Metabolic dysfunction associated fatty liver disease (MAFLD) is a global public health issue that is linked to metabolic dysfunction, and it affects approximately 38 % of the adult population worldwide [1-3]. MAFLD is increasingly recognized as a condition that impacts multiple systems in the body and is associated with a greater risk of chronic kidney disease (CKD) [4-7].
The previous terminology for nonalcoholic fatty liver disease (NAFLD) has various limitations, so a new definition for MAFLD was introduced. This new definition relies on specific diagnostic criteria that identify metabolic dysfunction [8-10]. The criteria for MAFLD have been widely validated and shown to be more effective at identifying patients at high risk of both hepatic and non-hepatic complications than the previous NAFLD criteria [11,12]. Recently, the term "metabolic dysfunction-associated steatotic liver disease" (MASLD) was proposed through the Delphi process. A new set of diagnostic criteria has been suggested for the diagnosis of MASLD [13].
Nevertheless, it is still uncertain whether the proposed MASLD criteria would better capture the clinical features and diverse outcomes of individuals with fatty liver disorders than the MAFLD criteria. Clarifying this issue is crucial because only a significant improvement in performance compared to the MAFLD criteria would justify the unavoidable risk of potential confusion due to introducing these criteria shortly after the MAFLD criteria.
Recent findings indicate that MAFLD is more effective than NAFLD in identifying people with CKD [14]. However, it is still unclear whether MASLD can more effectively identify people who are at greater risk of developing CKD (a significant consequence connected to FLD) than MAFLD can.
Therefore, the primary aim of this study was to assess the prevalence of CKD and abnormal urinary albumin levels in individuals identified using either the MAFLD or MASLD classification. To achieve this objective, we conducted a cross-sectional investigation using data from the 2017–2020 cycles of the U.S. National Health and Nutrition Examination Survey (NHANES).
2Patients and Methods2.1Study designThe cohort for this study was obtained from the most recent National Health and Nutrition Examination Surveys conducted between 2017 and 2020 (NHANES 2017–2020). These surveys were carried out by the National Center for Health Statistics, which is a part of the Centers for Disease Control and Prevention in the United States. The NHANES dataset, as well as additional information about the NHANES, can be accessed publicly at this link: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?Cycle=2017–2020.
2.2Clinical and laboratory dataThe following data were collected through self-reports: age, sex, and ethnicity. During the clinical evaluation, the following information was also gathered: body mass index (BMI), waist circumference (W.C.), blood pressure, presence or absence of type 2 diabetes mellitus (T2DM), hypertension, and dyslipidaemia (diagnosed based on standard criteria), as well as data on current alcohol usage.
Additional information on fasting blood total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase, platelet count, creatinine, and albumin were also obtained. The presence of an ongoing hepatitis C virus infection was determined by detecting viral RNA and/or using a validated antibody test. The presence of hepatitis B virus infection was confirmed by a positive surface antigen test.
2.3Vibration-controlled transient elastographyThe NHANES 2017–2020 cycles have vibration-controlled transient elastography (VCTE) undertaken using a FibroScan® Model 502 V2 Touch (Echosens), which was equipped with medium (M) and extra-large (XL) probes. The diagnosis of steatosis was determined by a median CAP cut-off of 263 dB/m, as established in previous studies. Significant fibrosis (≥F2) was defined by a median LSM of 8.0 kPa or greater [15,16].
2.4Diagnosis of MAFLD and MASLDMAFLD is diagnosed by hepatic steatosis and one of the following conditions: overweight or obesity, T2DM, or lean/normal weight with at least two metabolic risk abnormalities [8]. MASLD is diagnosed by hepatic steatosis, along with one or more of the specified five cardiometabolic risk factors [13]. Individuals who have MASLD and report higher alcohol use (ranging from 210 to 420 gs per week for men or 140 to 350 gs per week for females) were classified as having metabolic alcoholic liver disease (MetALD). Individuals who had both viral hepatitis and MASLD were classified as having MASLD combined with other liver diseases.
2.5Assessment of chronic kidney diseaseThe estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) algorithm, as previously explained. Chronic kidney disease (CKD) was defined as having either an eGFR ≤60 mL/min/1.73 m² or the presence of albuminuria. The stages of CKD were established based on the KDIGO guidelines [17].
2.6Statistical analysisContinuous variables are described as the median and range or the number of observations. Categorical variables are described by their frequencies and proportions. The differences between groups was assessed using the Wilcoxon rank-sum test for continuous variables and Fisher's exact test for categorical data. To identify the independent variables associated with CKD prevalence, a logistic regression model was applied. The results are reported as odds ratios (OR) with corresponding 95 % confidence intervals (C.I). A p value less than 0.05 indicated statistical significance. The data analysis was conducted using SPSS software.
2.7Ethical statementThe analyses conducted in that research program followed the specific recommendations provided by the NCHS. The NHANES was approved by the Centers for Disease Control and Prevention Research Ethics Review Board, and all adult participants signed informed consent forms.
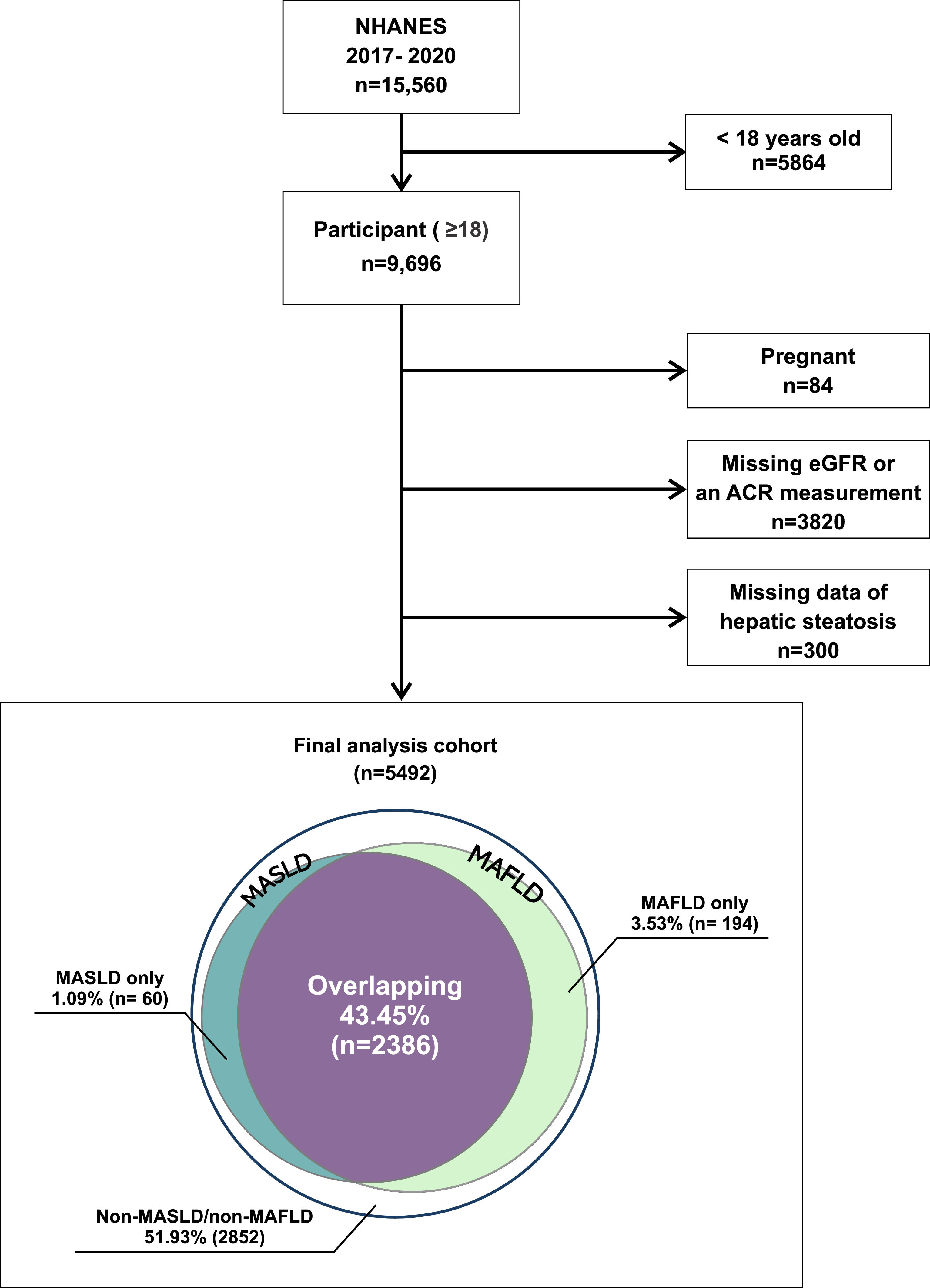
3Results3.1Study populationOut of the 9696 individuals aged 18 years or older in the NHANES dataset from 2017 to 2020, we excluded pregnant women and those with missing ultrasonography or essential clinical and laboratory data related to the outcome of interest. As a result, 4204 individuals were excluded from the study (Fig. 1). Therefore, the final analysis included a total of 5492 individuals. Within this cohort, 1585 participants (28.9 %) had evidence of CKD, 856 (15.6 %) of whom were classified as having CKD stages 1–2 and 729 (13.3 %) of whom were classified as having CKD stages 3–5.
MAFLD and MASLD were present in 47 % and 44.5 % of all participants, respectively. Additionally, 2.1 % had MetALD, and 0.8 % had MASLD with chronic viral hepatitis. Participants with both MAFLD and MASLD accounted for 43.4 % of the total cohort. Nonoverlapping MAFLD and MASLD were observed in 3.5 % and 1.1 %, respectively, of all participants. The remaining 51.9 % of the participants were classified as having none of the above (Fig. 1).
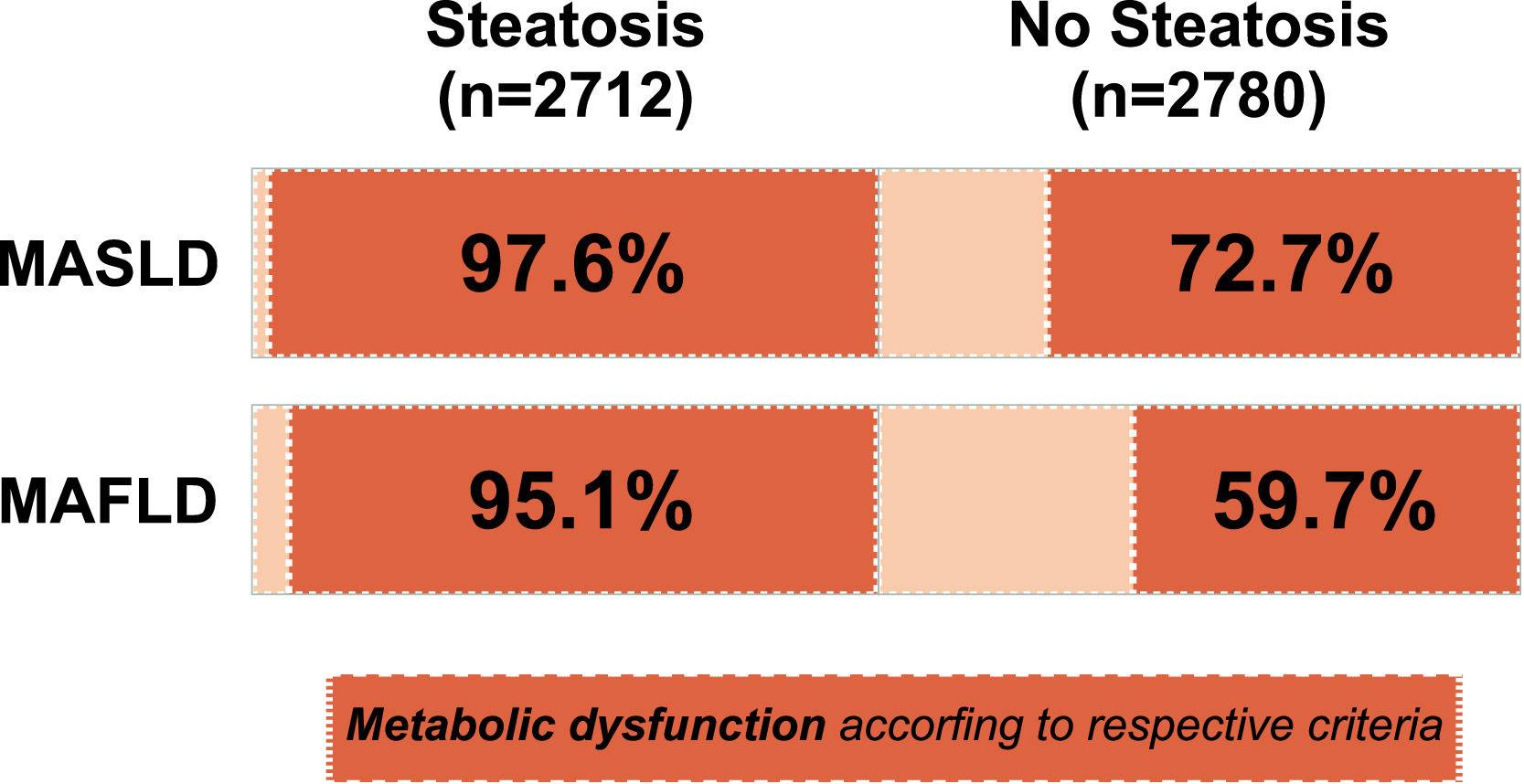
Notably, in the general adult U.S. population with no evidence of hepatic steatosis, 72.7 % of the participants were found to have at least one cardiometabolic risk factor for MASLD, while only 59.7 % of the participants were identified as having at least two risk factors according to the MAFLD criteria. In contrast, among the subjects with hepatic steatosis (n = 2712), 97.6 % and 95.1 % were diagnosed with MASLD and MAFLD, respectively (Fig. 2).
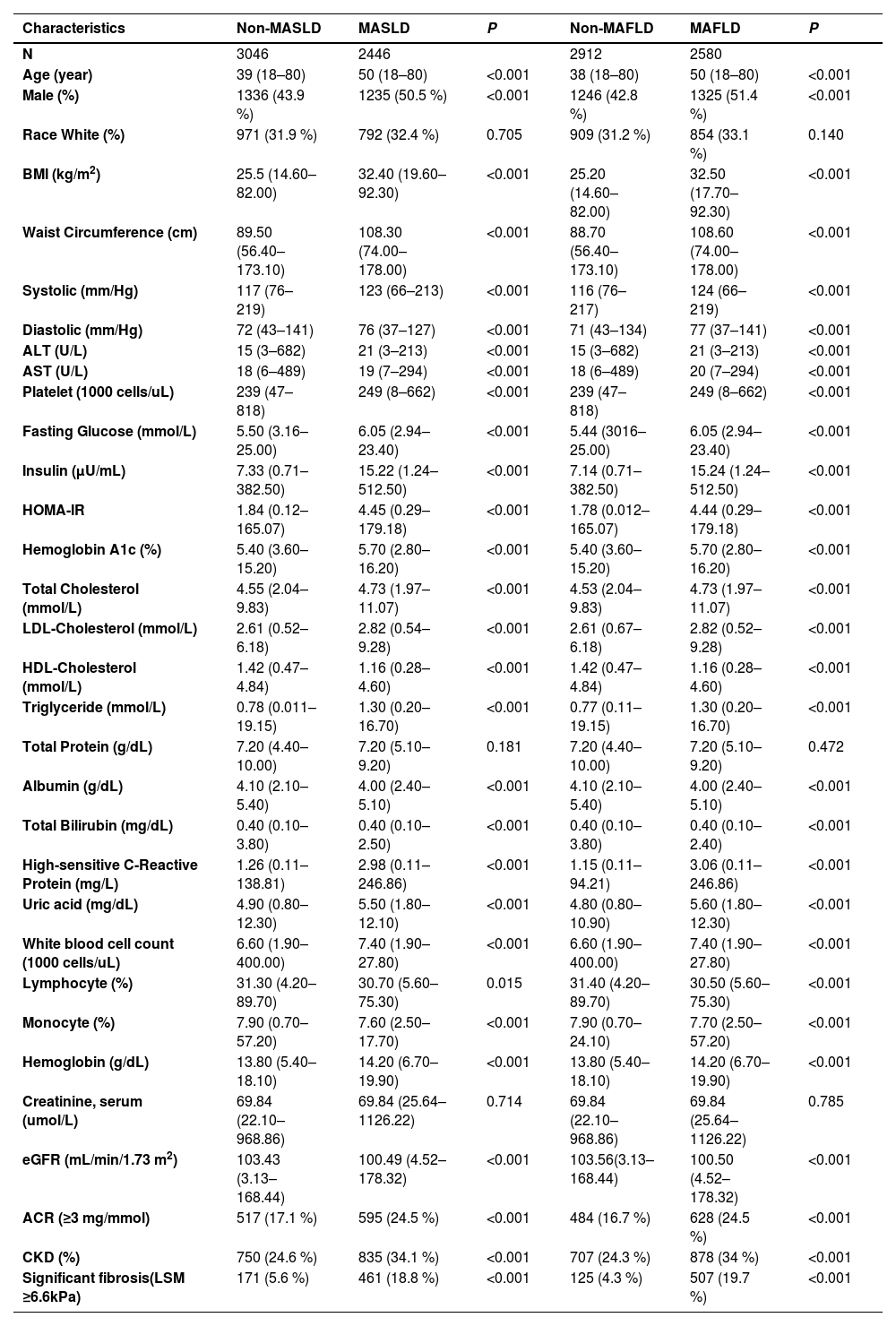
3.2Demographic and clinical characteristics of the participantsTable 1 depicts the clinical and biochemical characteristics of individuals diagnosed with MAFLD and MASLD. Compared to the non-MAFLD group, individuals with MAFLD showed a higher prevalence of males and advanced age; significantly greater BMI, HbA1c, fasting glucose, serum liver enzymes, liver stiffness measurement (LSM), and hs-CRP; and a more atherogenic lipid profile. There was a greater prevalence of hypertension and T2DM in individuals with MAFLD than in those without MAFLD. Additionally, they had higher urinary ACRs and lower eGFRs. Similar differences were also observed between subjects with MASLD and those without MASLD.
Comparison of baseline characteristics in subjects with and without MAFLD and in those with and without MASLD.
| Characteristics | Non-MASLD | MASLD | P | Non-MAFLD | MAFLD | P |
|---|---|---|---|---|---|---|
| N | 3046 | 2446 | 2912 | 2580 | ||
| Age (year) | 39 (18–80) | 50 (18–80) | <0.001 | 38 (18–80) | 50 (18–80) | <0.001 |
| Male (%) | 1336 (43.9 %) | 1235 (50.5 %) | <0.001 | 1246 (42.8 %) | 1325 (51.4 %) | <0.001 |
| Race White (%) | 971 (31.9 %) | 792 (32.4 %) | 0.705 | 909 (31.2 %) | 854 (33.1 %) | 0.140 |
| BMI (kg/m2) | 25.5 (14.60–82.00) | 32.40 (19.60–92.30) | <0.001 | 25.20 (14.60–82.00) | 32.50 (17.70–92.30) | <0.001 |
| Waist Circumference (cm) | 89.50 (56.40–173.10) | 108.30 (74.00–178.00) | <0.001 | 88.70 (56.40–173.10) | 108.60 (74.00–178.00) | <0.001 |
| Systolic (mm/Hg) | 117 (76–219) | 123 (66–213) | <0.001 | 116 (76–217) | 124 (66–219) | <0.001 |
| Diastolic (mm/Hg) | 72 (43–141) | 76 (37–127) | <0.001 | 71 (43–134) | 77 (37–141) | <0.001 |
| ALT (U/L) | 15 (3–682) | 21 (3–213) | <0.001 | 15 (3–682) | 21 (3–213) | <0.001 |
| AST (U/L) | 18 (6–489) | 19 (7–294) | <0.001 | 18 (6–489) | 20 (7–294) | <0.001 |
| Platelet (1000 cells/uL) | 239 (47–818) | 249 (8–662) | <0.001 | 239 (47–818) | 249 (8–662) | <0.001 |
| Fasting Glucose (mmol/L) | 5.50 (3.16–25.00) | 6.05 (2.94–23.40) | <0.001 | 5.44 (3016–25.00) | 6.05 (2.94–23.40) | <0.001 |
| Insulin (µU/mL) | 7.33 (0.71–382.50) | 15.22 (1.24–512.50) | <0.001 | 7.14 (0.71–382.50) | 15.24 (1.24–512.50) | <0.001 |
| HOMA-IR | 1.84 (0.12–165.07) | 4.45 (0.29–179.18) | <0.001 | 1.78 (0.012–165.07) | 4.44 (0.29–179.18) | <0.001 |
| Hemoglobin A1c (%) | 5.40 (3.60–15.20) | 5.70 (2.80–16.20) | <0.001 | 5.40 (3.60–15.20) | 5.70 (2.80–16.20) | <0.001 |
| Total Cholesterol (mmol/L) | 4.55 (2.04–9.83) | 4.73 (1.97–11.07) | <0.001 | 4.53 (2.04–9.83) | 4.73 (1.97–11.07) | <0.001 |
| LDL-Cholesterol (mmol/L) | 2.61 (0.52–6.18) | 2.82 (0.54–9.28) | <0.001 | 2.61 (0.67–6.18) | 2.82 (0.52–9.28) | <0.001 |
| HDL-Cholesterol (mmol/L) | 1.42 (0.47–4.84) | 1.16 (0.28–4.60) | <0.001 | 1.42 (0.47–4.84) | 1.16 (0.28–4.60) | <0.001 |
| Triglyceride (mmol/L) | 0.78 (0.011–19.15) | 1.30 (0.20–16.70) | <0.001 | 0.77 (0.11–19.15) | 1.30 (0.20–16.70) | <0.001 |
| Total Protein (g/dL) | 7.20 (4.40–10.00) | 7.20 (5.10–9.20) | 0.181 | 7.20 (4.40–10.00) | 7.20 (5.10–9.20) | 0.472 |
| Albumin (g/dL) | 4.10 (2.10–5.40) | 4.00 (2.40–5.10) | <0.001 | 4.10 (2.10–5.40) | 4.00 (2.40–5.10) | <0.001 |
| Total Bilirubin (mg/dL) | 0.40 (0.10–3.80) | 0.40 (0.10–2.50) | <0.001 | 0.40 (0.10–3.80) | 0.40 (0.10–2.40) | <0.001 |
| High-sensitive C-Reactive Protein (mg/L) | 1.26 (0.11–138.81) | 2.98 (0.11–246.86) | <0.001 | 1.15 (0.11–94.21) | 3.06 (0.11–246.86) | <0.001 |
| Uric acid (mg/dL) | 4.90 (0.80–12.30) | 5.50 (1.80–12.10) | <0.001 | 4.80 (0.80–10.90) | 5.60 (1.80–12.30) | <0.001 |
| White blood cell count (1000 cells/uL) | 6.60 (1.90–400.00) | 7.40 (1.90–27.80) | <0.001 | 6.60 (1.90–400.00) | 7.40 (1.90–27.80) | <0.001 |
| Lymphocyte (%) | 31.30 (4.20–89.70) | 30.70 (5.60–75.30) | 0.015 | 31.40 (4.20–89.70) | 30.50 (5.60–75.30) | <0.001 |
| Monocyte (%) | 7.90 (0.70–57.20) | 7.60 (2.50–17.70) | <0.001 | 7.90 (0.70–24.10) | 7.70 (2.50–57.20) | <0.001 |
| Hemoglobin (g/dL) | 13.80 (5.40–18.10) | 14.20 (6.70–19.90) | <0.001 | 13.80 (5.40–18.10) | 14.20 (6.70–19.90) | <0.001 |
| Creatinine, serum (umol/L) | 69.84 (22.10–968.86) | 69.84 (25.64–1126.22) | 0.714 | 69.84 (22.10–968.86) | 69.84 (25.64–1126.22) | 0.785 |
| eGFR (mL/min/1.73 m2) | 103.43 (3.13–168.44) | 100.49 (4.52–178.32) | <0.001 | 103.56(3.13–168.44) | 100.50 (4.52–178.32) | <0.001 |
| ACR (≥3 mg/mmol) | 517 (17.1 %) | 595 (24.5 %) | <0.001 | 484 (16.7 %) | 628 (24.5 %) | <0.001 |
| CKD (%) | 750 (24.6 %) | 835 (34.1 %) | <0.001 | 707 (24.3 %) | 878 (34 %) | <0.001 |
| Significant fibrosis(LSM ≥6.6kPa) | 171 (5.6 %) | 461 (18.8 %) | <0.001 | 125 (4.3 %) | 507 (19.7 %) | <0.001 |
ACR, albumin-to-creatinine ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; LDL, low density lipoprotein; LSM, liver stiffness measurement; MAFLD, metabolic dysfunction-associated fatty liver disease; MASLD, metabolic dysfunction-associated steatotic liver disease.
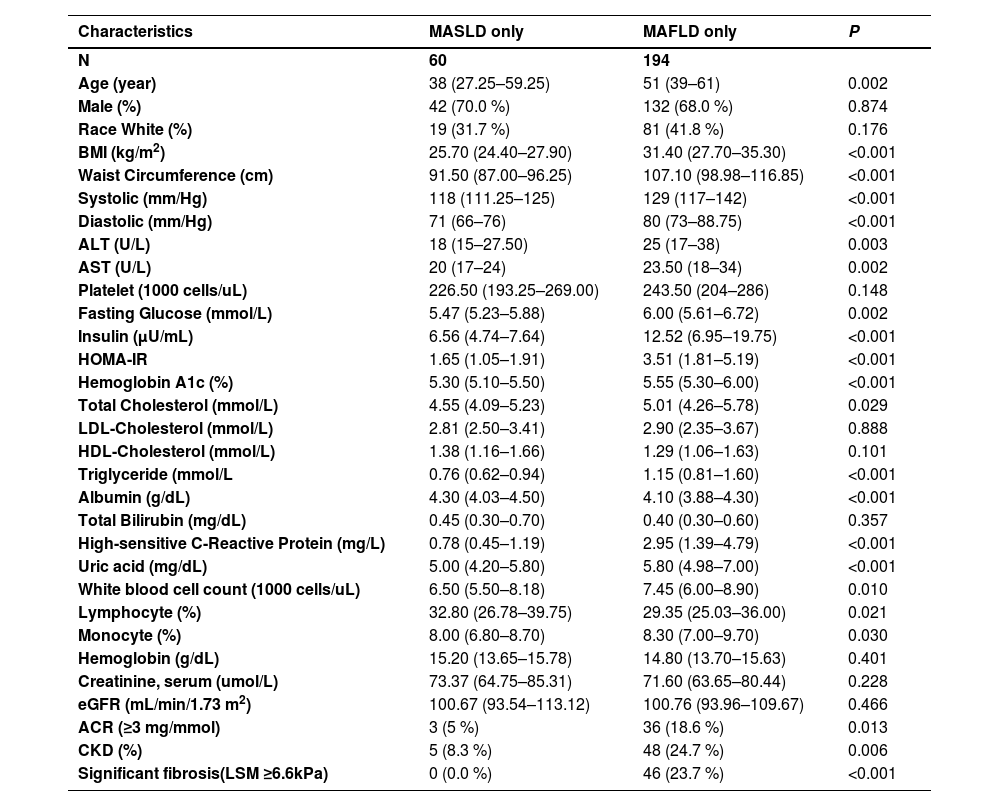
A comparison of nonoverlapping MAFLD patients and nonoverlapping MASLD patients revealed that MAFLD patients tended to be older and have a greater BMI and waist circumference. The MAFLD patients also had a worse metabolic profile, including a significantly greater prevalence of hypertension, T2DM, dyslipidaemia, and significant liver fibrosis, and greater levels of serum liver enzymes (AST and ALT) than did the MASLD patients (Table 2).
Comparison of baseline characteristics between the MASLD and MAFLD non-overlapping groups.
| Characteristics | MASLD only | MAFLD only | P |
|---|---|---|---|
| N | 60 | 194 | |
| Age (year) | 38 (27.25–59.25) | 51 (39–61) | 0.002 |
| Male (%) | 42 (70.0 %) | 132 (68.0 %) | 0.874 |
| Race White (%) | 19 (31.7 %) | 81 (41.8 %) | 0.176 |
| BMI (kg/m2) | 25.70 (24.40–27.90) | 31.40 (27.70–35.30) | <0.001 |
| Waist Circumference (cm) | 91.50 (87.00–96.25) | 107.10 (98.98–116.85) | <0.001 |
| Systolic (mm/Hg) | 118 (111.25–125) | 129 (117–142) | <0.001 |
| Diastolic (mm/Hg) | 71 (66–76) | 80 (73–88.75) | <0.001 |
| ALT (U/L) | 18 (15–27.50) | 25 (17–38) | 0.003 |
| AST (U/L) | 20 (17–24) | 23.50 (18–34) | 0.002 |
| Platelet (1000 cells/uL) | 226.50 (193.25–269.00) | 243.50 (204–286) | 0.148 |
| Fasting Glucose (mmol/L) | 5.47 (5.23–5.88) | 6.00 (5.61–6.72) | 0.002 |
| Insulin (µU/mL) | 6.56 (4.74–7.64) | 12.52 (6.95–19.75) | <0.001 |
| HOMA-IR | 1.65 (1.05–1.91) | 3.51 (1.81–5.19) | <0.001 |
| Hemoglobin A1c (%) | 5.30 (5.10–5.50) | 5.55 (5.30–6.00) | <0.001 |
| Total Cholesterol (mmol/L) | 4.55 (4.09–5.23) | 5.01 (4.26–5.78) | 0.029 |
| LDL-Cholesterol (mmol/L) | 2.81 (2.50–3.41) | 2.90 (2.35–3.67) | 0.888 |
| HDL-Cholesterol (mmol/L) | 1.38 (1.16–1.66) | 1.29 (1.06–1.63) | 0.101 |
| Triglyceride (mmol/L | 0.76 (0.62–0.94) | 1.15 (0.81–1.60) | <0.001 |
| Albumin (g/dL) | 4.30 (4.03–4.50) | 4.10 (3.88–4.30) | <0.001 |
| Total Bilirubin (mg/dL) | 0.45 (0.30–0.70) | 0.40 (0.30–0.60) | 0.357 |
| High-sensitive C-Reactive Protein (mg/L) | 0.78 (0.45–1.19) | 2.95 (1.39–4.79) | <0.001 |
| Uric acid (mg/dL) | 5.00 (4.20–5.80) | 5.80 (4.98–7.00) | <0.001 |
| White blood cell count (1000 cells/uL) | 6.50 (5.50–8.18) | 7.45 (6.00–8.90) | 0.010 |
| Lymphocyte (%) | 32.80 (26.78–39.75) | 29.35 (25.03–36.00) | 0.021 |
| Monocyte (%) | 8.00 (6.80–8.70) | 8.30 (7.00–9.70) | 0.030 |
| Hemoglobin (g/dL) | 15.20 (13.65–15.78) | 14.80 (13.70–15.63) | 0.401 |
| Creatinine, serum (umol/L) | 73.37 (64.75–85.31) | 71.60 (63.65–80.44) | 0.228 |
| eGFR (mL/min/1.73 m2) | 100.67 (93.54–113.12) | 100.76 (93.96–109.67) | 0.466 |
| ACR (≥3 mg/mmol) | 3 (5 %) | 36 (18.6 %) | 0.013 |
| CKD (%) | 5 (8.3 %) | 48 (24.7 %) | 0.006 |
| Significant fibrosis(LSM ≥6.6kPa) | 0 (0.0 %) | 46 (23.7 %) | <0.001 |
ACR, albumin-to-creatinine ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; HOMA-IR, homeostatic model assessment for insulin resistance; LDL, low density lipoprotein; LSM, liver stiffness measurement; MAFLD, metabolic dysfunction-associated fatty liver disease; MASLD, metabolic dysfunction-associated steatotic liver disease. eGFR data are expressed as median (interquartile range [IQR]).
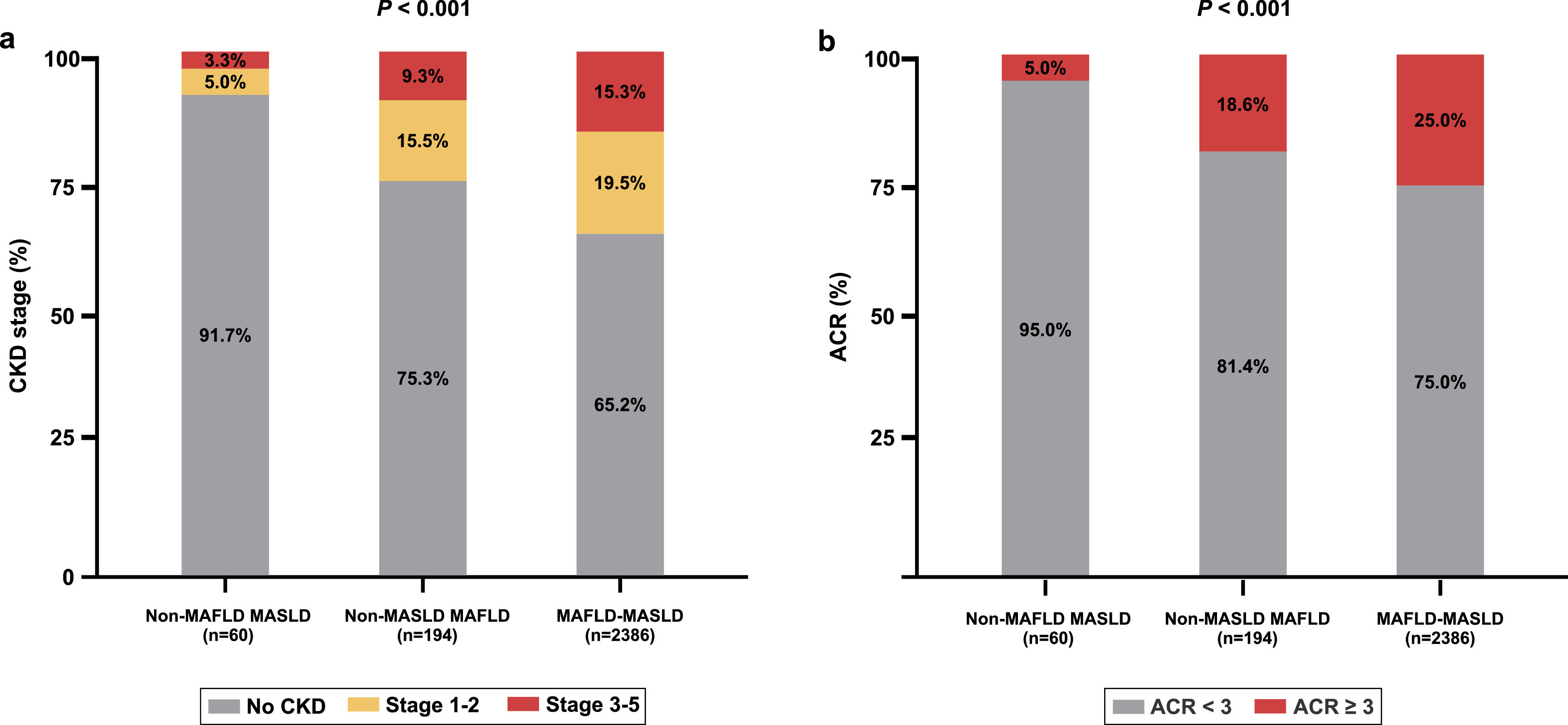
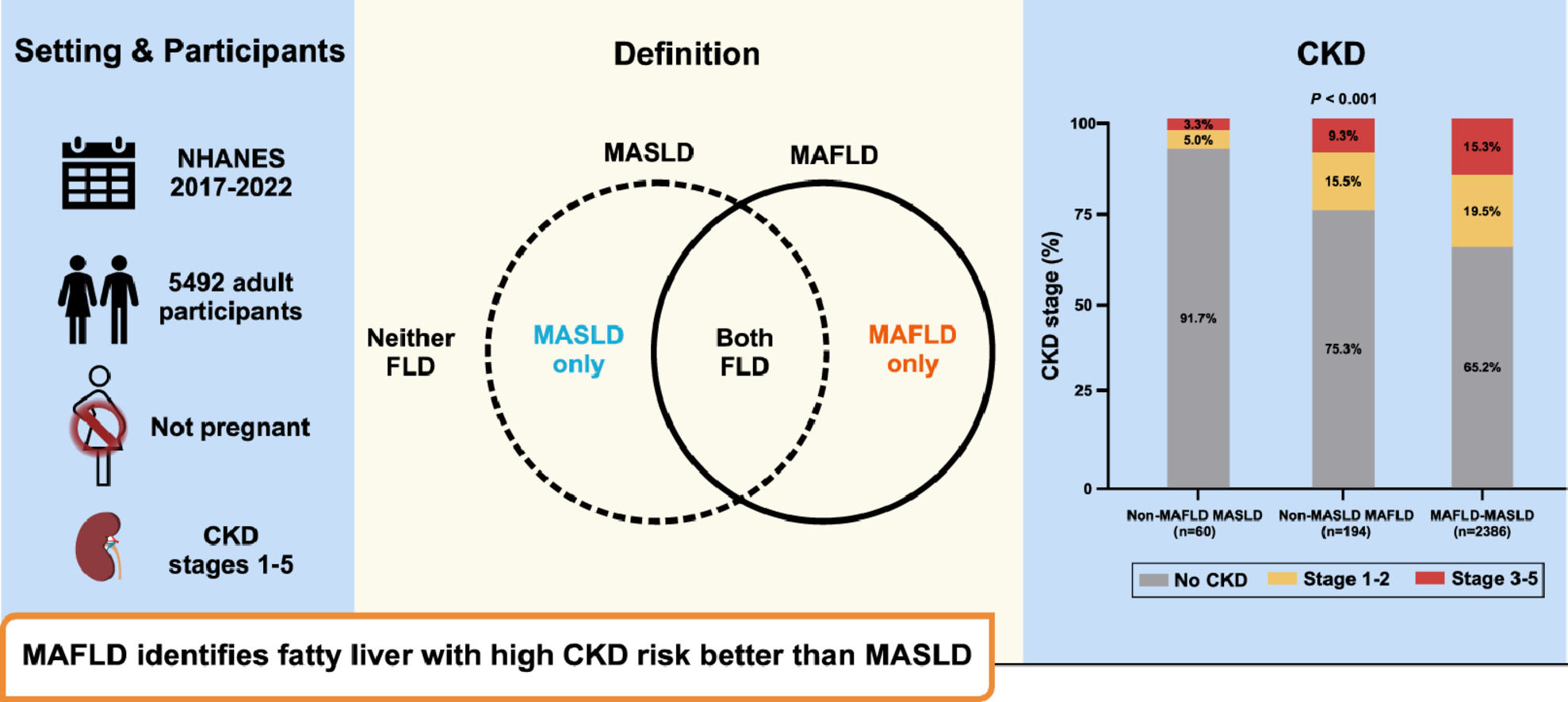
Fig. 3 shows a comparison of kidney function parameters and CKD stages between individuals with MAFLD and those with MASLD. Compared to those in the non-MAFLD-MASLD group, both the MAFLD-only and MAFLD-MASLD overlapping groups had significantly greater prevalences of both CKD and albuminuria.
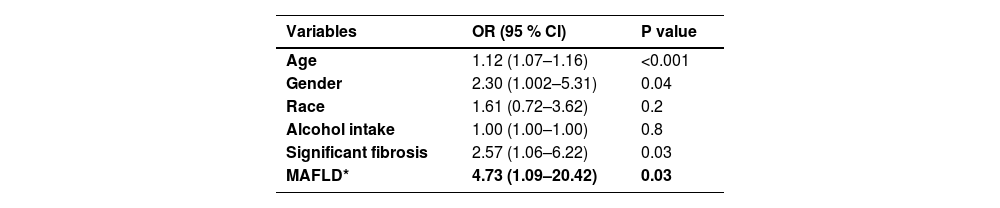
3.4Independent factors and profiles associated with decreased kidney functionTo account for potential confounding factors, we conducted a subsequent analysis comparing the nonoverlapping criteria of MAFLD and MASLD to determine their associations with the presence of CKD. This analysis utilized multiple logistic regression and was adjusted for age, sex, ethnicity, alcohol intake, and liver fibrosis severity. The results showed a significant association between MAFLD-only and CKD (OR 4.732; 95 % CI 1.09–20.42; P < 0.03) compared to MASLD-only (Table 3). Similar findings were observed for patients with an ACR ≥ 3.
Multivariate analysis of factors associated with the presence of CKD.
| Variables | OR (95 % CI) | P value |
|---|---|---|
| Age | 1.12 (1.07–1.16) | <0.001 |
| Gender | 2.30 (1.002–5.31) | 0.04 |
| Race | 1.61 (0.72–3.62) | 0.2 |
| Alcohol intake | 1.00 (1.00–1.00) | 0.8 |
| Significant fibrosis | 2.57 (1.06–6.22) | 0.03 |
| MAFLD* | 4.73 (1.09–20.42) | 0.03 |
OR, odds ratio; CI, confidence interval; MAFLD, metabolic dysfunction-associated fatty liver disease.
*MAFLD only group is compared to MASLD only group.
Next, we aimed to assess the extent to which excessive alcohol consumption contributed to the significant association between MAFLD and CKD. We compared the risk of CKD according to the presence of MetALD. However, there was no significant difference in the risk of CKD or ACR ≥ 3 between individuals with and without MetALD (p = 0.1 and 0.5, respectively).
4DiscussionIn the present study, we analysed the implementation of the recently proposed MASLD definition for FLD associated with metabolic dysfunction in clinical settings and compared it with the previously validated MAFLD definition. The main finding, which was based on a range of analyses, was that the MAFLD criteria were more accurate than the MASLD criteria in identifying individuals with hepatic steatosis with liver damage, metabolic dysfunction, and CKD. Additionally, individuals classified into the MASLD-only group appeared to be "healthier" than those classified into the MAFLD-only group or the overlapping MAFLD/MASLD group. In addition, the patients in the MASLD-only group had the lowest prevalence of albuminuria and of an eGFR ≤60 mL/min/1.73 m², even when compared to those with no steatosis. Overall, our data indicate that the MAFLD criteria were more effective than the MASLD criteria in identifying individuals at risk of CKD.
In a paradigm shift from the dogma of NAFLD that has persisted for four decades, the term MAFLD was introduced in 2020, along with an updated definition that requires the presence of metabolic dysfunction [8]. These criteria have been extensively evaluated and proven to be more effective in identifying hepatic and extrahepatic consequences than the traditional NAFLD criteria [14,18,19]. A previous investigation using the NHANES database indicated that the MAFLD criteria are superior to the NAFLD criteria in accurately identifying individuals with fatty liver who are at a heightened risk of developing CKD [14]. Similarly, another study of 28,890 Japanese subjects revealed that MAFLD predicts the new onset of CKD better than fatty liver disease or NAFLD [20]. These findings collectively demonstrate that for identifying individuals with fatty liver who are at high risk of developing CKD, the MAFLD criteria are not only more accurate than the NAFLD criteria but are also more accurate than the new MASLD criteria. Similar findings were recently reported for cardiovascular disease and in the paediatric population [21,22]. A recent consensus statement on MAFLD and CKD was put forth4, and various scores, including MAFLD fibrosis related scores, were demonstrated to predict risk of CKD among MAFLD patients [23].
Notably, the MASLD criteria appear to be quite broad and lack specificity. In the present study, 97.6 % of patients with steatosis met these criteria, as did 72.7 % of patients without steatosis. Similarly, among patients with steatosis, 95.1 % met the criteria for MAFLD, while only 59.7 % of subjects without steatosis met the criteria. Multiple other studies have reported similar results. In a study from Austria, the MASLD criteria were met by 99.0 % of patients with steatosis and 95.4 % of patients without steatosis [24]. Moreover, MAFLD was identified in 95.3 % of those with steatosis and 69 % without steatosis [20]. Additionally, a study from India showed that 77.2 % of individuals without steatosis met the criteria for MASLD, while 33.9 % met those for MAFLD [25]. These findings suggest that the MASLD criteria are highly inclusive and lack granularity. In contrast, the MAFLD criteria are more stringent, striking the right balance and identifying the subgroup of patients with steatosis and profound metabolic dysfunction that are less commonly found among subjects without steatosis. Overall, our findings cast doubt on whether a switch from MAFLD to MASLD is justified, and further studies are needed to address this issue.
This study has several advantages, as it is the first comparative analysis to examine the correlation between MAFLD and MASLD classifications and the risk of CKD in a community-based population. However, this study has several limitations inherent to the NHANES database. These limitations include the absence of a biopsy for histological validation, the cross-sectional nature of the study, the lack of a validated CAP cut-off in MetALD patients, and potential biases in self-reported alcohol intake.
5ConclusionsIn conclusion, the MAFLD criteria are more effective than the MASLD criteria for accurately identifying a cohesive group of individuals at high risk for metabolic dysfunction and CKD. Additionally, the criteria for MASLD appear to lack specificity. Therefore, further prospective cohort studies are needed to examine the likelihood of developing CKD in individuals with MAFLD compared to those with MASLD.
Author contributionsAll authors contributed equally to the conception and design of the study, data interpretation, and drafting and critical revision of the manuscript.
ME is supported by National Health and Medical Research Council of Australia (NHMRC) Program Grant (APP1053206) and Project and ideas grants (APP2001692, APP1107178 and APP1108422).