Outcomes of liver transplantation (LT) with donors after circulatory death (DCD) have been considered suboptimal due to higher rates of ischemic cholangiopathy, especially when the super-rapid recovery (SRR) technique is used. This study aimed to compare the incidence of complications between recipients receiving DCD vs those receiving donors after brain death (DBD) in a large-volume liver transplant centre.
MethodsWe performed a retrospective cohort study (LT from January 2015 to December 2018) comparing recipients who underwent a LT with DCD vs. a control group of LT with DBD, matched 1:1 without replacement by propensity score matching that included the following variables: LT indication, recipient sex and age, donor age and MELD score.
Results51 recipients with DCD-LT (29 SRR, 22 normothermic regional perfusion [NRP]) were matched with 51 DBD-LT recipients. Biliary complications were more frequent in DCD, 10% (n=5), all with SRR technique, vs 2% (n=1) in the DBD group, p=0.2. Two patients (4%) suffered primary graft non-function in the DCD group (1 SRR and 1 NRP) versus zero in the DBD group (p=0.49). Postoperative bleeding and reinterventions were also higher in the DCD group: 7 (13.7%) vs 1 (1.95%) and 8 (15.7%) vs 2 (3.9%) respectively (p=0.06 and 0.09). On the 1st postoperative day AST/ALT peak was higher in DCD (p≤0001). The incidence of rejection, vascular complications, renal injury, hospital stay, and readmissions were similar in both groups. Cumulative 1-, 2-, 3- and 4-year graft and patient survival were also similar.
ConclusionsDCD donors are an adequate option to increase the donor pool in LT, achieving similar graft and patient survival rates to those achieved with DBD donors, especially when the NRP technique is used.
Despite multiple strategies, particularly the use of elderly donors, a significant imbalance between liver offer and demand [1] is still a reality in most transplant centres, and donation after circulatory death (DCD) represents an important source to expand the donor pool. Although concerns related to warm ischemia and damage to the biliary tree have been described, the introduction of normothermic regional reperfusion (NRP) has improved outcomes both in Spain and elsewhere [2]. Spain remains the world leader in organ transplantation, registering 1034 liver transplants in 2020 [3]. Yet, like elsewhere, numbers are insufficient to satisfy organ demand. The use of DCD with NRP has allowed to increase the overall organ donor pool in recent years.
The interest in DCD LT experimented a growth in the late 80s [4]. DCD donors represent a specific type of extended criteria donors, for whom death is declared based on cardiopulmonary criteria rather than cessation of brain function [5]. Following long-term success with kidney transplantation from DCD [6], specialists have focused on liver grafts. Based on an increasing experience in Spanish centres, a Consensus Statement of DCD in LT recipients was recently held organized by the Spanish Liver Transplantation Society [1,2,7–10].
At first, uncontrolled DCD grafts without NRP were used cautiously, due to the high incidence of ischemic cholangiopathy (IC), primary graft non-function (PNF), vascular complications - such as arterial thrombosis-, acute rejection and renal insufficiency, among others [11–15]. The most common complication of this technique is IC, as the biliary tree seems to be more sensitive to warm ischemia and ischemia-reperfusion than hepatocytes [16]. The IC risk differs related to the type of DCD and the liver recovery technique used [2,17–26]. As described previously, the general use of NRP technique has reduced the rate of IC [2,20]; in addition, a better donor selection criteria, including donor time arrest of less than 20 minutes in Maastricht other than III and donor age under 70 years [9,25,27], has also resulted in better outcomes. Of note, the recent extended use of controlled DCD with normothermic reperfusion has shown to substantially improve outcomes with series demonstrating results that are close to those achieved using DBD donors [2,13–15].
Interestingly, DCD grafts have shown to be an important source of organs for patients with hepatocellular carcinoma (HCC) on the waiting list. These patients tend to have lower functional MELD scores, one of the factors that was initially thought to improve outcomes when using DCD donors [28–30], making DCD a good option for reducing their time on the waiting list before these candidates exceed the Milan criteria.
While the use of these DCD is extended in the clinical practice, there are still some unknown aspects and the number of high levels and control studies comparing different technics is small.
We aimed to examine the incidence of complications in DCD graft recipients and compare the results with patients receiving DBD grafts during the same period in a large-volume liver transplant centre where the DCD procurement has also evolved over the last years, with the introduction of NRP as the standard of care.
2Materials and methods2.1Study designWe performed a retrospective cohort study, including adult patients (aged ≥ 18 years) undergoing LT with DCD donors from January 1st, 2015 to December 31st, 2018 in La Fe University Hospital, with a minimum follow-up of 6 months after LT. These patients were compared with LT recipients with DBD donors selected by propensity score matching during the same period.
Exclusion criteria were: retransplantation, split livers, multiorgan transplantation and MELD score ≥ 30.
Data were acquired from the prospectively maintained liver transplant database of our hospital, and from patients’ medical records.
2.2Ethical statementExemption from informed consent requirements was approved owing to the retrospective nature of the study. Many participants had likely passed or moved by their places of residence.
The study protocol conformed to the ethical guidelines in the 1975 Declaration of Helsinki. This Is reflected by the approval of the study from the La Fe Health Department (2019-016-1).
2.3OutcomesThe primary endpoint was patient and graft survival (death-uncensored) from LT date until the end of follow-up, death, or retransplantation. Two groups were compared: DCD and DBD graft recipients.
Secondary endpoints were incidence of PNF and early graft non-function; biliary complications (leak, IC, strictures) diagnosed by either magnetic resonance cholangiography or by T-tube cholangiography; vascular complications (portal vein thrombosis, hepatic artery stenosis or thrombosis); renal failure at 1st month, cellular rejection (confirmed by liver biopsy, using the Banff classification); postoperative bleeding; reinterventions; length of hospital stay and number of readmissions; and liver function test values during the first six months after transplantation.
2.4DefinitionsPNF was defined as an immediate graft failure, with poor initial function requiring retransplantation or leading to death within seven days after LT, without any identifiable cause of graft failure [31]. Early graft non-function was defined according to Olthoff definition [32].
IC was defined as the set of disorders characterized by multiple diffuse strictures affecting the graft biliary system in the absence of concomitant hepatic artery thrombosis or stenosis, secondary to an impaired blood supply [33,34], evaluated by T-Tube cholangiography on day 4 and month 2 after LT (done by protocol in every LT recipient carrying a T-Tube); or by magnetic resonance cholangiography done on a clinical basis when cholestatic enzymes were altered.
Vascular complications (portal vein thrombosis and hepatic artery stenosis or thrombosis) were evaluated by Doppler ultrasound at 24 hours, day 7 and month 2 after LT or when needed on a clinical basis.
Postoperative bleeding was defined as a drop in haemoglobin level > 3 g/dL and/or any post-operative transfusion of red blood cells (RBC) for falling haemoglobin and/or the need for invasive re-intervention to stop bleeding [35].
Renal failure was defined as a glomerular filtration (eGFR) under 60mL/min/1,73m2.
2.5Collected variables- -
Pre-transplant recipient variables: sex and age, indication for LT, body mass index (BMI, kg/m2), MELD (only for patients with chronic liver disease), presence of renal failure (eGFR < 60 mL/min), cardiovascular risk factors (arterial hypertension, diabetes mellitus, hypercholesterolemia or hypertriglyceridemia; only if requiring pharmacological therapy), presence of previous portal vein thrombosis.
- -
Related to donor and surgery: donor age and sex; DCD cause of death; type of DCD (controlled or uncontrolled, according to Maastricht classification); extraction technique (NRP or SRR); cold ischemia time (minutes); warm ischemia time (minutes); NRP time (minutes); type of biliary anastomosis.
- -
Liver function test values: alanine aminotransferase or ALT (UI/L), aspartate aminotransferase or AST (UI/L), gamma glutamyl transferase or GGT (UI/L), alkaline phosphatase or AP (UI/L), lactate dehydrogenase or LDH (UI/L), total bilirubin (mg/dL), albumin (g/dL), and prothrombin activity (%) on days 1, 7 and 14 post-LT, and at months 1, 3 and 6 after LT.
Withdrawal of the therapeutic support was made in the operating room. Recovery was made either with NRP with pre-mortem canulation or with the SRR technique. Donor organ procurement was always made by the same surgical team.
During NRP, serum transaminases, pH, LDH, lactate levels and CPK were monitored to assess organ function at 20, 40, 60 and 80 minutes. Lactate levels were recommended to be stable or decreasing during NRP, with no absolute cut-off value. Levels under three times the normal value at any time and under four times at the end of the procedure were recommended. According to protocol, livers were discarded when transaminases levels exceeded greater than four times the normal value or deteriorate.
Pump flow during NRP was 1.7–4 L/min at 37°C, temperature 35.5–37.5°C, pH 7.35–7.45 and hematocrit >20%. Heparin, 8.4% bicarbonate (as required) and two units of red cell concentrate (range 0–4) were added to maintain these parameters.
All donors received heparin at a dose of 3mg/kg before pre-mortem canulation. In SRR, heparin was administered before extubation and in NRP heparin was initiated before femoral artery and vein canulation.
Warm ischemia time was defined as the interval from the extubation of the donor to the initiation of organ perfusion. Cold ischemia time was defined as the time from perfusion with preservation solution of organs during the recovery to the beginning of the implant phase in the recipient [26]. NRP time was defined as the period of time between NECMO system was initiated after a 5-minutes period of continuous cardiorespiratory arrest, until the organ perfusion with Celsior solution.
Surgical procedure in the recipient was the same in both DBD and DCD grafts, using the piggy-back technique. Biliary duct anastomosis technique was chosen depending on the anatomy, according to the size of the biliary duct of both donor and recipient, with use of duct-to-duct anastomosis or T-tube biliary anastomosis [36]; or exceptionally use of hepaticojejunostomy when necessary. DCD recipients usually underwent an end-to-end choledocho-choledochostomy with a T-tube, and exceptionally was made with no T-tube insertion according to surgeons’ intraoperative decision.
2.7ImmunosuppressionThe immunosuppression protocol was the same in both DBD and DCD recipients, with Tacrolimus and Mycophenolate Mofetil. Basiliximab was used in those with prior renal failure or acute renal failure in the immediate postoperative days together with delayed and low dose-tacrolimus. Prednisone was typically used in those with an immune mediated LT indication.
2.8Criteria for DCD donor selectionThe inclusion and exclusion criteria for DCD donor selection depend on whether the recovery of the liver graft is controlled or uncontrolled, according to the modified Maastricht classification (Table 1) [37]. Only Maastricht II and Maastricht III DCDs are eligible for liver transplants in our centre.
- -
Inclusion criteria in uncontrolled DCD (Maastricht II): patient with any type of cardiorespiratory arrest; graft with good consistency, homogeneous appearance after cold perfusion, no congestion signs, absent or mild steatosis.
- -
Exclusion criteria in uncontrolled DCD (Maastricht II): age ≥ 55, time from cardiac arrest until the start of advanced life support measures over 15 minutes, time from cardiac arrest until arrival at the hospital over 120 minutes; warm ischemia time over 150 minutes; total time from the cardiac arrest to liver extraction over 4 hours; cold ischemia time over 8 hours; physical signs of injecting drug use; existing bleeding lesions in thorax and abdomen; existing systemic infections or neoplastic diseases; multi-organ failure; bowel ischemia (as it can suggest there is bad perfusion in other organs); graft with heterogeneous consistency or moderate to severe steatosis.
- -
Inclusion criteria in controlled DCD (Maastricht III): patients admitted to hospital who suffer cardiac arrest after the intentional withdrawal of life-sustaining treatment; graft with good consistency, homogeneous appearance after cold perfusion, no congestion signs, absent or mild steatosis.
- -
Exclusion criteria in controlled DCD (Maastricht III): age ≥ 65; Intensive Care Unit (ICU) length of stay over 7 days; time from initiation of life-sustaining therapy withdrawal to the established cardiac arrest over 60 minutes; functional warm ischemia time over 30 minutes; physical signs of injecting drug use; existing bleeding lesions in thorax and abdomen; existing systemic infections or neoplastic diseases; multi-organ failure; bowel ischemia; graft with heterogeneous consistency or moderate to severe steatosis.
Modified Maastricht Classification of DCD.
| Definitions | |
|---|---|
| Maastricht I | Uncontrolled DCD, found dead. |
| Maastricht II | Uncontrolled DCD, witnessed cardiac arrest with unsuccessful resuscitation. IIA: out-of-hospital.IIB: in-hospital. |
| Maastricht III | Controlled DCD, withdrawal of life-sustaining therapy |
| Maastricht IV | Uncontrolled controlled, cardiac arrest while life-brain dead. |
DCD, Donation after Circulatory Death.
There were no absolute criteria for the selection of DCD recipient; yet patients with primary sclerosing cholangitis or patients undergoing re-LT were typically not considered for this technique.
2.10Statistical analysesControl cases were selected with propensity score matching method. Patients were matched 1:1 without replacement. Matching variables were indication for LT, recipient sex, recipient age, donor age and MELD score.
Propensity score matching was performed using R version 3.6 for Windows.
A descriptive analysis was performed for all the studied variables, summarising continuous variables in mean or median and standard deviation (SD) or quartiles 1 (Q1) and 3 (Q3), and qualitative variables in absolute and relative frequencies.
Normal distribution of outcome variables was confirmed with Kolmogorov-Smirnov test. Student's t and Mann-Whitney U tests were used for quantitative variables, and Chi-square and Fisher exact test for qualitative variables.
Graft and patient survival analysis were performed with Kaplan-Meier survival curves. A p value of < 0.05 was considered significant for all analysis.
Data analysis was performed using SPSS version 20.0 for Mac.
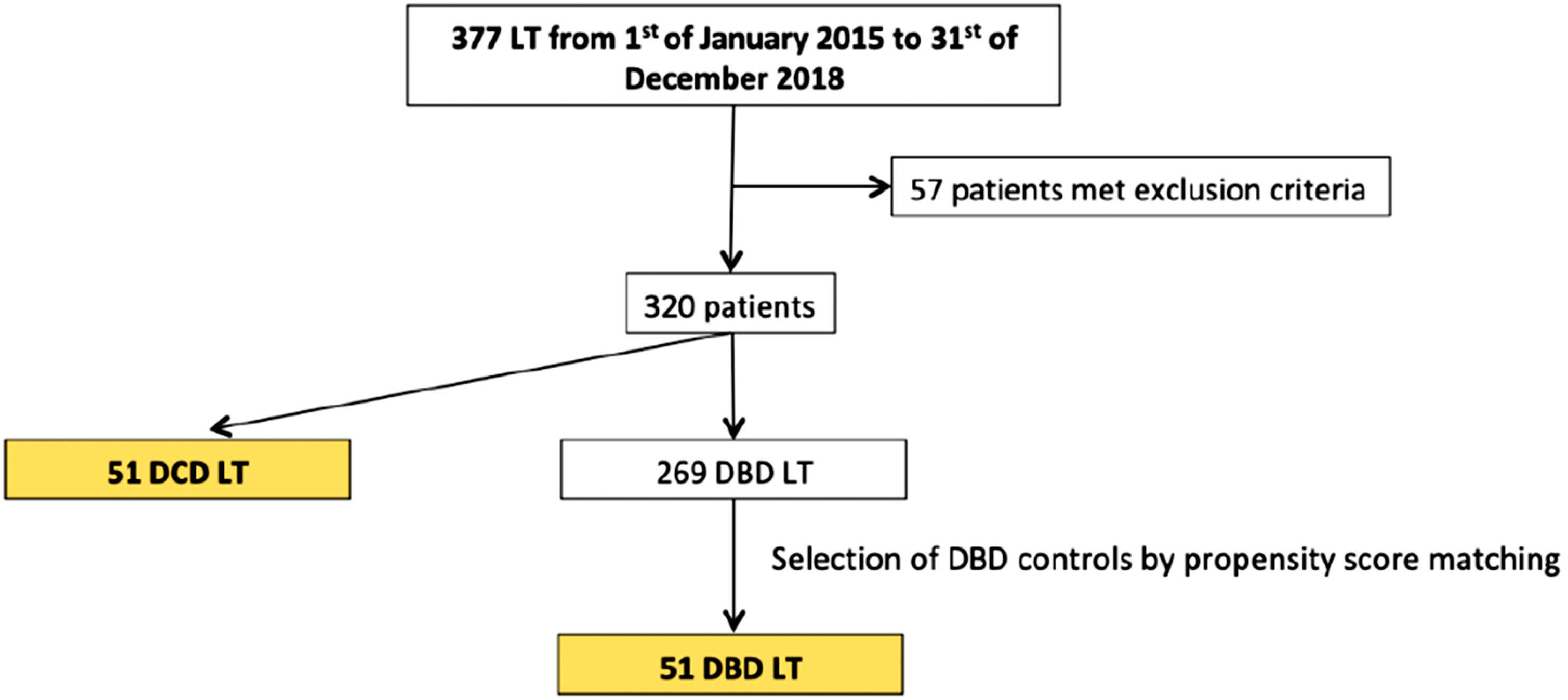
3ResultsA total of 377 liver transplants were performed during the study period. Fifty-seven recipients were excluded from the study due to: MELD score > 30 (n = 7), paediatric LT (n = 13), multiple-organ transplantation (n = 13), retransplantation (n = 22) and split LT (n = 2), with 269 patients that fulfilled the inclusion criteria. Of those, 218 received a DBD donor graft and 51 patients received a DCD donor graft.
During the study period a total of 86 DCD donors were offered but only 51 were considered for LT; 35 were discarded mainly due to suboptimal macroscopic aspect, atherosclerosis, ischemia signs or long cardiac arrest. Propensity score matching was performed to match recipients of DCD grafts with a control group of 51 patients receiving DBD grafts (see Fig. 1).
All patients had at least 6 months of follow-up, with a mean follow-up of 27.4±13.6 months in DBD group and 23.1±12.6 in DCD group.
3.1Baseline featuresBaseline features are shown in Table 2. Most donors were men; donor mean ages were 54.4±15.5 and 53.5±14.5 years in DBD and DCD respectively. Causes of DCD death were: cerebrovascular accident in 25 (49%), anoxia in 13 (25%), trauma in 6 (12%) and respiratory failure in 7 (14%).
Baseline characteristics.
| DBD (n=51) | DCD (n=51) | |
|---|---|---|
| RECIPIENT CHARACTERISTICS | ||
| Recipient age (years) | 59.2 ± 5.5 | 59.3 ± 6 |
| Recipient sexMaleFemale | 39 (76.5%)12 (23.5%) | 38 (74.5%)13 (25.5%) |
| Presence of cardiovascular risk factors | 27 (52.9%) | 22 (43.1%) |
| BMI (kg/m2) | 28.7 ± 4.9 | 27.9 ± 4.8 |
| Presence of renal failure before LT | 11 (21.6%) | 11 (21.6%) |
| Portal vein thrombosis at the time of surgery | 6 (11.8%) | 13 (25.5%) |
| MELD | 11 (8-17) | 13 (9-18) |
| Indication for LTViral cirrhosisAlcoholic cirrhosisHCCOthers | 4 (7.8%)11 (21.6%)35 (68.6%)1 (2%) | 8 (15.7%)11 (21.6%)31 (60.8%)1 (2%) |
| DONOR CHARACTERISTICS | ||
| Donor age (years) | 54.2 ± 15.5 | 53.5 ± 14.5 |
| Donor sexMaleFemale | 32 (62.7%)19 (37.3%) | 31 (60.8%)20 (39.2%) |
| SURGICAL FEATURES | ||
| Controlled/uncontrolled DCDMaastricht IIIMaastricht IIa | 49 (96.1%)2 (3.9%) | |
| Recovery techniqueSRRNRP | 29 (56.9%)22 (43.1%) | |
| Cold ischemia time (minutes) | 257 ± 100.2 | 304.6 ± 80 |
| Warm ischemia time (minutes) | 45.5 ± 11.5 | 49.5 ± 18 |
| NRP (minutes) | 106 (54-280) | |
| Type of biliary anastomosisDuct-to-duct anastomosis without T-tubeT-tube biliary anastomosisHepaticojejunostomy | 23 (45.1%)27 (52.9%)1 (2%) | 8 (15.7%)42 (82.4%)1 (2%) |
| FOLLOW-UP TIME (MONTHS) | 27.4 ± 13.6 | 23.2 ± 12.6 |
DBD, Donation after Brain Death; DCD, Donation after Cardiac Death; BMI, Body Mass Index; LT, Liver Transplantation; MELD, Model of End-stage Liver Disease; HCC, Hepatocellular carcinoma; SRR, Super Rapid Recovery; NRP, Normothermic Regional Perfusion.
Values expressed as mean ± standard deviation, median (interquartile range) or number (percentage) as appropriate.
Most recipients were men. Recipient's mean ages were 59.2±5.5 and 59.3±6 in DBD and DCD groups, respectively. HCC was the leading indication for LT (68.6% in DBD and 60.8% in DCD), followed by alcoholic cirrhosis (21.6% in both groups).
About half (52.9%) of the patients in the DBD group had at least one cardiovascular risk factor; 21.6% had renal failure before LT and 25.5% had portal vein thrombosis at time of surgery. The median MELD score at transplantation was 11 (8-17) in the DBD group and 13 (9-18) in the DCD group.
Regarding surgery, recovery was made with SRR in 29 patients (56.9%) and with NRP with pre-mortem canulation in 22 (43.1%) in the DCD group. The most common type of biliary anastomosis was duct-to-duct anastomosis without T-Tube in the DBD group, 45.1%, and T-Tube biliary anastomosis in the DCD group, 82.4% (Table 2).
3.2Biochemical dataPeak AST and ALT at day 1 after transplant was higher in the DCD group vs the DBD group (1369 UI/L [749-2311] vs 694 UI/L [349-1026]) (p≤0.001), as well as bilirubin at day 7 (3.6 g/dL [1.9-6.2] vs 7.1 g/dL [2.6-9.4]) (p=0.024). There were no other significant differences in blood parameters between both groups during follow-up (see Table 3, post-transplant complications and outcomes).
Post-transplant complications and outcomes.
| DBD (n=51) | DCD (n=51) | p value | |
|---|---|---|---|
| PNF | 0 (0%) | 2 (3.9%) | 0.49 |
| Early graft non-function | 9 (17.6%) | 11 (21.6%) | 0.62 |
| Biliary complicationsBile leakIschemic cholangiopathyBiliary stenosis | 1 (1.95%)001 (1.95%) | 5 (9.8%)3 (5.9%)2 (3.9%)0 | 0.2 |
| Vascular complicationsHepatic artery stenosisHepatic artery thrombosisHepatic artery pseudoaneurysmPortal vein thrombosis | 2 (3.9%)01 (1.95%)01 (1.95%) | 2 (3.9%)1 (1.95%)01 (1.95%)0 | 1.3 |
| Acute cellular rejection | 3 (5.9%) | 2 (3.9%) | 1 |
| Postoperative bleeding | 1 (1.95%) | 7 (13.7%) | 0.06 |
| Reinterventions | 2 (3.9%) | 8 (15.7%) | 0.09 |
| Transfusion during surgeryRBCPlateletsFFP | 1 (0-2.5)1 (0-2)1,5 (0-2) | 2 (0-4)2 (0-2)2 (0-3) | 0.160.030.22 |
| AST day 1 (UI/L) | 694 (349-1026) | 1369 (749-2311) | 0.000 |
| ALT day 1 (UI/L) | 529 (279-914) | 885 (549-1550) | 0.001 |
| Bilirubin day 7 (mg/dL) | 3.6 (1.9-6.2) | 7.1 (2.6-9.4) | 0.024 |
| eGFR (ml/min) 1st month | 83 ± 19.6 | 80 ± 23.9 | 0.5 |
| Renal failure post-LT | 5 (9.8%) | 8 (15.7%) | 0.38 |
| Hospital stay | 15 | 14 | 0.53 |
| Readmissions | 0 (0-1) | 0 (0-1) | 0.85 |
| Retransplantation | 1 (1.95%) | 2 (3.9%) | 0.55 |
DBD, Donation after Brain Death; DCD, Donation after Cardiac Death; PNF, Primary Non-Function; RBC, Red Blood Cells; FFP, Fresh Frozen Plasma; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; eGFR, estimated Glomerular Filtration Rate; LT, Liver Transplantation.
Values expressed as mean ± standard deviation, median (interquartile range) or number (percentage) as appropriate.
*Because of its extension, only values with significant differences are included in the table. There was no significant difference between other parameters (GGT, AP, LDH, albumin and prothrombin activity on days 1, 7 and 14 post-LT, and 1st, 3rd and 6th month after LT; AST and ALT on days 7 and 14 post-LT, and 1st, 3rd and 6th month after LT; and bilirubin on days 1 and 14 post-LT, and 1st, 3rd and 6th month after LT).
Clinical outcomes are shown in Table 3. PNF occurred in 2 (3.9%) DCD recipients (one of them died 24 hours after LT; the other one was retransplanted 48 hours later), whereas in the DBD group none of the patients suffered this complication (p=0.49). 9 and 11 patients in DBD and DCD respectively suffered early allograft disfunction defined by Olthoff (p=0.62).
Six patients developed biliary complications: 5 (9.8%) in the DCD and one (1.95%) in the DBD group (p=0.2). The five DCD organs had been retrieved with the SRR technique. Complications were 3 bile leaks, all of them in the anastomosis site, 2 IC and 1 biliary stenosis. One patient with IC died 53 days after LT and the other required retransplantation 114 days after LT.
Postoperative bleeding and reinterventions were also higher in the DCD group: 7 (13.7%) vs 1 (1.95%) and 8 (15.7%) vs 2 (3.9%), respectively, yet without reaching statistical significance.
The number of patients with vascular complications did not differ between groups (3.9% in both): 1 hepatic artery stenosis and 1 hepatic artery pseudoaneurysm in the DCD group, versus 1 portal vein thrombosis and 1 hepatic artery thrombosis in the DBD group.
Mean eGFR at the month 1 after LT was 83.04 mL/min/1.73m2 in the DBD group vs 80.04 mL/ min/1.73m2 in the DCD group (p=0.4). The number of patients with renal failure at month 1 was similar between groups (5 and 8 in DBD and DCD, respectively; p=NS).
No differences were found in the development of acute cellular rejection between groups (5.9% in DCD vs 3.9% in DBD; p=NS).
The length of hospital stay (in the immediate post-transplant period), as well as the number of readmissions during the first 6 months after LT were also similar in both groups (Table 3).
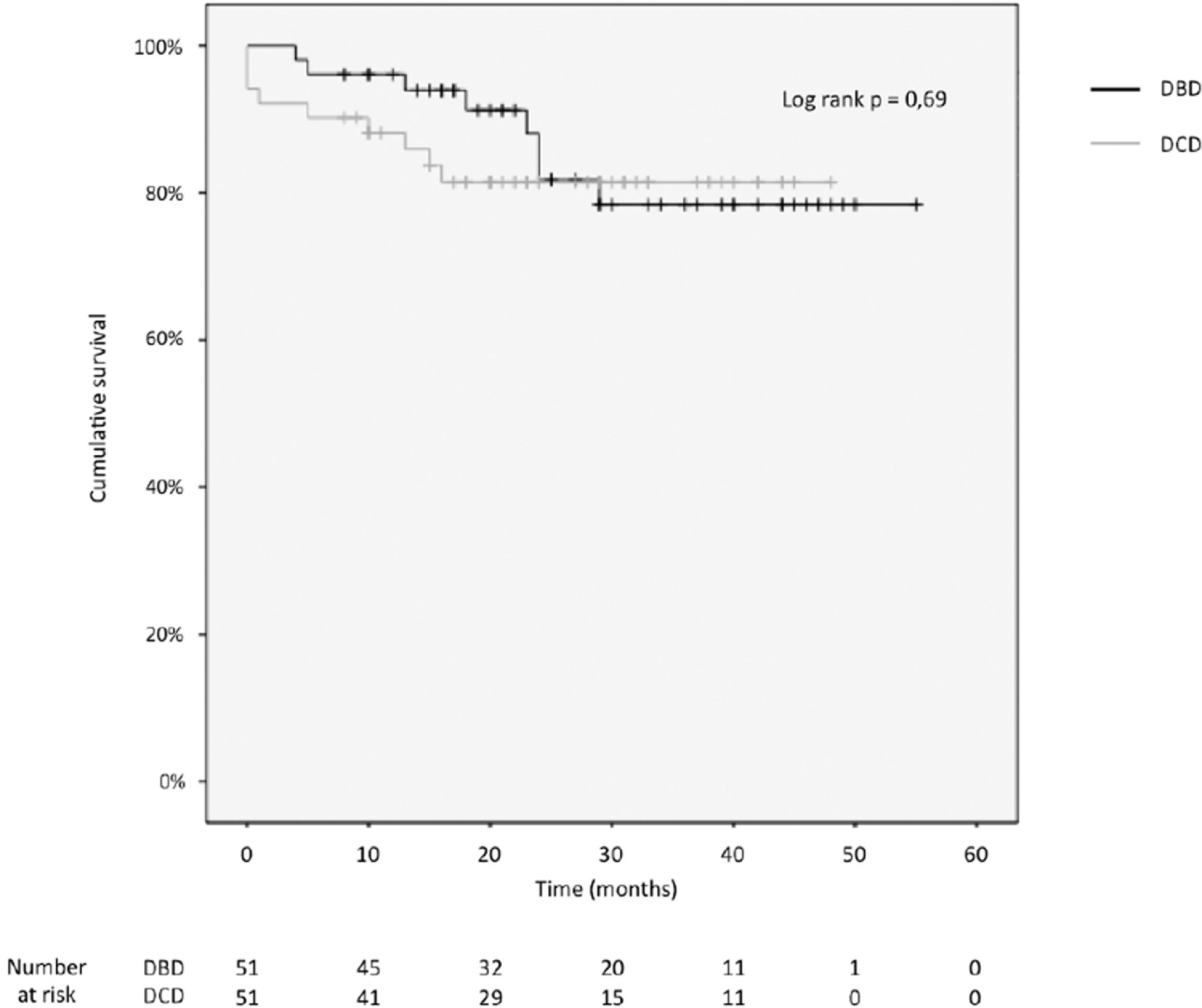
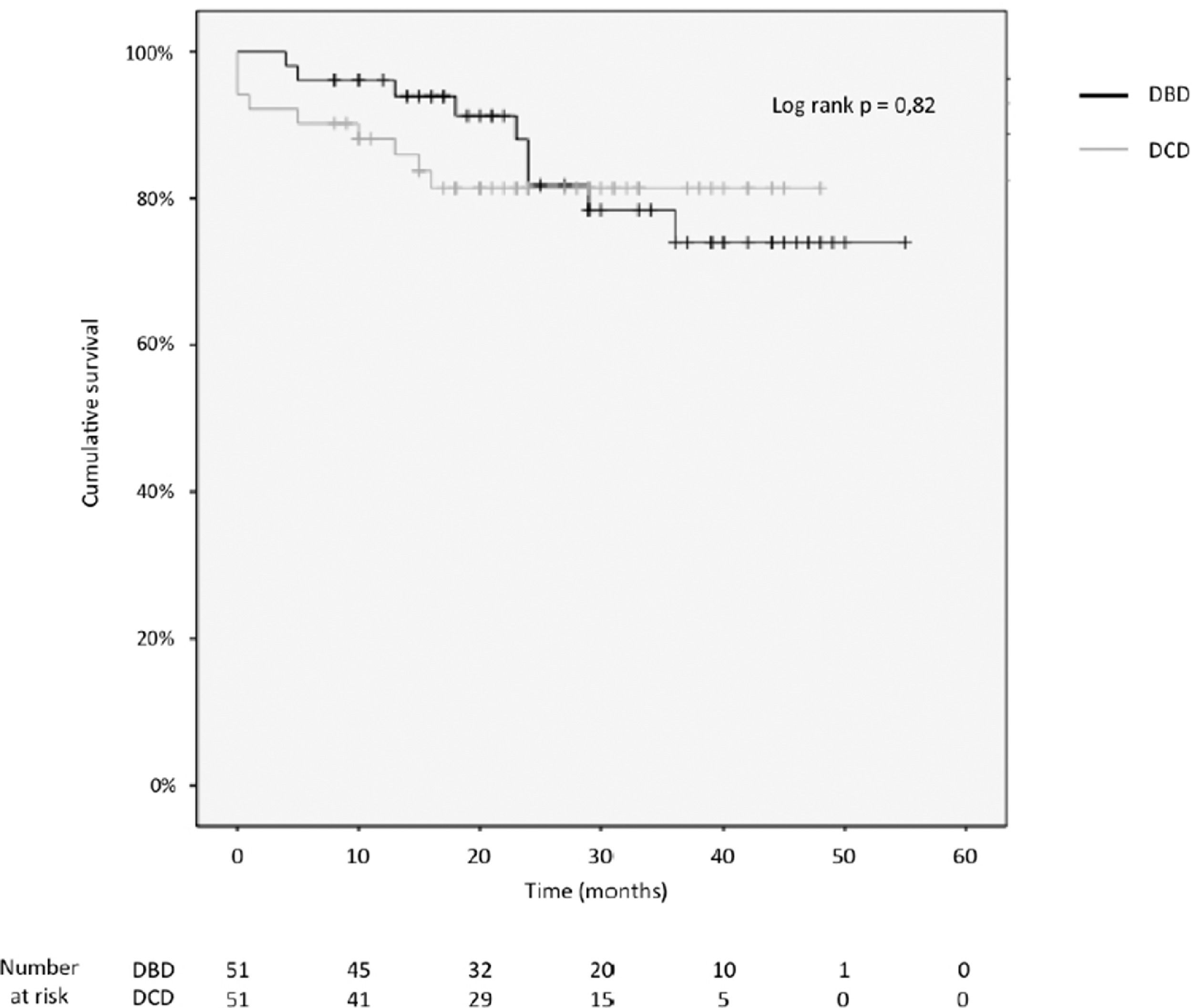
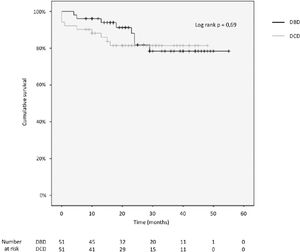
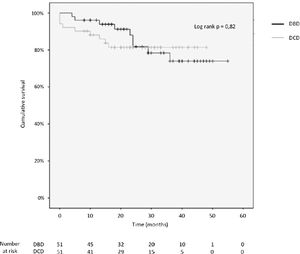
3.4Patient and graft survivalOverall graft and patient survival showed no significant differences in the Kaplan-Meier analysis, p=0.8 and p=0.67, respectively (Figs. 2 and 3), with a mean follow-up of 27.4±13.6 months in the DBD group and 23.1±12.6 in DCD group.
The cumulative 1-, 2-, 3- and 4-year patient survival rates were 80%, 80%, 80% and 80% in the DCD group, and 88%, 78%, 78% and 78% in the DBD group (log rank test = 0.69; Fig. 2).
Actuarial 1-, 2-, 3- and 4-year graft survival rates were 80%, 80%, 80% and 80% in the DCD group, and 88%, 78%, 71% and 71% in the DBD group (log rank test = 0.82; Fig. 3).
Seventeen patients died during the follow-up (8 in DBD and 9 in DCD). The main causes of death in DCD were septic/haemorrhagic shock in 5 patients (55%), 4 of them in the early post-transplant period and de novo cancer (4 patients, 50%), and septic shock (2 patients, 25%) in DBD. Early death within the first 6 months occurred in 7 patients (5 in DCD and 2 in the DBD group).
The rate of retransplantation was also similar in both groups: 2 re-transplantations were made in the DCD group, 1 due to PNF, performed 48 hours after LT, and 1 due to IC, one year later; in turn, in the DBD group only one retransplantation was needed, due to acute hepatic artery thrombosis on the fourth postoperative day.
4DiscussionDespite the lack of well-done controlled studies comparing the results of DCD vs. DBD donors, the use of DCD donors has substantially increased in Spain in recent years, particularly since the introduction of NRP, expanding the donor pool to 37.4 donors per million in the year 2020 [3].
Although there is still controversy in several studies regarding the potential increase of complications with the use of this type of grafts, most recent series have demonstrated similar incidence of complications between DCD and DBD [1,2,9,10,17,19,38,39]. Reasons that explain improved results are likely related to the learning curve of the surgical technique, the implementation of NRP in most centres and a better selection of DCD donors and recipients.
Of note, despite the fact that SRR was still used in our study at the beginning of the program (currently NRP is the only extraction technique in our centre due to the evidence of higher complications with SRR, which had led to avoiding it), we did not find any statistically significant difference between the incidence of complications in DCD compared to the DBD group. Yet, some clinical differences merit to be highlighted. We observed a slightly higher incidence of biliary complications in DCD recipients and a higher transaminase peak in the DCD group. However, no clinical differences in graft rejection, PNF, renal failure, patient and graft survival, and re-transplantation were found.
Biliary complications developed in 6 patients during the follow-up (5 in DCD vs. 1 in DBD). Of note, 5 out of 6 occurred in patients transplanted with DCD and SRR technique; however, 3 of them were related to leak at the biliary anastomosis, a complication not always related to the use of DCD donors. As it is known, IC is an established complication related to DCD, widely attributed to the warm ischemia time, specific to these grafts [1,40]. Its incidence varies from 8% to 38% in different series and it is related to the type of DCD donor and technique used [2,17–26]. More specifically, this complication ranges between 15-31% vs. 8-16% with uncontrolled vs. controlled DCD, respectively, and between 0-8% vs. 27-31% depending on the use of SRR or NRP recovery technique [2,20]. In our series, the type of biliary complication (biliary anastomosis leak) could not be only attributed to DCD completely due to the site of the presentation.
A higher incidence of postoperative bleeding and reinterventions also occurred in the DCD group, without reaching statistical significance. As for IC, these complications occurred preferentially when using the SRR technique.
While the peak of AST, ALT, and bilirubin were higher in the first week in the DCD group, no differences were observed in further follow-up comparisons, with results similar to other series [1,4,9,10,15].
An increased frequency of renal failure has been described with DCD grafts, potentially related to liver ischemia-reperfusion injury [14]. While only a few series have analysed long-term renal function in this setting [14,41], most did not find a significant increase in the incidence of chronic kidney disease in DCD recipients. Our study showed no differences in the incidence of renal failure at one-month post-LT, also when only including those without renal impairment before LT. The strict policy of our hospital to prevent renal impairment, with the regular use of basiliximab in patients with preLT renal failure, together with the delay in introducing tacrolimus at low doses, may explain the lack of differences.
The favourable outcomes seen in our patients may be partially related to the low MELD scores in both groups. The included MELD score for this study was functional MELD, with no added exception points. Of note, HCC was the leading indication in both groups and in addition, exclusion criteria for the study were patients with MELD>30. Moreover, a short waiting list at our centre (below 6 months) could also explain these results. Yet, we tried to avoid a selection bias by using a propensity score matching approach.
Limitations to this study are related to its observational nature, the retrospective data collection, the non-random distribution of DCD grafts and the relatively small sample size; yet it included homogeneous matched cohorts and a long follow-up period.
It is also worth mentioning that the use of NRP and machine perfusion devices has allowed decreasing both warm ischemia time and ischemia-reperfusion damage [2,17], translating into a lower incidence of IC [1,2,17,19,39]. Additionally, Spanish law allows premortem cannulation [42], which further reduces warm ischemia time. During the last four years, all DCD LT in our centre has been performed using normothermic extracorporeal membrane oxygenation (NECMO) with premortem cannulation, reaching similar results to those obtained with DBD LT; evidence that has been supported by recent publications [1,2,17,19,39]. However, as this study includes all DCD LT performed from January 2015 to December 2018, it still comprises a high percentage of DCD performed with SRR, a currently extinct technique in our group. The use of donors with SRR at the beginning of the program was used with very strict selection criteria, which could explain the low percentage of ischemic cholangiopathy and other complications (see Supplementary Table). Due to the availability of the NECMO machine not only at our hospital, but also with a portable NECMO and an on call team, we soon joined the new technique with promising better results. Nowadays, criteria such as donor age and ICU stay are not so strict due to the better outcomes obtained with NRP recovery.
Furthermore, the standard use of T-tube in DCD LT has also been abandoned by our group; currently, it is only used depending on the biliary duct diameter, so better results are possible with these recent changes, and further studies are needed.
5ConclusionDCD donors are an adequate option to increase the donor pool in LT, achieving similar long-term graft and patient survival and ischemic cholangiopathy rates to those achieved with DBD donors, especially when NRP technique with machine perfusion device is used the currently adopted approach at our LT program.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributionsMFV: bibliography research, design of the study, data collection, data analysis and interpretation, manuscript writing, critical revision of the article and submission of the paper. PPV: design of the study, data collection, critical revision of the article, final approval of the version to be published. SBS: design of the study, data collection, critical revision of the article, final approval of the version to be published. RLA: design of the study, critical revision of the article, final approval of the version to be published. MB: design of the study, critical revision of the article, final approval of the version to be published. MP: design of the study, critical revision of the article, final approval of the version to be published. EM: design of the study, manuscript writing, critical revision of the article, final approval of the version to be published. VA: design of the study, data analysis and interpretation, manuscript writing, critical revision of the article, final approval of the version to be published.
The authors thank nurses and doctors in the Hepatology and HBP Surgery and Transplants Units for the management of patients included in this study, and the anesthetists from the postoperative Intensive Care Unit for the intensive care management of all transplant recipients.