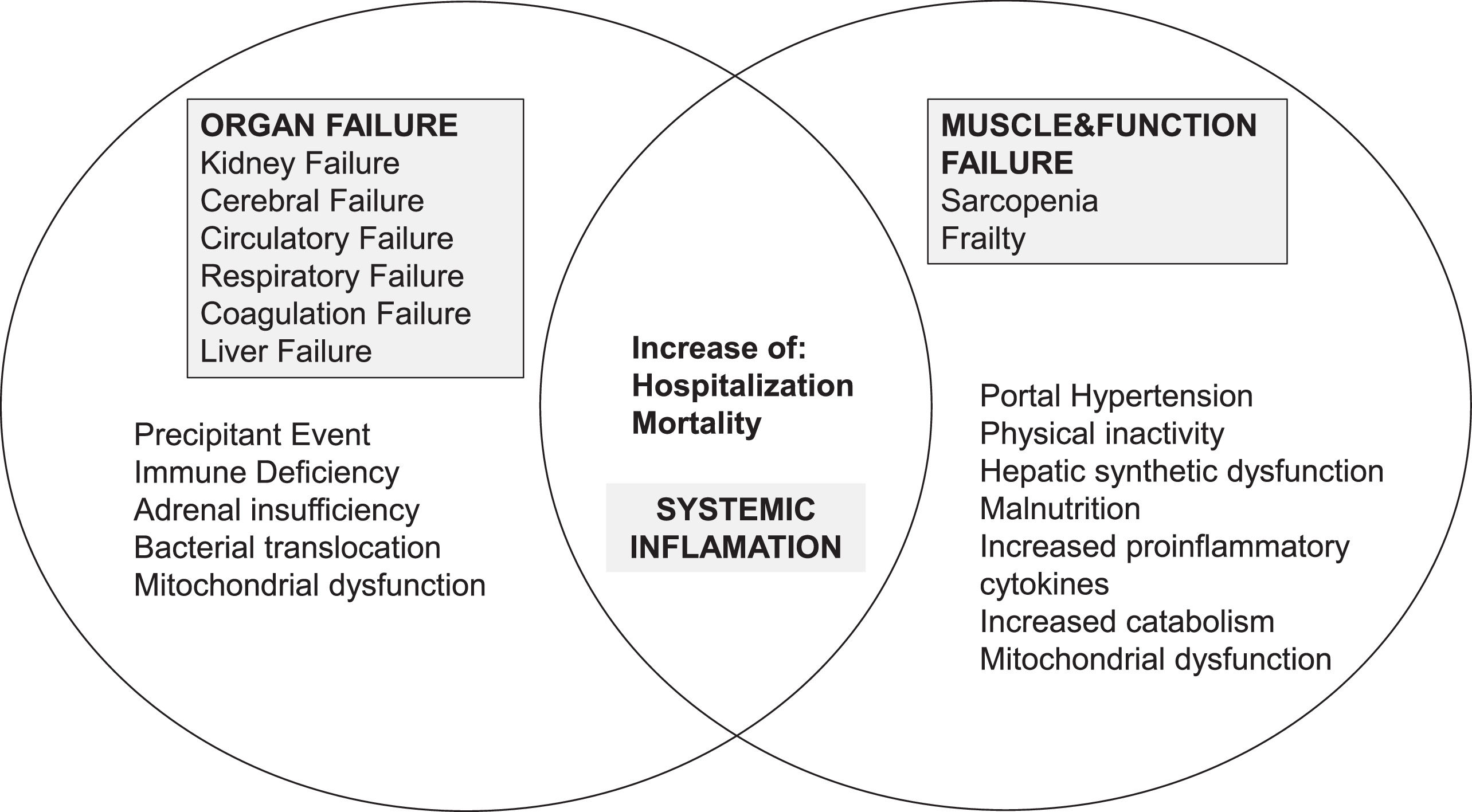
The aim of this comprehensive review is to present the available clinical information regarding different assessment options for frailty and sarcopenia in patients with ACLF and briefly describe their impact and implications in this subgroup of patients. We will successively display data regarding 1) frailty and sarcopenia in cirrhosis in both the in- and outpatient setting; 2) frailty and sarcopenia in the critical illness setting; 3) frailty in patients with ACLF; 4) sarcopenia in patients with ACLF; and 5) finally discuss clinical implications, limitations, and future directions. A proposed interaction between frailty and sarcopenia with ACLF is depicted in Fig. 1. A PubMed search using the search terms “sarcopenia,” “frailty,” “cirrhosis,” “ACLF,” “critically ill,” and related terms was conducted in August 2023. Hepatology and LT scientific societies' statements or guidelines have also been included.
2Frailty and sarcopenia in patients with cirrhosis2.1Frailty in patients with cirrhosis. Outcomes and measurementFrailty has been largely accepted as a factor related to worse outcomes in patients with cirrhosis. Furthermore, its impact goes beyond LT and affects post-LT outcomes [2,4-6,15,30-34]. In a large multicentric cohort, those LT candidates identified as frail had a 3-, 6- and 12-month WL mortality of 13 %, 22 %, and 35 %, compared to a 2 %, 6 %, and 11 % for those non-frail candidates (p < 0.001). In this study, frailty was the only variable related to WL mortality independently of the MELDNa score or the presence of ascites or encephalopathy [4]. Importantly, functional impairment over time is related to a higher risk of mortality. Those patients with a 0.1 unit worsening every three months in their baseline LFI have a 2-fold increased risk of death (or delisting) while in the WL [35].
The working group for the study of frailty from the American Society of Transplantation advocated first for the incorporation of frailty measurement in every LT candidate, not only at baseline but also over time, and second, for the use of standardized tools. They propose the so-called frailty tool kit, composed of four scores, to evaluate frailty in the different scenarios that we can face in the LT setting [10]. First, the six-minute walk test is an objective measure, only validated in the outpatient setting, which is associated with WL mortality [36,37]. Second, the Karnofsky Performance Status (KPS) has been evaluated in the inpatient and outpatient settings and correlates well with WL mortality [38], mortality after hospitalization in patients with cirrhosis [7], and mortality after LT [39]. Likewise, the activities of daily living (ADL) scale correlates well with mortality in the inpatient and outpatient setting [2,32], including patients in the WL [32,40,41], with the need of discharge to a rehabilitation hospital [32], and mortality after LT [39]. Fourth, the liver frailty index (LFI) has been largely validated in the outpatient setting, proving its relation with WL mortality [1,4,35], and mortality after LT [5]. An LFI <3.2 identifies frail patients, between 3.2–4.3 pre-frail patients, and an LFI ≥4.4 identifies robust patients.
Evaluation focused on the inpatient setting will be discussed further in subsequent sections.
2.2Sarcopenia in patients with cirrhosis. Outcomes and measurementSimilarly, sarcopenia has a deleterious impact on LT candidates before and after the LT. In a retrospective multicentric study, including 496 patients with cirrhosis, those sarcopenic had a significantly higher WL mortality than those who were not sarcopenic (70 % increased risk of WL mortality for men and 182 % for women) [11].
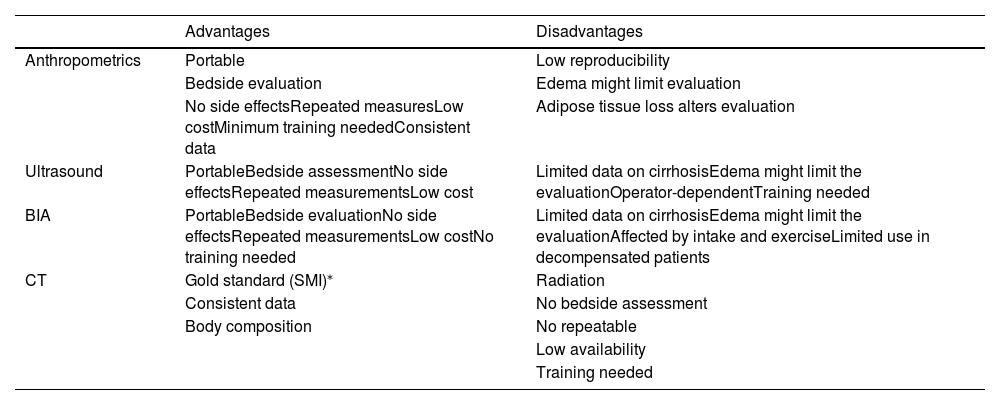
The advantages and disadvantages of tools to evaluate sarcopenia in patients with cirrhosis are summarized in Table 2.
Tools that can be used to evaluate sarcopenia in patients with cirrhosis.
| Advantages | Disadvantages | |
|---|---|---|
| Anthropometrics | Portable | Low reproducibility |
| Bedside evaluation | Edema might limit evaluation | |
| No side effectsRepeated measuresLow costMinimum training neededConsistent data | Adipose tissue loss alters evaluation | |
| Ultrasound | PortableBedside assessmentNo side effectsRepeated measurementsLow cost | Limited data on cirrhosisEdema might limit the evaluationOperator-dependentTraining needed |
| BIA | PortableBedside evaluationNo side effectsRepeated measurementsLow costNo training needed | Limited data on cirrhosisEdema might limit the evaluationAffected by intake and exerciseLimited use in decompensated patients |
| CT | Gold standard (SMI)⁎ | Radiation |
| Consistent data | No bedside assessment | |
| Body composition | No repeatable | |
| Low availability | ||
| Training needed |
ACLF, Acute-on-chronic liver failure; BIA, Bioelectrical impedance analysis; CT, computed tomography; SMI, skeletal muscle index.
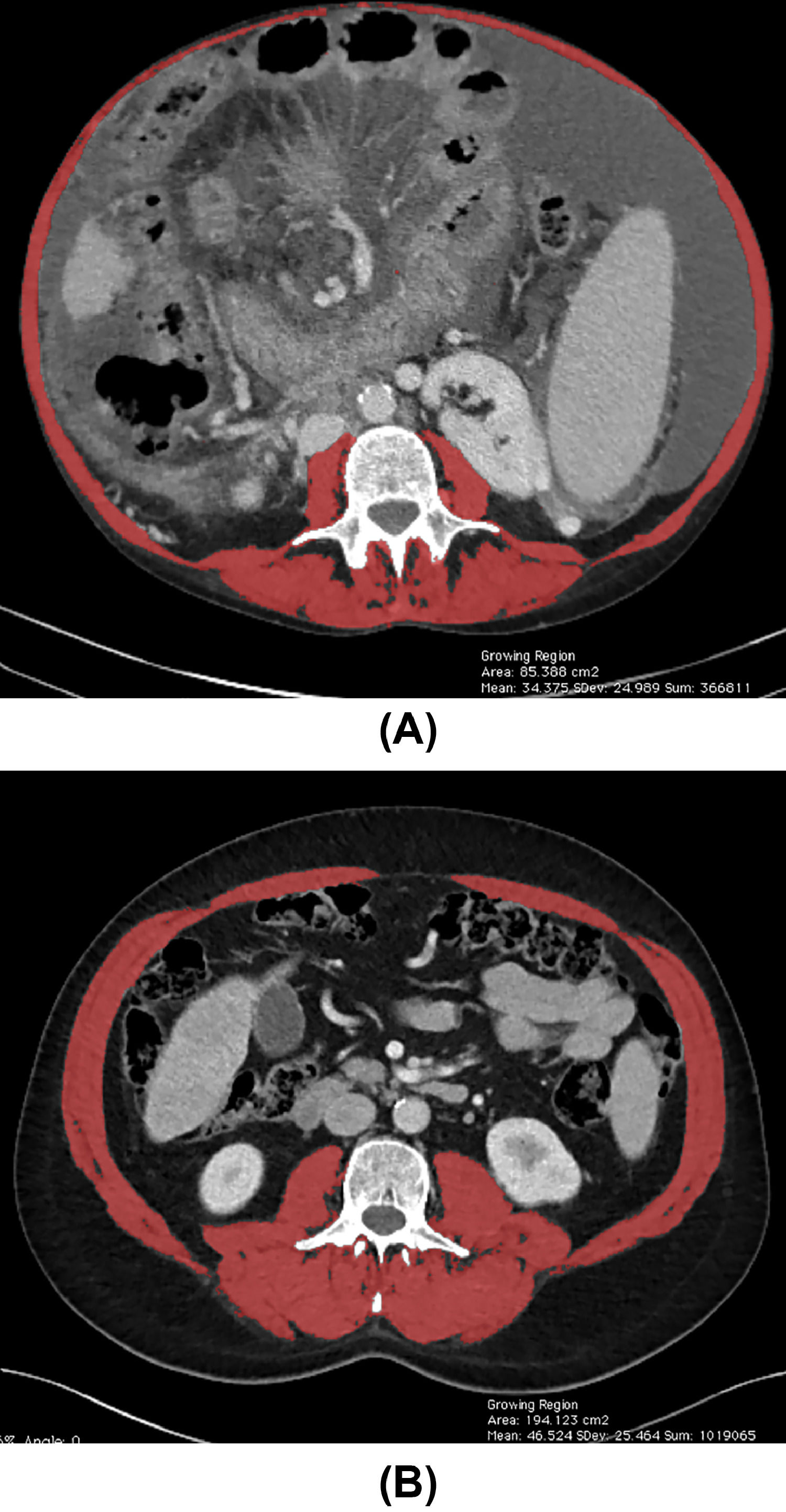
According to AASLD and the FLEXIT (Fitness, Life Enhancement, and Exercise in Liver Transplantation) consortium, skeletal muscle index (SMI) assessed by computed tomography (CT) is the current gold standard to identify sarcopenia among patients with cirrhosis. The FLEXIT consortium defines sarcopenia in patients with cirrhosis by a cut-off value of SMI <50 cm2/m2 in male and <39 cm2/m2 in female patients, respectively, measured in cross-sectional imaging at L3 vertebral level [10,11]. Fig. 2 illustrates total muscle area quantification at L3 vertebral level measured in an abdominal CT.
Total muscle area quantification at the level of the third lumbar vertebra using abdominal CT images from two patients with cirrhosis. (A) Female patients with low SMI (32.21 cm2/m2) and (B), and male patients with high SMI (67.17 cm2/m2), as indicated by the red shading. SMI, skeletal muscle index. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Muscle characteristics (quantity and quality) have also proved to have an impact on post-LT outcomes. This large study evaluated 277 living donor LT (LDLT) recipients; skeletal muscle mass was evaluated by SMI, muscle quality by intramuscular adipose tissue content (IMAC), and visceral adiposity by visceral-to-subcutaneous adipose tissue area ratio (VSR) using CT. Cut-off values were defined by evaluating 657 healthy LDLT donors according to sex. Those LT recipients with lower SMI (HR 2.355, 95 %CI 1.399–3.907, p = 0.002) and higher IMAC (HR 2.179, 95 %CI 1.336–3.632, p = 0.002) or VSR (HR 2.373, 95 %CI 1.441–3.939, p = 0.001) had an increased risk of post-LT mortality [42].
Anthropometry is an easy-to-use and inexpensive tool to evaluate sarcopenia. Midarm muscular circumference (MAMC) and triceps skinfold thickness are useful strategies, but they have important limitations, mainly related to lack of concordance and low reproducibility. Also, measures are affected by fluid retention and loss of adipose tissue [43-45].
Handgrip strength has also been used to evaluate sarcopenia in LT candidates, basically in the outpatient setting. The handgrip strength has proved to correlate well with WL outcomes in a cohort of 292 LT candidates prospectively evaluated in the outpatient clinic [46]. In this study, and after adjustments, in a multivariate (MV) model, handgrip strength remained significantly associated with WL mortality (p = 0.008), while muscle mass was not (p = 0.35), suggesting that functional test might be better associated with outcomes than muscle mass alone. However, results are not consistent, and in a previously mentioned study evaluating frailty in the inpatient setting, handgrip alone did not show any relation to outcomes [47].
Thigh muscle ultrasound has also been used to evaluate muscle mass in patients with cirrhosis in the outpatient setting. One-hundred and fifty-nine patients with cirrhosis were included in a prospective study to develop a model to identify patients with sarcopenia, using as the gold standard the SMI determined by CT or magnetic resonance. Thigh muscle thickness, in combination with BMI, was able to identify cirrhotic patients with sarcopenia, with an area under the receiver operating characteristic (AUROC) value of 0.78 for women and 0.89 for men. This study did not evaluate outcomes but demonstrated the feasibility of this approach [45].
A retrospective study including 136 patients with cirrhosis compared the ability of bioelectrical impedance analysis (BIA) and SMI to identify sarcopenia [48]. Phase angle (PhA), estimated by BIA, was able to identify sarcopenia with a sensitivity of 94 %, being defined by a cut-off value of PhA <5.4° in female patients and <5.6° in male patients. Importantly, its correlation with SMI was not affected by the presence of ascites. Patients with sarcopenia, identified by either SMI (HR 0.95, 95 %CI 0.90–0.99, p = 0 0.035) or PhA (HR 0.61, 95 %CI 0.42–0.88, p = 0.009) had a higher mortality.
2.3Relationship between frailty and sarcopenia in patients with cirrhosisFrailty and sarcopenia are interrelated constructs, and in clinical practice, both are frequently identified in the same patient and bring together similar information but reveal different aspects, which deserve to be taken into account, understanding each entity separately. Operational definitions are largely accepted in clinical practice and research, as displayed in previous sections of this manuscript. Despite limited data, there are some studies measuring sarcopenia and frailty in the same cohort, showing a discordant correlation between both entities. Importantly, these studies evaluating sarcopenia and frailty in the same individuals have important limitations, as did not use the current definition of sarcopenia evaluated by SMI, and for the evaluation of frailty, different approaches were used, increasing the difficulty to compare data and to generalize results [46,49,50]. More data on the behavior of both entities would be desirable to enhance the knowledge of this challenging binomial.
2.4Inpatient frailty and sarcopenia measurement in patients with cirrhosisPatients with cirrhosis in need of an in-hospital stay due to cirrhosis decompensation have inherent particularities that deserve some considerations regarding frailty and sarcopenia evaluation.
First, despite the large number of studies evaluating the LFI in the outpatient setting, there is less data regarding its applicability in those ill patients requiring hospital admission and with limitations to perform tests based on physical performance. Recently, a study carried out in a multi-center cohort of 211 hospitalized patients with cirrhosis demonstrated that LFI measurement was feasible in this setting and associated with LOS, mortality, and discharge to a rehabilitation hospital. However, only 64 % of the patients were able to complete the three tests. Interestingly, handgrip evaluation alone (completed by 99 % of the patients) was not related to outcomes [47].
As previously mentioned, KPS and ADL have proved to correlate well with outcomes in patients in need of hospital admission.
Despite not being included in the frailty tool kit, other scales have been used to evaluate frailty in the inpatient setting.
One of them is the Braden scale, which is comprised of six domains: skin sensory perception, moisture, activity, mobility, nutrition, and friction (ability to hold a comfortable position in a chair and bed). A score of 23 indicates no risk of skin breakdown, whereas a score below 16 indicates a high risk of nosocomial pressure ulcers. The Braden scale has been shown to predict 90-day mortality after discharge, LOS, need for discharge to a rehabilitation facility, as well as early disability-related outcomes and increased LOS after LT [32,51].
Another tool that has been used to evaluate patients with cirrhosis is the Hospital Frailty Risk Score (HFRS). This score uses population-level data, using the International Classification of Diseases (ICD) 10 to define frailty. HFRS has not been evaluated as well in the cirrhosis literature as the other scores discussed beforehand, but in the last few years, there have been some liver publications incorporating this score and finding a good correlation with outcomes [52]. This retrospective study included 16,561 in-hospital patients with cirrhosis and 6061 with any grade of ACLF. The baseline pre-admission frailty was the value considered for the analysis of in-hospital-related outcomes. Those patients with cirrhosis identified as frail had an increased risk of ACLF-related hospitalization, but frailty did not impact short-term ACLF-related mortality [53]. This study brings together a combined approach, as a previous frailty assessment based on population-level data is used to evaluate inpatient outcomes.
Regarding sarcopenia, it is worth mentioning two multicentric retrospective studies from the same group, evaluating the role of body composition in 126 and 116 critically ill patients with cirrhosis undergoing urgent evaluation for LT [54,55]. The first one was focused on sarcopenia [54], and the second on sarcopenic visceral obesity (SVO) [55]. In the first study, an SMI cut-off value of 48 cm2/m2 was used to identify 46 % of sarcopenic men in this cohort. In the MV analysis restricted to men, sarcopenia remained related to a higher risk of post-LT mortality (HR 4.39, 95 %CI 1.49–12.97, p = 0.007). In women, no association was found [54]. In the second study, pre-established cut-off values of SMI were used for men and women (<50 cm2/m2 in males and <39 cm2/m2 in females) [10]. Fifty-five percent and 35 % of men and women were respectively identified as sarcopenic. The cut-off value for VSR was identified per sex by means of a time-dependent ROC curve method (≥1.54 for men and ≥1.37 for women). Subsequently, SVO was defined as a combination of sarcopenia and VSR. Twenty percent of the cohort was sarcopenic visceral obese. In the MV, only SVO remained related to increased post-LT mortality (HR 3.50, 95 %CI 1.10–11.15, p = 0.03) [55].
These two studies probably included patients with ACLF; however, no ACLF definition was used, and the criteria of non-elective hospitalization for LT evaluation was the only one used for the cohort selection. Therefore, these studies cannot be considered to have evaluated sarcopenia in patients with ACLF.
2.5Training and nutritional interventions in patients with cirrhosisThe deleterious impact of sarcopenia and frailty in patients with cirrhosis is clear, as it is their worsening while in the WL [1,7,14,35,40,56]. Interventions to reverse the damage and improve sarcopenia or frailty metrics, and more importantly, outcomes are not well-known. In the LT setting, diverse attempts at prehabilitation and nutritional interventions have been made to improve the metrics and outcomes of these patients.
Regarding exercise interventions, combined or not with nutritional supplementation, there are multiple trials proving that supervised interventions can improve frailty or sarcopenia metrics; limitations of these studies are the small number of patients involved, restrictions in access to training programs outside of clinical trials, which is related to the limitation to maintain this type of intervention over time, aside from cirrhosis-related barriers to exercise, like fluid overload, fatigue, daytime somnolence, hepatic encephalopathy, or anemia among others [57-60].
There are some experiences in home-based training programs in patients in the LT WL with different results. A multicentric US randomized trial (STRIVE) included 58 and 25 patients in the intervention and control group, respectively. Frailty was assessed by means of the LFI, liver function tests, and quality of life parameters. After a 12-week intervention consisting of an initial face-to-face coach visit, followed by weekly counseling coaching with a 30-minute video-guided exercise program, no significant differences were found. Although, some non-significant improvements were observed in the LFI and quality of life metrics. This study failed to demonstrate that prehabilitation was able to significantly improve LFI. Importantly, only 14 % of the patients adhered to the training video for 10–12 weeks [61].
Lack of adherence is probably one of the main limitations to carrying out home-based interventions. Incorporating smartphone applications is another strategy that has been tried in the study conducted by Duarte-Rojo et al. [62]. Thirty-one patients were enrolled in this prospective intervention study; 21 completed the led-in phase, and 15 finished the study. Coach intervention was combined with a smartphone application for 12 weeks; this strategy was called mobile-assisted telehealth regimens to increase exercise (MATRIX). Among the 15 patients who completed the intervention, a significant improvement in LFI and 6MWT was observed. (P = 0.03 and P = 0.005 respectively). The impact on outcomes was not evaluated.
Regarding nutritional interventions alone, there have been some attempts to improve post-LT outcomes with this strategy, many of them not evaluating frailty or sarcopenia parameters. This review summarizes nutritional interventions specifically in the LT setting, stratifying them according to the time of intervention in relation to the LT [63]. Among the 14 studies included, only three evaluated body composition parameters (frailty was not evaluated) and one nutritional parameter. Other experiences in patients with cirrhosis, not focused on the LT setting, have shown some effect of supplementation with branched-chain amino acids (BCAA) on muscle in patients with cirrhosis. A study including 21 patients and after 48 weeks of BCAA supplementation showed that 52.4 % of patients ameliorated hypoalbuminemia, while 47.6 % presented decreased serum albumin. Among those 11 patients with improved albumin levels, all of them also showed an improvement in IMAC, and six showed an increase in SMI (P = 0.01 for both) [64]. Another study including 82 patients and after 24-week BCAAs supplementation showed an increase in hand grip strength (P < 0.001) and a non-significant decrease in muscle mass (P = 0.33) [65].
3Frailty, sarcopenia, and critical illnessFrailty is a common phenomenon among intensive care unit (ICU) admitted patients and affects not only elderly people but also younger patients [66]. In this setting, outside the liver-specific literature, muscle and frailty have been evaluated following different strategies that we briefly present.
Handgrip dynamometry has been used in this setting. In a multi-center study published by Ali et al. [67], patients admitted to an ICU and ventilated for at least five days were considered for the study. One-hundred and thirty-six were finally included, as they survived and were awaked. These patients underwent strength measurement. In this general ICU cohort, cirrhosis as a comorbidity was reported in 5 % of the patients. The 25.7 % of the cohort was identified as having severe weakness. Handgrip strength was independently associated with higher mortality (OR 4.5, 95 %CI 1.5–13.6; p = 0.007) and a 41 % (95 %CI, 56 %−19 %; p = 0.001) reduction in ICU-free days.
Another ICU-based study evaluated muscle strength in patients receiving mechanical ventilation for a primary pulmonary problem. One hundred twenty critically ill patients were enrolled [68]. This study demonstrated, first, that evaluation of muscle function by handgrip dynamometer in patients receiving mechanical ventilation was feasible, and second, identified the following factors as related to an increased muscle weakness: the number of days of mechanical ventilation, older age, and female sex.
Another insightful study combined muscle ultrasound, muscle biopsy, and the ratio of protein to DNA to prospectively evaluate muscle mass on days 1, 3, 7, and 10 after ICU admission [69]. Among the 63 patients included, 9.5 % had liver cirrhosis. Patients were recruited within 24 h of ICU admission, and serial rectus muscle ultrasound and biopsies were done (35 patients had muscle biopsies on days 1 and 7 of ICU stay, and 28 were assessed using all three methods on days 1 and 7). Importantly, the rectus femoris cross-sectional area decreased a 12.5 % (95 %CI, 15.8 %−9.1 %; p = 0.002) from days 1 to 7 and a 17.7 % (95 % CI, 20.9 %−4.8 %; p < 0.001) at day 10. Among the 28 patients with all three evaluations, the fiber cross-sectional area decreased by 17.5 % (95 CI%, 5.8 %−29.3 %), and the ratio of protein to DNA was 29.5 % (95 % CI, 13.4 %−45.6 %).
A correlation between the number of organs failing and the muscle was also observed (p < 0.001). On days 3 and 10, the negative change in the rectus femoris cross-sectional area was greater among those with more than one organ failing (p = 0.03 and p < 0.001, respectively). This change was also greater among those with more than three organs failing (p < 0.001) and more evident by day 10. The MV analysis demonstrated that age, bicarbonate level at admission, and the ratio of PaO2 to FiO2 were factors associated with a > 10 % loss in the rectus femoris cross-sectional area at day 10 (p < 0.001).
BIA has also been used within the ICU arena to investigate whether PhA and frailty (Korean Modified Barthel Index) were associated with the outcomes of critically ill patients. This prospectively designed study included 97 ICU-admitted patients [70]. Both PhA and frailty were demonstrated to be factors predicting the outcomes of these patients. Low values of PhA were associated with increased mortality (p = 0.042) and a longer ICU stay (5.6 days vs. 9.8 days, p = 0.016), and frailty was associated with more days of mechanical ventilation (2.3 days vs. 7.1 days; p = 0.018).
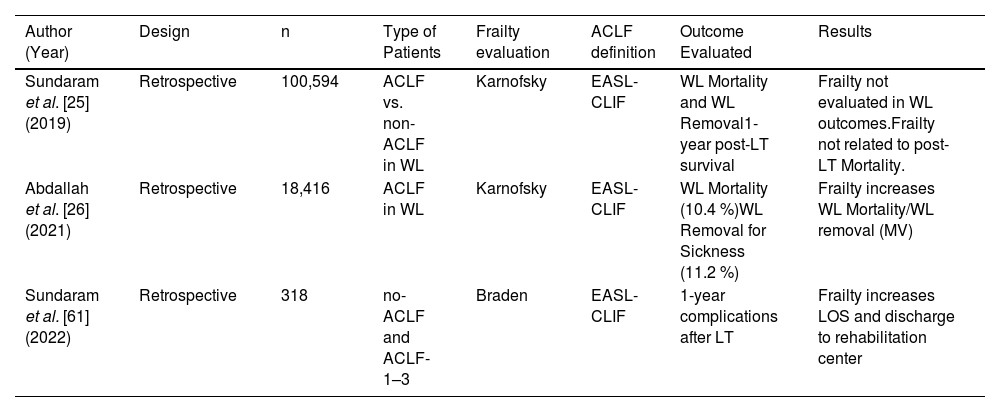
4Frailty in patients with ACLFA summary of the three studies evaluating frailty as a factor involved in the prognosis of LT candidates and recipients with ACLF is presented in Table 3. None of the studies included any test requiring patient collaboration; two evaluated the KPS score, and the other one was the Braden scale.
Clinical studies describing frailty evaluation and ACLF.
| Author (Year) | Design | n | Type of Patients | Frailty evaluation | ACLF definition | Outcome Evaluated | Results |
|---|---|---|---|---|---|---|---|
| Sundaram et al. [25] (2019) | Retrospective | 100,594 | ACLF vs. non-ACLF in WL | Karnofsky | EASL-CLIF | WL Mortality and WL Removal1-year post-LT survival | Frailty not evaluated in WL outcomes.Frailty not related to post-LT Mortality. |
| Abdallah et al. [26] (2021) | Retrospective | 18,416 | ACLF in WL | Karnofsky | EASL-CLIF | WL Mortality (10.4 %)WL Removal for Sickness (11.2 %) | Frailty increases WL Mortality/WL removal (MV) |
| Sundaram et al. [61] (2022) | Retrospective | 318 | no-ACLF and ACLF-1–3 | Braden | EASL-CLIF | 1-year complications after LT | Frailty increases LOS and discharge to rehabilitation center |
ACLF, Acute-on-chronic liver failure; EASL-CLIF, European Association Study Liver- Chronic Liver Failure Consortium; LOS, length of stay; LT, liver transplantation; MV, multivariate; WL, waitlist.
The study by Sundaram et al., [25] evaluated a retrospective United Network for Organ Sharing (UNOS) cohort of 100,594 LT candidates. Frailty was evaluated by KPS but did not show any impact on post-LT mortality. This study showed that the proportion of patients with KPS >80 % was lower as the grade of ACLF increased (14.5 %, 7.5 %, and 2.5 % for ACLF grades 1-3, respectively, p < 0.001). Different factors were related to increased mortality after LT in the MV analysis: a donor risk index ≥1.7, the need for mechanical ventilation, and a number of four or more organs failing. Time from listing to transplant within 30 days was associated with lower post-LT mortality. A KPS ≥80 % was not associated with 1-year post-LT mortality risk (HR 0.76, 95 %CI 0.55–1.06).
The study by Abdallah et al. [26] is another retrospective UNOS-based study that included 18,416 LT candidates and evaluated frailty among the factors involved in the prognosis of LT WL candidates with ACLF. Frailty was captured by KPS. The authors developed a new score that improved the current ones in use to predict WL mortality in this population, including frailty in the evaluation. Recipient age, etiology of liver disease, ACLF grade, MELD score, race, obesity, sex, and KPS (HR 1.24, 95 %CI 1.11–1.38, p < 0.001) were the variables related to outcomes that composed the scoring model. This combination allowed us to better identify patients at a higher risk of death than any of the individual scores evaluated.
The last study assessed frailty by means of the Braden scale in a retrospective multi-center cohort of 318 LT recipients requiring ICU admission before the LT. The proportion of frail patients increased with the grade of ACLF (4.7 %, 14.8 %, 13.5 %, and 20.9 %, respectively, for ACLF 0–3; p < 0.001). In an adjusted analysis, frailty was related to a longer LOS and a higher need for discharge to a rehabilitation center, while it was not related to the post-LT length of dialysis or 30-day readmission [71].
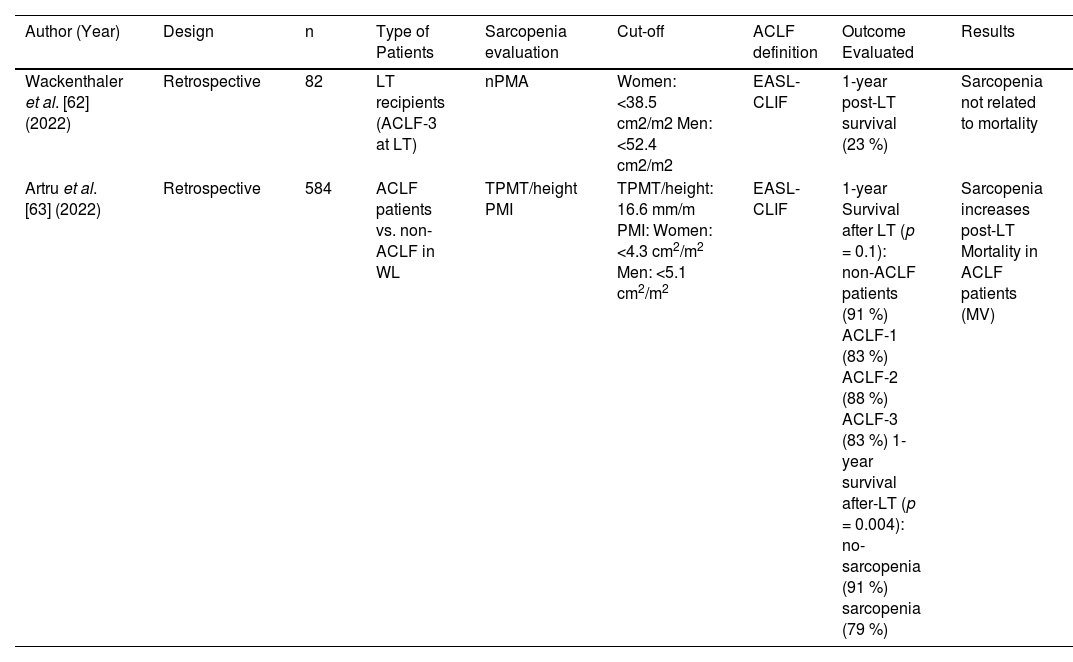
5Sarcopenia in patients with ACLFOnly two studies have been identified to evaluate the impact of sarcopenia on patients with ACLF in the LT setting. These studies are summarized in Table 4. Both studies are of a retrospective nature and evaluate muscle mass by CT, but none of them defined sarcopenia by SMI.
Clinical studies describing sarcopenia evaluation and ACLF.
| Author (Year) | Design | n | Type of Patients | Sarcopenia evaluation | Cut-off | ACLF definition | Outcome Evaluated | Results |
|---|---|---|---|---|---|---|---|---|
| Wackenthaler et al. [62] (2022) | Retrospective | 82 | LT recipients (ACLF-3 at LT) | nPMA | Women: <38.5 cm2/m2 Men: <52.4 cm2/m2 | EASL-CLIF | 1-year post-LT survival (23 %) | Sarcopenia not related to mortality |
| Artru et al. [63] (2022) | Retrospective | 584 | ACLF patients vs. non-ACLF in WL | TPMT/height PMI | TPMT/height: 16.6 mm/m PMI: Women: <4.3 cm2/m2 Men: <5.1 cm2/m2 | EASL-CLIF | 1-year Survival after LT (p = 0.1): non-ACLF patients (91 %) ACLF-1 (83 %) ACLF-2 (88 %) ACLF-3 (83 %) 1-year survival after-LT (p = 0.004): no-sarcopenia (91 %) sarcopenia (79 %) | Sarcopenia increases post-LT Mortality in ACLF patients (MV) |
ACLF, Acute-on-chronic liver failure; EASL-CLIF, European Association Study liver- Chronic Liver Failure Consortium; LT, liver transplantation; MV, multivariate; PMA, Psoas muscle area; PMI, psoas muscle index; TIPS, transjugular intrahepatic portosystemic shunt; TPMT/height transversal right psoas muscle thickness at the umbilical level/height; WL, waitlist.
In the first study, 82 patients with ACLF grade 3 who underwent LT were included in a retrospective analysis [72]. Normalized psoas muscle area (nPMA) was used to evaluate sarcopenia, with different cut-off values for women and men. In this study, sarcopenia did not show any relation with outcomes. However, a score composed of image-based parameters (splenomegaly, liver atrophy, and cava diameter ratio) was able to predict 1-year post-LT survival.
In the study by Artru et al. [73], sarcopenia was evaluated as the primary predictor of post-LT mortality in a retrospective cohort of 584 LT candidates. Sarcopenia was captured by measuring the transversal psoas muscle thickness at the umbilical level/height (TPMT/height) and the psoas muscle index (PMI) at the L3-L4 level. One-year patient survival after LT was 91 %, 83 %, 88 %, and 83 % for non-ACLF and ACLF 1–3, respectively. In the MV analyses, the only factor associated with 1-year patient survival after LT in this ACLF cohort was sarcopenia (HR 0.82, 95 %CI 0.68–0.9, p = 0.03). This association remained in women and was only a trend in men in a sensitivity analysis according to sex. Overall, survival was significantly lower for those sarcopenic patients [75 % (95 %CI 65 %−85 %)] when compared to those non-sarcopenic [88 % (95 %CI 84 %−92 %)], p = 0.007.
In summary, we can say that frailty and sarcopenia have been scarcely taken into account in studies evaluating LT-related outcomes in patients with ACLF, probably because of their retrospective nature, difficulties in capturing these entities, and lack of data in registry-based studies. Among the five studies reported in this review, three have found that performance status or sarcopenia has an impact on WL or post-LT mortality of patients with ACLF.
6Clinical implications, limitations, and future directionsPatients with ACLF have differential characteristics, such as OF, that lead on multiple occasions to the need for extrahepatic organ support and ICU admission.
In the setting of critical illness, the physiologic reserve has a decisive relevance, as the catabolic state is exacerbated, and those frail or sarcopenic patients might not have enough reserve to face the critical situation [74].
In patients with ACLF, frailty, and sarcopenia might be used as additional tools to guide futility decision-making [75]. This is of special relevance for a clinical situation where our common tools to establish a prognosis do not work that well. As previously exposed, MELD fails to capture the risk of death in patients with ACLF, underestimating their mortality risk [25,26].
While it is clear that MELD and MELDNa fail to capture the risk of death in different subpopulations of patients with cirrhosis, such as women, frail or sarcopenic patients, or patients with ACLF, there is no unique solution to serve all these patients. The different allocation policies around the world should be periodically reviewed to mitigate disparities in access to LT. Some changes are being made to improve prioritization to LT, as implementation of MELD 3.0 in some regions. Regarding patients with ACLF, there is one recently communicated pilot experience in the United Kingdom. Those 48 patients included in the WL with ACLF-3 were prioritized independently of their MELD/MELDNa score (prioritization tier). After a median WL time of 3 (2–5 days), 81 % received an LT, with a 1-year post-LT survival of 80 %. The mortality among those not transplanted was 100 %. Prioritization beyond MELD seems to be needed for these patients, with a very limited window for LT. While waiting for more data in this regard, this approach may lead the way forward [76].
The attempts that have been made to improve the current scores used to estimate post-LT survival in this setting, like TAM or SALT-M scores, have not yet led to a model or score accepted in clinical practice to predict pre- or post-LT survival or to make decisions regarding prioritization or futility in this setting.
Importantly, sarcopenia and functional status might play a significant role, but they have scarcely been evaluated in this specific setting. The relation of sarcopenia and frailty with poor outcomes both before and after LT in patients with cirrhosis has been largely established, and it is to be expected that sarcopenia and frailty might have a similar or even greater role in the outcomes of LT candidates with ACLF.
It is important to underscore that these patients might be unable to complete any test requiring collaboration, so frailty evaluation would have to rely on tests that do not require cooperation from the patient, such as KPS or the Braden test. In this scenario, the evaluation of sarcopenia can be performed with the tools described above, as supported by the critically ill literature. In this regard, CT evaluation can provide body composition data, and in addition to sarcopenia, the role of subcutaneous adipose tissue index, visceral adipose tissue index, VSR, SVO, and muscle attenuation or myoesteatosis might add valuable information in this arena [77-81].
The main limitations of the presented studies are the low number of studies, some of them with few patients evaluated, and their retrospective nature. Most of the literature regarding ACLF does not consider frailty or sarcopenia as variables of study. Also, neither the definitions of frailty or sarcopenia nor the tests used were homogenous, limiting the ability to compare studies [2,10]. These shortcomings in the field may also guide future directions.
In looking toward the future, it is imperative to incorporate the assessment of sarcopenia and/or frailty in all studies examining outcomes among patients with ACLF. Such assessments should be conducted systematically. The use of standardized and common definitions, as proposed by the frailty and sarcopenia expert opinion working groups, will be beneficial for clinical practice and research [2,10,15]. More specifically focused on this setting, recently published EASL guidelines establish a strong recommendation to evaluate sarcopenia using SMI if a CT is done. LFI evaluation is suggested in non-bedridden patients as a weak recommendation [20].
The role of muscle ultrasound and BIA needs further research in this scenario; their accessibility, the potential to perform repeated measurements, and lack of side effects make them valuable tools, especially in critically ill patients, for whom bedside evaluations might be preferable, allowing repeated assessments.
In light of the study by Artru et al., the evaluation of differences between women and men should also be included [73]. This is in consonance with previous descriptions that women and men with similar MELDNa present different frailty scores; likewise, the prevalence and impact of body composition differ by sex in patients with cirrhosis [11,54,82].
Regarding LT for patients with ACLF, there are three main needs: first, to identify those patients who would benefit from LT, recognizing those who are not good candidates, second, to evaluate interventions that might help to improve prognosis, and third, LT prioritization for these patients should be improved according to their actual risk of death. The evaluation of body composition and/or frailty might be of great use as it might have a role in the first two aspects, also evaluating interventions. Those frail or sarcopenic patients would be less likely to recover than non-frail or non-sarcopenic patients despite the same degree of OF. Despite limitations, the data we have gathered so far suggests a relationship between sarcopenia, frailty, and outcomes in patients with ACLF. Most of the studies presented in this review are not from the LT setting; however, some of this data can be useful in reinforcing and broadening the relationship between frailty and sarcopenia and critical illness and ACLF [83-85]. Studies in this population considering sarcopenia and/or frailty are needed. The evaluation of sarcopenia might be preferable in these patients in a scenario where other metrics might not be possible.
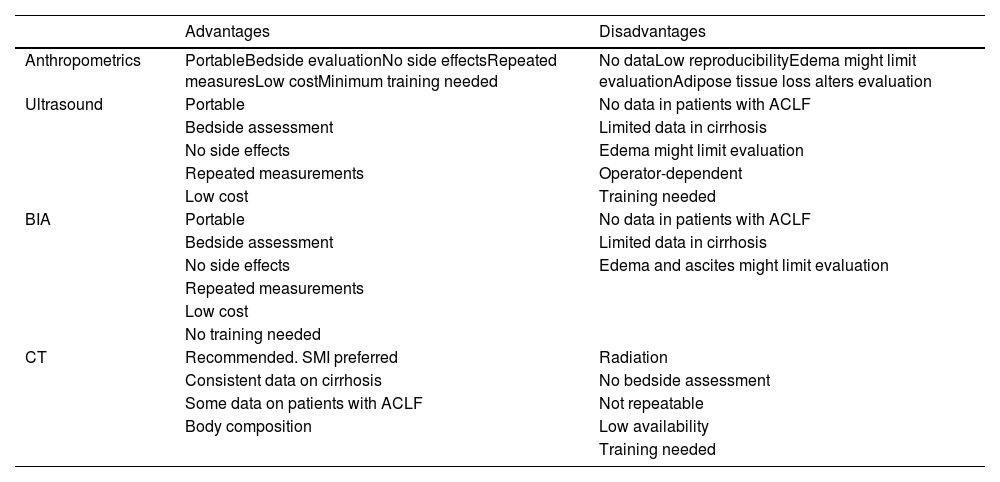
More difficult to assess are the interventions that could improve sarcopenia and frailty, especially in this setting when the time to LT is so limited. It seems reasonable that in the context of patients with ACLF, the efforts might be directed not only to reverse but to avoid deterioration. The clinical situation of patients with ACLF is extremely dynamic, as should be our ability to capture their improvement or deterioration. The possibility of performing repeated measurements would be key to monitoring sarcopenia and frailty, giving extra value to those tools that allow us to perform repeated evaluations without side effects or any other limitations, such as ultrasound, BIA, or even frailty evaluation. Table 5 summarizes the advantages and disadvantages of potential tools to evaluate sarcopenia in patients with ACLF.
Tools that can be used to evaluate sarcopenia in patients with ACLF.
ACLF, Acute-on-chronic liver failure; BIA, Bioelectrical impedance analysis; CT, computed tomography; SMI, skeletal muscle index.
Malnutrition is common in patients with cirrhosis and related to impaired outcomes. Also, the risk of malnutrition increases during hospitalization and ICU admission [15,86-90]. The Royal Free Hospital Nutrition Prioritizing Tool (RFHNPT) and the Royal Free Hospital Global Assessment (RFH-SGA) as cirrhotic-specific tools have been recommended as useful to evaluate malnutrition in patients with cirrhosis [15,63,91]. Hand grip strength is also considered a measure of malnutrition [1,35].
Acknowledging the lack of specific data in patients with ACLF, EASL ACLF guidelines recommend that these patients achieve a calorie intake of 30–35 kcal/kg/day, as well as a minimum protein intake of 1.2–1.5 g/kg/day, that can be increased to 2 g/kg/day. Micronutrients should also be supplied, and long fasting should be avoided. Based on the absence of evidence, AASLD ACLF guidelines recommend a standard nutritional formula since there is no proven benefit from BCAA formulas. There is agreement among societies to optimize nutritional status in these patients [20,21,92].
In the specific context of patients with ACLF, no experiences with training or nutrition interventions are reported aside from some data on ICU patients. None of the above-mentioned training interventions would be feasible both because of the inability of patients to carry out the prescribed physical activity and, importantly, because of timing, as these patients have a very limited time window for LT. Nutritional interventions might be feasible, even using a feeding tube, but the success of these strategies might be time-limited, as the minimum length needed to observe any effect is not known. One valuable objective might be to avoid deterioration in these patients, even if no improvement is achieved. As these patients have not been included in most of the reported studies, data evaluating the optimization of nutritional status and physical condition in patients with ACLF are needed.
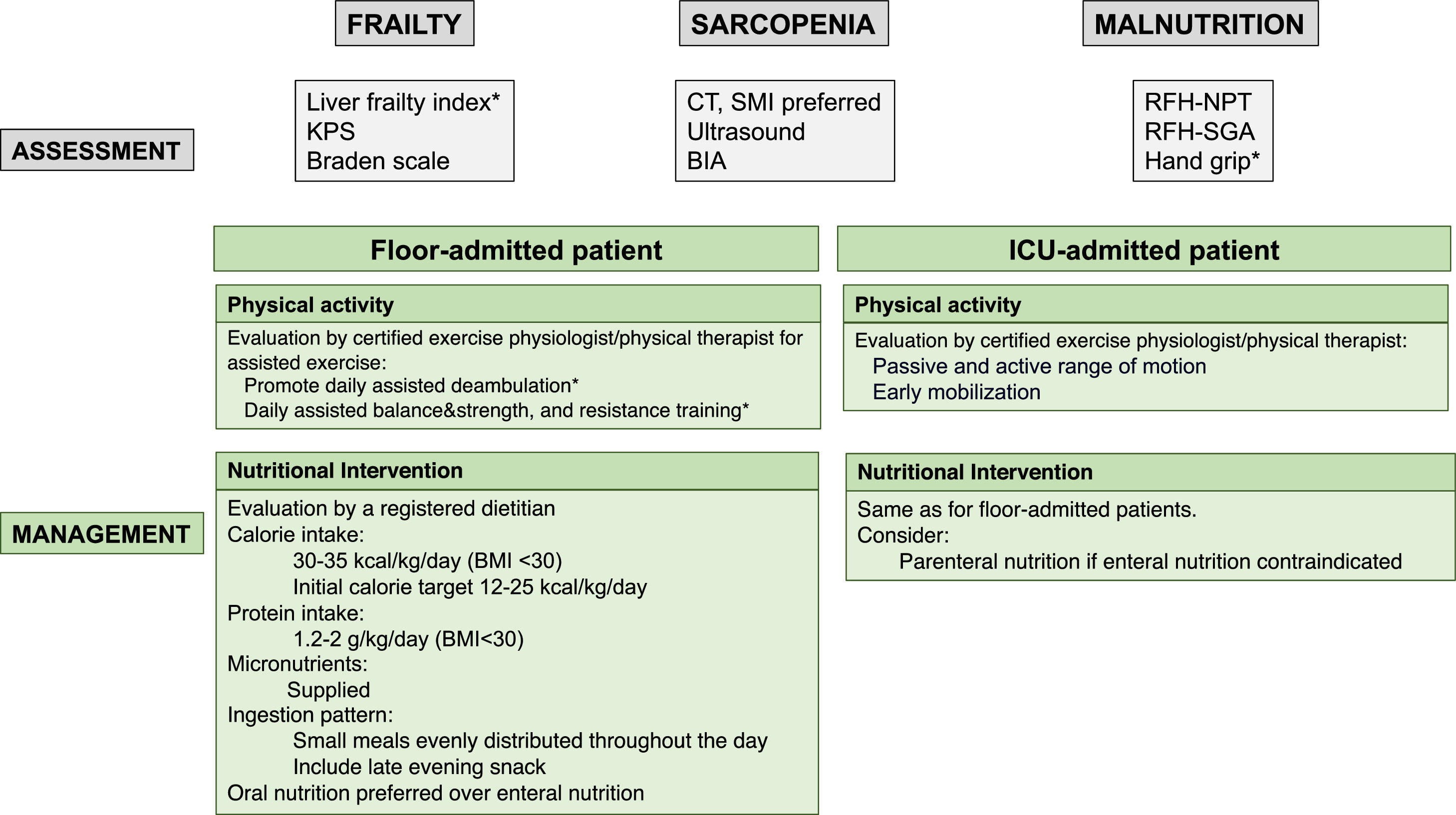
An algorithm for the assessment and management of sarcopenia, frailty, and malnutrition in patients with ACLF is proposed in Fig. 3.
Proposed assessment and management of frailty, sarcopenia, and malnutrition with specific tools for patients with ACLF according to their clinical situation. BIA, bioelectrical impedance; BMI, body mass index; CT, computed tomography; KPS, Karnofsky performance scale; RFH-NPT, Royal Free Hospital-Nutritional Prioritizing Tool; RFH-SGA, Royal Free Hospital; SMI, skeletal muscle index.
*If the clinical condition allows.
The information gathered from the limited literature that brings together information on sarcopenia and/or frailty in patients with ACLF in the LT setting grants to implement their systematic evaluation in this scenario. Those patients with ACLF who are frail or sarcopenic have a greater risk of impaired outcomes and mortality. This field in expansion will benefit from this approach, as other areas of study of patients with cirrhosis have done before. Sarcopenia and frailty evaluation in patients with ACLF might contribute to identifying those better candidates for LT, as well as those patients too sick to undergo a LT. The evaluation of body composition appears to be the most reliable tool in this setting.
Author contributionsConceptualization: IC-V, MS-T. Writing original draft: IC-V, MS-T. Software: SQ. Writing-review & editing: LC, SQ, VV.
Isabel Campos-Varela's research activity is funded by grant PI19/00330, funded by Instituto de Salud Carlos III and co-funded by the European Union (ERDF/ESF, “A way to make Europe”/“Investing in your future”). Macarena Simon-Talero's research activity is funded by grant PI21/00312, funded by Instituto de Salud Carlos III and co-funded by the European Union (ERDF/ESF, “A way to make Europe”/“Investing in your future”). CIBERehd is supported by Instituto de Salud Carlos III. The work was independent of all funding.