Liver cirrhosis accounts for over 2 million deaths annually worldwide. A subset of these patients – those with alcoholic hepatitis and decompensated cirrhosis, have abysmal short-term survival. Liver transplant is the only intervention of proven survival benefit; however organ availability is a major limitation. It is thus imperative to assess potential benefit of experimental therapies as a bridge to transplant.
Stem cell therapies have shown some promise in patients with end-stage liver disease. Of these, bone-marrow derived hematopoietic stem cells have generated the most interest. Animal as well as human data suggest biological plausibility of stem cell translocation from bone marrow to liver, giving credence to cytokine therapies based on bone marrow stimulation. Granulocyte colony stimulating factor has been the most frequently used cytokine for this purpose. This intervention has shown encouraging results in terms of safety as well as survival benefits in small clinical trials. The evidence, however, is sparse and heterogeneous.
In this review we describe the biological plausibility, mechanisms of action, and clinical evidence of the use of cytokine based stem cell therapy in patients with end-stage liver disease.
bone marrow derived stem cells
>Child Pugh score
erythropoietin
granulocyte colony stimulating factor
Maddrey's discriminant function
Mayo model for End-stage Liver Disease
standard medical therapy
transplant free survival
Liver disease is one of the most common causes of morbidity and mortality across the world, being responsible for almost 2 million deaths annually. Of these, over half are caused by complications of advanced cirrhosis [1]. With a considerable male preponderance and median age at diagnosis in the early fifties, it predominantly affects men in the working age group [2–4]. Lately there has been a disproportionate increase in cirrhosis-related deaths among people in their 20s and 30s, driven mainly by alcohol [2]. In addition to high mortality, cirrhosis is also responsible for considerable a high degree of morbidity, poor quality of life, healthcare expenditure, and indirect economic burden from disability related unemployment [5].
Patients with decompensated cirrhosis have a median survival of two years [6] – a duration shorter than that for many advanced metastatic malignancies. Liver transplantation is the only definitive treatment option for these patients. However, even in the most advanced healthcare setups, there is a tragic disparity between the demand and availability of transplantation. In the United States, approximately 7500 liver transplants were performed in 2018, which represents a mere 5% of all patients with decompensated cirrhosis [7]. This disparity is far greater in developing and underdeveloped economies mainly due to financial constraints, limited access to transplant centres, lack of information, and low rates of organ donation.
Severe alcoholic steatohepatitis constitutes a sinister subset of patients decompensated cirrhosis, with decompensated cirrhosis, which constitutes even higher morbidity and mortality. Conventional treatment options like corticosteroids, pentoxifylline and N-acetyl-cystine, have failed to demonstrate a convincing impact on survival [8]. Corticosteroids had been the mainstay of therapy for long, however, a large multicentre randomized clinical trial clearly showed no improvement in 90-day and one-year survival with either prednisolone or pentoxifylline treatment [9]. Liver transplantation remains the only intervention with proven mortality benefit in this population [10,11]. However, as these patients have recent alcohol consumption, they are disqualified by most transplant programmes until the stipulated abstinence period (usually 6 months, although the required duration of abstinence is currently in evolution at many centres). Up to 2/3rd of patients may not survive this period of abstinence. There is thus a substantial unmet need for newer therapies to extend survival so as to provide a bridge to transplant.
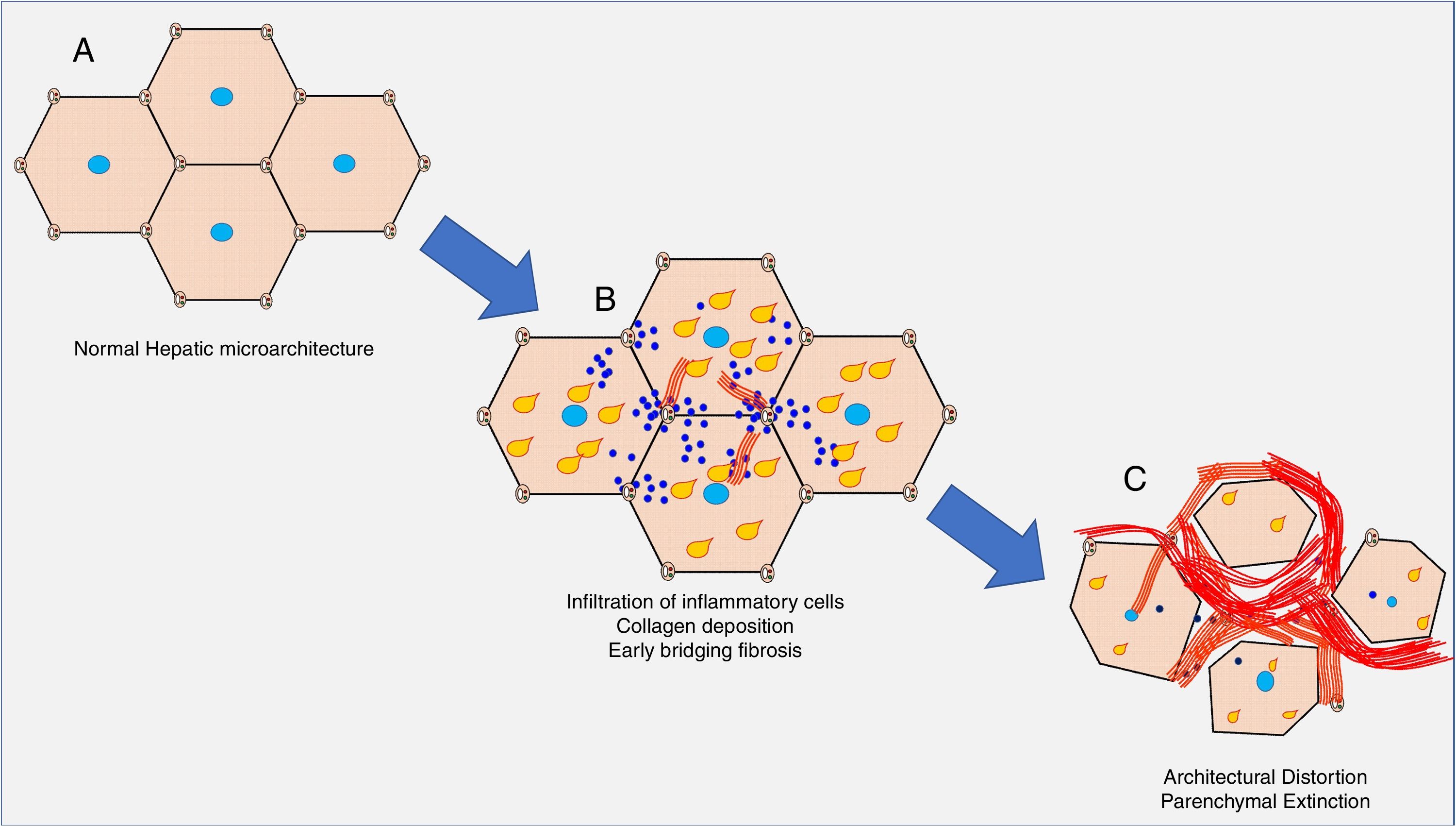
The clinical consequences of decompensated cirrhosis are essentially a combination of hepatic parenchymal extinction, portal hypertension, immune dysregulation, and a proinflammatory milieu conducive to oncogenesis. The chronic inflammation of the hepatic parenchyma leads to perisinusoidal collagen deposition, which progresses to portoportal and portocentral bridging fibrosis, and in turn, formation of cirrhotic nodules. In this process, the porto-central sinusoidal flow is compromised leading to increased sinusoidal resistance, causing portal hypertension. In addition, this impaired sinusoidal blood flow to hepatic parenchyma leads to cellular undernutrition and hypoxia, causing extinction of hepatic parenchyma, and further exacerbating inflammation and fibrosis (Fig. 1).
Simplified schematic of evolution of cirrhosis. A. Normal hepatic microarchitecture. B. Chronic inflammation and/or steatosis (yellow tear drops) lead to fibrosis (red bands), which progresses to porto-portal and porto-central fibrotic bands. This leads to impaired hepatic microcirculation, increased sinusoidal resistance, and increase in portal pressures. C. The fibrotic bands become more extensive, further distorting architecture, leading to parenchymal destruction and exacerbating portal hypertension.
Hematopoietic stem cell therapies have been under evaluation for patients with liver disease since 2005, when initial trials suggest a role of these cells in liver regeneration [12]. The principle hypothesis is that pluripotent hematopoietic stem cells have the potential to translocate to liver and differentiate into hepatocytic lineage, thereby repopulating the extinguished hepatic parenchyma. In addition, these cells are thought to aid in regression of fibrosis. Various small studies involving infusion of marrow-derived stem cells into the portal vein, peripheral vein, or hepatic artery have since demonstrated safety and potential for improvement in liver function [13–17]. While these procedures were tedious and cumbersome to perform, they provided a proof of concept for technically simpler interventions.
Colony stimulating factors mobilize pluripotent hematopoietic stem cells from the bone marrow in to the peripheral circulation, which then localize into visceral organs like liver, and may dedifferentiate to hepatocytic lineage. A beneficial effect on cirrhosis related immune dysfunction is also postulated. Many studies have now explored the potential of this pathway in patients with cirrhosis, with mixed results. We here review the evidence on efficacy of stem cell induction by colony stimulating factors in patients with severe alcoholic hepatitis and decompensated cirrhosis.
2Bone marrow stem cells in liver: preliminary evidenceBone marrow and liver share certain similarities in embryonic origin. Liver is the dominant site for erythropoiesis in the foetus until the marrow matures and takes over. Even in adulthood, low levels of haematopoiesis may occur in the liver from the hepatic pool of resident progenitor cells. This may become dominant in conditions of stress or loss of marrow stem cell niche [18]. This embryonic and functional congruence between the bone marrow and liver justifies exploring a possibility of bone marrow derived stem cell differentiation into hepatocytic lineage.
Bone marrow has the potential to disseminate various stem cell populations into the systemic circulation in times of stress. Some of these bone-marrow derived stem cells (BMSCs) are pluripotent stem cells with potential for multidirectional differentiation. Both animal models and human studies have reported the existence of BMSC derived hepatocytes. Myeloablated female mice who underwent bone marrow transplantation were reported to have Y-chromosome containing hepatocytes and cholangiocytes within 7 days of procedure [19]. A retrospective assessment of archived human specimens of gender mismatched recipients of bone marrow transplants and liver transplants confirmed these findings, showing opposite gender hepatocytes and cholangiocytes within the liver [20]. It thus became clear that BMSCs had the potential to translocate into the liver and undergo hepato-cholangiolar differentiation. Moreover, the extent of stem cell engraftment into the liver was proportionate to degree of hepatic injury, indicating it may have been a reparative process [19,20]. The differentiation of BMSCs to functional hepatocytes was further supported by mouse model of type-1 tyrosinemia using fumarylacetoacetate hydrolase deficient mice. This study demonstrated bone marrow derived cells of myelomonocytic differentiation convert into functional hepatocytes with fumarylacetoacetate hydrolase production [21].
3Autologus stem cell transfusion: early trialsMouse studies reporting differentiation of BMSCs into functional hepatocytes with capability of albumin production encouraged further trials in this area [22]. The initial preclinical trials in fulminant hepatic failure reported a reduction in mortality when animal models were transfused with stem cells [23,24]. The early human trials transfused bone marrow aspirate derived BMSCs into peripheral vein, portal vein, or hepatic artery, and showed improvement in disease severity [14,15,25]. Patients experienced reductions in serum bilirubin and prothrombin time, and increase in albumin as well as alpha-fetoprotein indicating hepatic regeneration and improved synthetic capacity. Similar results were thereafter replicated by other researchers in controlled trials, both randomized and non-randomized, using stem cells derived from bone marrow as well as umbilical cord [16,17,26–28].
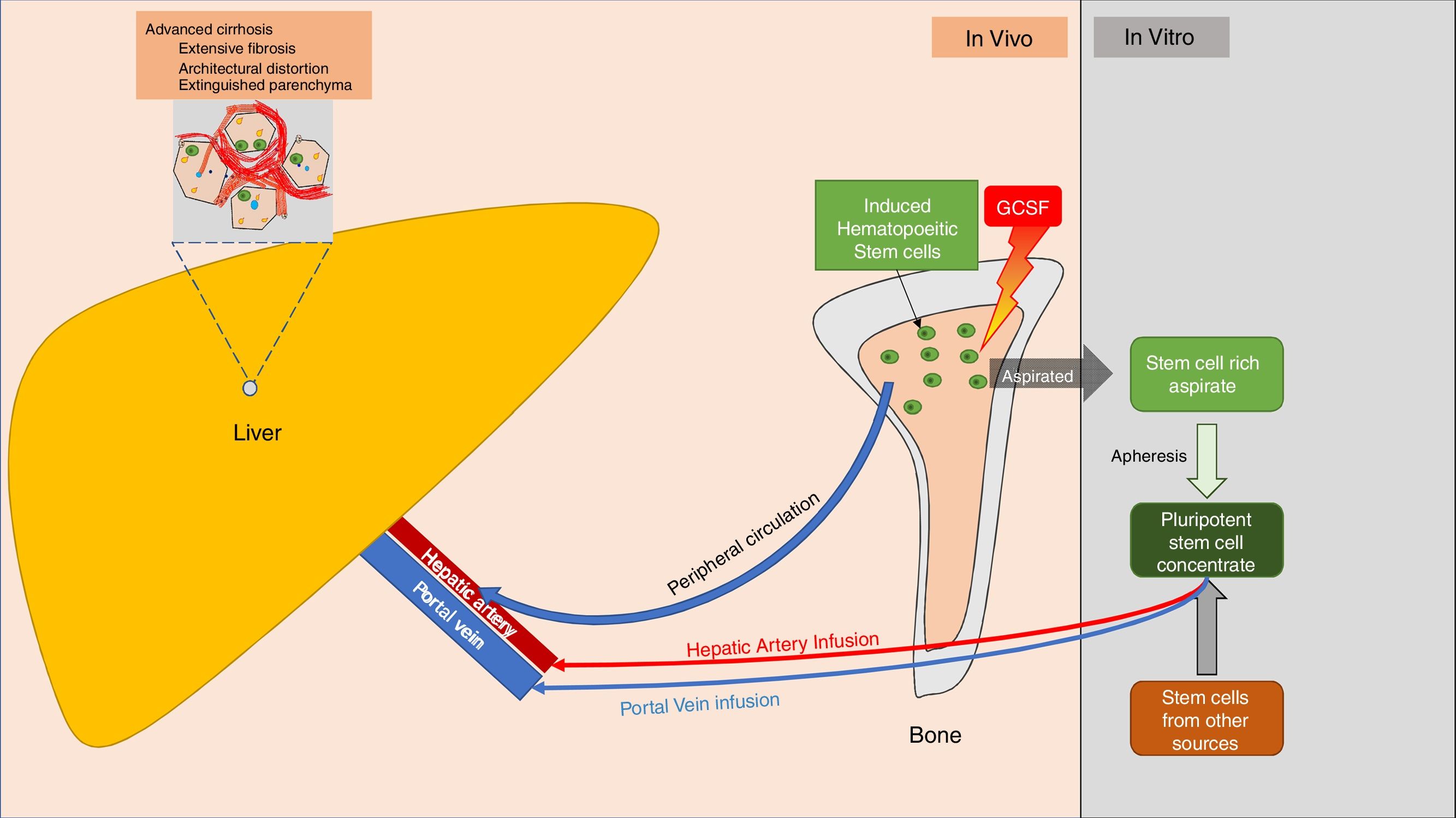
For most of these studies, BMSCs were harvested in an autologous manner from iliac crest bone marrow aspirate, and infused into the liver via peripheral vein, portal vein, or hepatic artery infusion. Nearly all studies showed that the procedures were safe and well tolerated [26]. However, the therapy was not widely accepted. This was mainly due to the need for specialized biotechnology to retrieve, separate, and maintain autologous stem cells, and the invasive procedures required for infusion. Despite these limitations, these studies furnished the proof of concept for safety and potential merits of BMSCs in hepatic regeneration and rejuvenation (Fig. 2).
Schematic of routes for stem cell localization into the hepatic parenchyma. The source of stem cells may be stimulated bone marrow, umbilical cord stem cells, or other potential sources of pluripotent stem cells. Stem cells (depicted as green, round cells) localize to liver parenchyma.
Circulating hematopoietic stem cells have the potential to engraft into liver, skin and gastrointestinal tract, and undergo differentiation into the corresponding local lineage [29]. Granulocyte colony stimulating factor (GCSF) is the most frequently used cytokine for mobilization of hematopoietic stem cells from bone marrow to the systemic circulation, and can increase the circulating hematopoeitic stem cell pool by a factor of 1000 [30]. In patients with haematological malignancies planned for bone marrow transplant, GCSF is thus used during periods of induced remission to mobilize autologous hematopoietic progenitor cells into peripheral circulation for aphaeretic retrieval [31]. However, retrieval and maintenance of viable BMSCs, and infusing them back into the circulation is technically challenging, especially in patients with severe disease. Therefore, attempts were made to stimulate the bone marrow using GCSF, and allow engraftment of the circulating pool of hematopoietic stem cells onto the liver [13,32–34].
GCSF, or filgrastim, is a recombinant 175 amino acid protein produced by Escherichia coli transfected with the human granulocyte stimulating factor gene. It is commonly administered as a subcutaneous injection, and reaches peak concentration in 4h [31]. It is currently licensed by the US-Food and Drug Administration for use in
- •
Neutropenic patients undergoing myelosupressive chemotherapy
- •
Mobilization of hematopoeitic progenitor cells for leucoapharetic collection
- •
Chronic severe neutropenia.
GCSF is usually well tolerated, with common side-effects being mild-moderate bone pains (24%) and headache (7%). However, severe side effects have rarely been reported, which include allergic reactions, splenic rupture, acute respiratory distress syndrome, and precipitation of sickle cell crisis [31]. Profound leucocytosis, which may seem alarming, is actually an expected therapeutic response when used in non-neutropenic setting.
GCSF is known to mobilize CD34+ stem cells to the liver. These cells are thought to participate in hepatic proliferation, either by transdifferentiation into hepatic lineage, or by activation of endogenous repair via paracrine secretions. Moreover, it also has an action on the immunoparetic state of decompensated cirrhosis by way of neutrophil reconstitution improving oxidative burst, increased dendritic cells improving antigen presentation, and reduced CD8 T cell secretion of IFN-γ. Moreover, it is also thought to have an antifibrotic effect over time [35–37].
5Hematopoeitic stem cell stimulation: alcoholic hepatitisAlcoholic hepatitis is a state of advanced hepatic decompensation, further complicated by a combination of hyperinflammation and immunoparesis. Sepsis is common in these patients, and is probably a consequence of intestinal barrier dysfunction and neutrophil exhaustion in the background of hepatic failure. However, recent alcohol intake, coexistent sepsis, and frequent multi-organ dysfunction often preclude to early transplantation, which is the only intervention with an unequivocal survival benefit in this situation. There is thus a very high rate of short term mortality, and most patients with severe alcoholic hepatitis are not able to reach the point of transplantation. An intervention which can effectively improve survival over 3–6 months can provide an effective bridge to facilitate the opportunity of liver transplantation.
The following properties of GCSF form the rationale behind its use
- (a)
stem cell proliferation, mobilization, and localization into the liver, leading to hepatocytic differentiation
- (b)
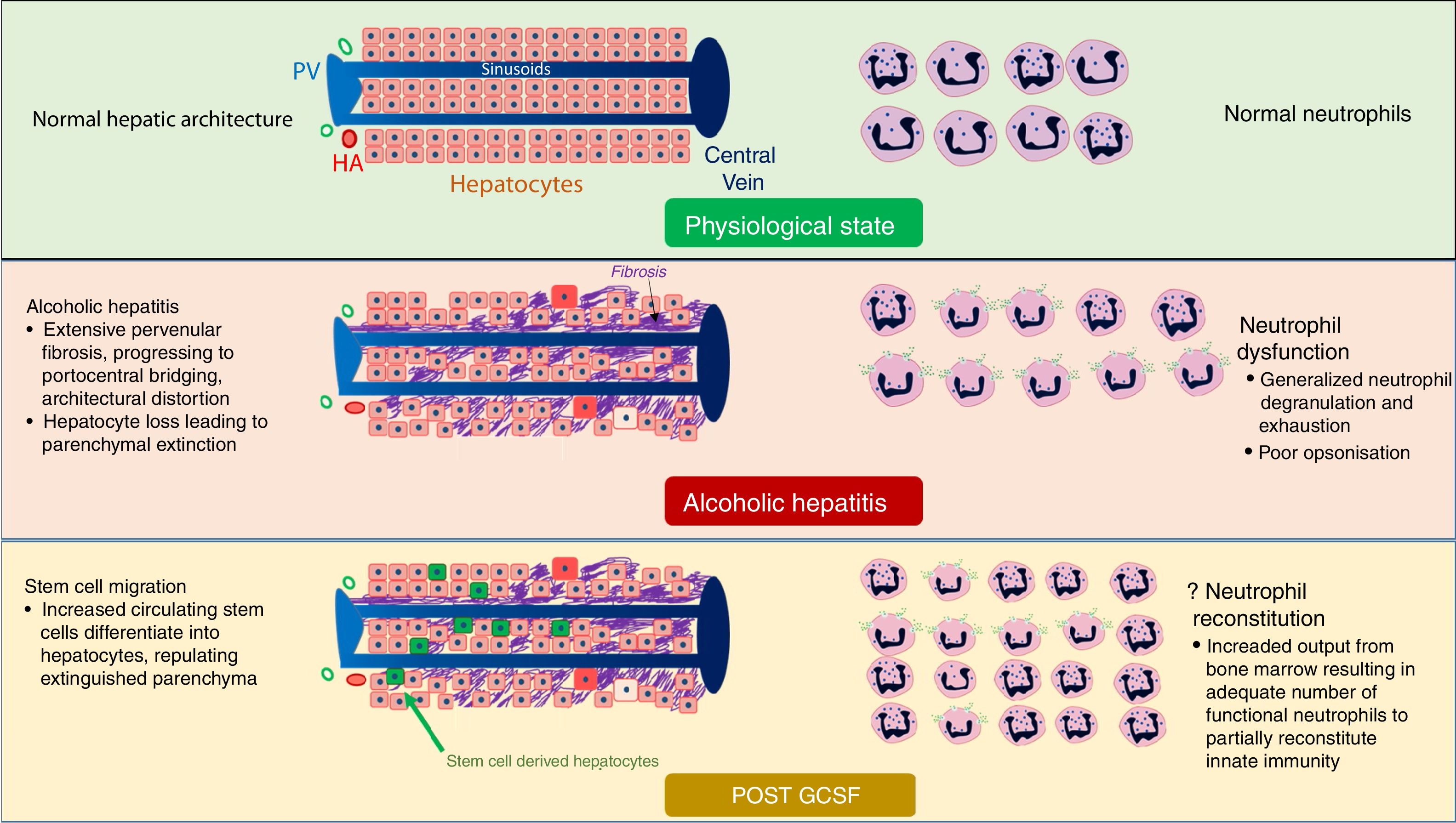
neutrophil proliferation, counteracting neutrophil exhaustion and innate immunoparesis [38–40] (Fig. 3)
Fig. 3.Mechanism of action of GCSF in patients with alcoholic hepatitis. GCSF likely leads to repopulation of extinguished parenchyma with new hepatocytes, improving liver function (Left half of figure). GCSF also stimulates bone marrow to produce more functional neutrophils, possibly reducing infections (Right half of figure). BD, Bile Ductule; HA, Hepatic Arteriole; PV, Portal Venoule.
(0.54MB). - (c)
good safety profile
A clinical trial on use of GCSF in severe alcoholic hepatitis was conducted by Singh et al. This trial included patients with clinical diagnosis of AH, and randomized them into standard medical therapy with or without GCSF. Standard medical therapy included pentoxyphylline [33]. None of the patients were given corticosteroids. GCSF was administered at a dose of 5mcg/day for 5 days. Each arm had 23 patients with a median MELD of 26–27. This trial reached its primary end point, with markedly improved 90-day survival in the GCSF group (78% vs. 30%). There was a trend towards an increase in circulating hematopoietic stem cells (CD34+) at the end of GCSF therapy, however, CD34+ cells in hepatic tissue were not assessed. A follow-up trial by the same group recruited 57 patients, and added a third arm with N-acetylcysteine combined with GCSF. The GCSF arms had a combined survival of 78% versus 30% in the control group at 90 days [41]. The addition of N-acetylcysteine had no effect on survival.
It is well known that patients with alcoholic hepatitis treated with corticosteroids, who do not show a favourable response based on the Lille model, have a poor survival and increased rates of infections [9,42]. A recent trial used GCSF for steroid non-responsive alcoholic hepatitis. The results were encouraging, with a considerable 90-day survival advantage (64% vs. 29%, Table 2), along with a reduction in infection rates in the GCSF group. Moreover, the response was durable, with survival benefit carrying over up to 12 months of followup [43] (Table 1). The trial faced some concerns regarding high mortality rates (71%) in the control arm as compared to previously published literature [44]. However, the patient population was much sicker in this trial (median MELD 27). A comparison with a trial having similar severity of disease was a landmark trial on early liver transplantation in alcoholic hepatitis [45]. Here, the median MELD score was 28.5, and mortality rate in the control group was 77%.
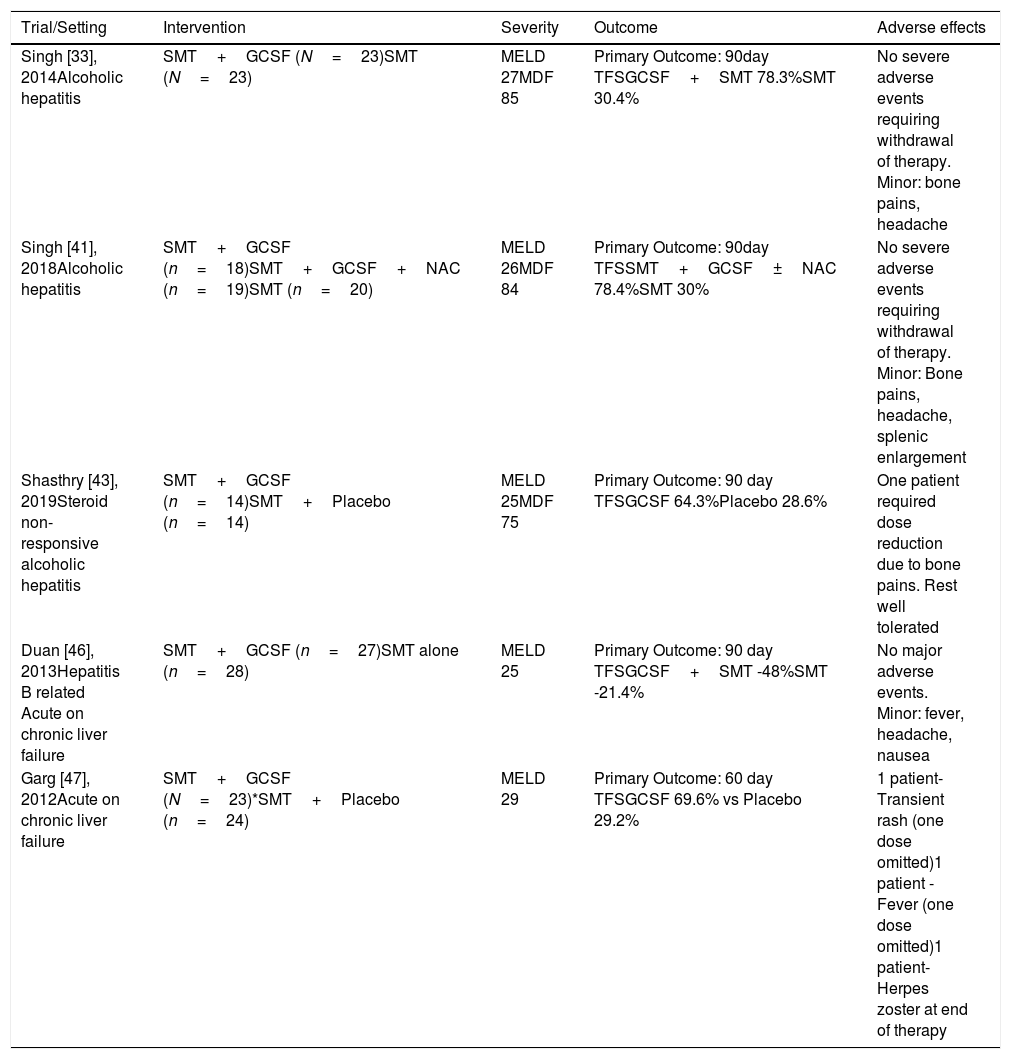
Trials with GCSF in alcoholic hepatitis and acute on chronic liver failure.
| Trial/Setting | Intervention | Severity | Outcome | Adverse effects |
|---|---|---|---|---|
| Singh [33], 2014Alcoholic hepatitis | SMT+GCSF (N=23)SMT (N=23) | MELD 27MDF 85 | Primary Outcome: 90day TFSGCSF+SMT 78.3%SMT 30.4% | No severe adverse events requiring withdrawal of therapy. Minor: bone pains, headache |
| Singh [41], 2018Alcoholic hepatitis | SMT+GCSF (n=18)SMT+GCSF+NAC (n=19)SMT (n=20) | MELD 26MDF 84 | Primary Outcome: 90day TFSSMT+GCSF±NAC 78.4%SMT 30% | No severe adverse events requiring withdrawal of therapy. Minor: Bone pains, headache, splenic enlargement |
| Shasthry [43], 2019Steroid non-responsive alcoholic hepatitis | SMT+GCSF (n=14)SMT+Placebo (n=14) | MELD 25MDF 75 | Primary Outcome: 90 day TFSGCSF 64.3%Placebo 28.6% | One patient required dose reduction due to bone pains. Rest well tolerated |
| Duan [46], 2013Hepatitis B related Acute on chronic liver failure | SMT+GCSF (n=27)SMT alone (n=28) | MELD 25 | Primary Outcome: 90 day TFSGCSF+SMT -48%SMT -21.4% | No major adverse events. Minor: fever, headache, nausea |
| Garg [47], 2012Acute on chronic liver failure | SMT+GCSF (N=23)*SMT+Placebo (n=24) | MELD 29 | Primary Outcome: 60 day TFSGCSF 69.6% vs Placebo 29.2% | 1 patient-Transient rash (one dose omitted)1 patient -Fever (one dose omitted)1 patient-Herpes zoster at end of therapy |
CPS, Child Pugh score; SOFA, sequential organ failure assessment; GCSF, granulocyte colony stimulating factor; MDF, Maddrey discriminant function; MELD, Mayo model for end-stage liver disease; SMT, standard medical therapy, TFS, transplant free survival.
The utility of GCSF in patients with acute on chronic liver failure was also examined in two randomized controlled trials [34,46]. The trial from India included 47 patients randomized to either standard medical therapy (SMT) or SMT plus GCSF. Twenty-three patients (15 had alcoholic hepatitis) with a median MELD score of 29 were included in the GCSF treatment arm. Patients with alcoholic hepatitis were treated with pentoxyphylline but not steroids, and patients with hepatitis B were treated with tenofovir. There was a considerable difference in 60 day survival between the GCSF and the placebo arms (70% vs. 29%). There was also a significantly higher number of CD34+ cells in protocol liver biopsies in the GCSF group at 30 days, giving credence to the biological plausibility of this treatment.
ACLF due to flare of hepatitis B is classically associated with very high mortality rates, even in presence of effective antivirals [47,48]. A randomized trial from China evaluated the role of GCSF versus no GCSF in this population of patients. All participants were treated with entecavir. There was a considerable survival advantage in the GCSF arm, with 90-day survival over twice the control arm (Table 1) [46].
These studies from 3 different centres in India, and one from China, suggest a potential role of GCSF in patients with alcoholic hepatitis and acute on chronic liver failure. Most of the patients included in these studies were severely ill, as indicated by their median MELD score of greater than 25, and Maddrey's discriminant function score of greater than 75. The control groups in each study had dismal survival with standard medical therapy, which was similar to previously published literature in comparable patients. Moreover, the GCSF therapy was easy to administer, and was well tolerated in all studies. In addition to survival advantage, there was improvement in all measures of severity of liver disease.
Interestingly, most of the survival advantage was seen in the first 2–3 weeks of therapy, as evidenced by early splitting of survival curves. Whether this benefit was derived from improvement in hepatic synthetic function or improved acute infection control is difficult to separate from these studies. Nevertheless, the clear early survival advantage may provide the initial stabilization in carefully selected acutely ill patients, providing a potential bridge to transplant, or improvement in the likelihood of long-term survival if transplantation is not available.
6GCSF in decompensated cirrhosisImprovement in liver function with GCSF mediated stem cell mobilization was shown in two early trials from Italy and Switzerland [13,32]. These studies paved way for subsequent randomized clinical trials, which used GCSF in combination with other growth factors in patients with stable decompensated cirrhosis.
Prajapati et al., in their single centre, open labelled randomized trial evaluated the role of GCSF as an adjuvant to standard medical therapy [49]. They used computer generated randomization to allot 253 patients to GCSF versus standard medical therapy only groups. GCSF was given to 126 patients, and 6-month transplant free survival was assessed. They demonstrated an increase in peripheral blood CD34 cells at the end of 5 days of GCSF therapy. None of the patients developed any severe adverse effects, and there was a clear survival benefit at 6 months in the GCSF arm (79% versus 68%, P=0.025).
Kedarisetty et al. evaluated the impact of multiple doses of GCSF administered in combination with darbopoetin, an erythropoietin(EPO) analogue, to 29 patients with decompensated cirrhosis [50]. Darbopoetin was added to GCSF as there was mouse-model evidence of a hepatoprotective effect of erythropoietin in fulminant hepatic failure [51]. This study showed a considerable difference in 6-month mortality (Table 2). Also, there was better improvement in the severity of liver disease in the treatment arm, as evidenced by improvement in Child–Pugh, MELD and Maddery Discriminant Function scores. Improved regeneration of liver in the treatment arm were associated with higher CD34+ and CD 133+ (another marker of hematopoietic stem cells) cells on liver biopsy, as well as higher levels of α-fetoprotein in the treatment arm. A recent follow-up trial to darbopoetin-GCSF combination aimed to assess the incremental effect of EPO to GCSF in decompensated cirrhosis [52]. They randomized 30 patients each to GCSF and GCSF+EPO arms. The combination arm showed a statistically non-significant trend towards improved survival at 2 months. Moreover, the combination arm showed better improvement in Child–Pugh score and ascites control. There was reduction in hepatic inflammation as well as parenchymal necrosis in both groups on paired histology, and immunohistochemical analysis showed increased levels of CD34+ cells and pro-regenerative CD163 macrophages. Additionally, there was reduction in smooth muscle actin depositing myofibroblasts.
Trials with GCSF in compensated and decompensated cirrhosis.
| Trial/Setting | Intervention | Severity | Outcome | Adverse events |
|---|---|---|---|---|
| Kedarisetty [50], 2015Decompensated cirrhosis | GCSF+Darbopoetin (N=29)Placebo (N=26) | MELD 22CPS 11 | Primary Outcome:1 year TFSGCSF+darbopoetin-68.6%Placebo-26.9%Decreased need for therapeutic paracentesesBetter improvement in Child and MELD scoresReduced probability of sepsis | No major difference in adverse effects between groups |
| Prajapati[49], 2017Decompensated cirrhosis | GCSF+SMT (N=126)SMT (127) | MELD 17CTP 10 | Primary Outcome:6 month TFSGCSF+SMT -79%SMT alone– 68% | No significant adverse effects requiring drug modificaiton |
| Verma[37], 2018Decompensated cirrhosis | SMT+GCSF±GH (N=23)SMT+GCSF without GH (N=21)SMT (N=21) | MELD 15CPS 9 | Primary Outcome: 1 year TFSGCSF arms combined- 84%SMT without GCSF - 47.6%Improved ascites control, quality of lifeReduced infection rates | No major adverse events. Mild adverse events included bone pains, back pain, fatigue |
| Anand[52], 2019Decompensated cirrhosis | GCSF+EPO (N=30)GCSF+Placebo(30) | MELD 15CPS 9 | Primary Outcome: 1-Year mortalityGCSF+EPO 16.6%GCSF+Placebo 36.7%Better ascites and encephalopathy control in combination armResponse better in Child class B (compared to C), MELD<16 | Major: One episode of hematemesis in EPO group.Minor: Urticaria, flulike symptoms, coagulopathy related mucosal bleeding |
| Newsome[54], 2018Compensated cirrhosis | SMT (N=27)SMT+GCSF (N=26)SMT+CD133 cell infusion (N=28) | MELD 13CPS 7 | Primary Outcome: Change in MELD score at 90 daysNo improvement in liver function and fibrosisIncreased adverse events | Severe adverse events:SMT- 12%, Variceal bleed, hepatic decompensation, hypoglycaemiaGCSF group –11%, Ascites, variceal bleedStem cell Infusion group -43%, Progression of liver disease, sepsis, ascites, encephalopathy, cardiac failure |
| De[53] 2020Decompensated cirrhosis | GCSF (N=50)SMT (N=50) | MELD 15CTP 10 | Primary outcome: 12 month survival higher in GCSF groupImproved quality of life, liver functionImproved ascites control | No drug limiting adverse events |
| Philips[55]2019 (Real world)Decompensated cirrhosis | GCSF (N=100)Historical Controls (24) | MELD 24CPS 11 | 6-month survival lower in GCSF groupModified intention to treat group- survival 48% vs 75% in historical controls | GCSF group had more events of sepsis, intensive care unit admissions, decompensation events, and liver cancer |
BMSC. bone marrow stem cells; CPS, Child Pugh score; GH, growth hormone; MDF, Maddrey discriminant function; MELD, Mayo model for end-stage liver disease; SMT – standard medical therapy; TFS, transplant free survival.
Mouse models have shown improved liver regeneration after hepatectomy in response to growth hormone. Thus, in an attempt to further augment hepatic regeneration, Verma et al. added multiple cycles of growth hormone to GCSF therapy in patients with decompensated cirrhosis [37]. They used multiple cycles of growth factors, which had previously shown improved CD34+ cell mobilization from bone marrow, potentially aiding in better regeneration [13]. They randomized patients with decompensated cirrhosis into 3 arms – GCSF, GCSF+ growth hormone, and standard medical therapy. There was a significant improvement in the primary end-point of 1 year survival in patients who received GCSF with or without growth hormone (84% vs. 46%). as well as in quality of life, ascites control and liver stiffness. However, there was no significant difference among the GCSF groups with or without growth hormone (Table 2). Another trial was recently published by the same group, which randomized 100 patients to multiple courses of GCSF for a year, versus standard medical therapy [53]. They were able to replicate the previous results, with the intervention arm having a survival of 74% at 1 year against 42% in the control arm. They also noted an improvement in severity of liver disease, better ascites control, and improved quality of life.
These trials show an improvement in synthetic function of the liver, possibly by increase in hepatocyte population and regression of fibrosis. Hepatocyte proliferation is possibly secondary to repopulation of the extinguished hepatic parenchyma by BMSC derived hepatocytes, however a stimulatory action of GCSF on native hepatocytes has also been proposed. The marked reduction in markers of liver fibrosis is more difficult to explain in the given timeline, and is possibly a consequence of reduction in hepatic inflammation rather than regression of established fibrosis (although GCSF mediated regression of scarring via currently undescribed mechanisms cannot be excluded).
Not all clinical trials are in agreement with a positive effect of stem cell therapies in decompensated cirrhosis. A recent multicentre randomized control trial from UK evaluated the efficacy of stem cell therapy (Both GCSF and infused stem cells) in patients with compensated cirrhosis [54]. They failed to find any significant difference in disease severity or non-invasive measures of liver fibrosis. However, this trial's enrolment criteria may have significantly hindered the likelihood of determining a positive outcome as most patients had well compensated cirrhosis, and the median MELD score was 13. GCSF's benefit presumably includes repopulation of liver with functional hepatocytes, and most of these patients probably already had a significant reserve population of functional hepatocytes remaining in their liver parenchyma. The required sample size to assess a difference at these scores would likely be much larger, and the required follow-up much longer than 1 year to detect any possible difference in clinical outcomes in these patients with early cirrhosis. Moreover, the compensated cirrhotic patient population is clinically, and pathophysiologically, very different from the decompensated cirrhosis/severe alcoholic hepatitis patient populations. Therefore, the generalizability of this study to the decompensated clinically unwell patient populations is questionable. The main importance of this study is that GCSF is probably of no benefit if patients have compensated cirrhosis and have relatively stable disease. Conversely, the erythropoietin+GCSF study [52] suggested that therapeutic benefits were derived more by patients who had lower grade of ascites and MELD <16. However, all participants still had decompensated cirrhosis.
While still under investigation, some centres have started using GCSF for cirrhosis in clinical practice as off-label use. A recent real-world retrospective review of use of GCSF in decompensated cirrhosis was recently published from southern India, where 100 patients with a median MELD score of 24 were compared with matched historical controls [55]. The results were quite the opposite from other studies published from northern India, with poorer survival, increased sepsis, and higher rates of hepatocellular carcinoma. The severity of liver disease in this study was much higher than three of the four published studies from this region [37,49,52] (Table 2). Moreover, they remarked that patients with Child Pugh score >11 and MELD score >25 did worse with GCSF therapy. While this was a retrospective chart review for an off-label use drug, there may be some interesting takeaways from this experience. It is possible that only patients within a therapeutic window of severity of liver disease derive benefit from this therapy. This possibility is further substantiated by the work of Anand et al., who showed that among patients with decompensated cirrhosis, patients with more advanced disease had poorer bone marrow reserve and impaired regenerative response to growth factors as compared to those with less severe disease [52]. That said, a possibility that the results may not be reproducible in all settings should also be considered.
7ConclusionThere has been a mounting interest in stem cell-based therapies in nearly all medical subspecialties over the last few years. The isolation, maintenance, and instillation of these delicate cells has always been a highly specialized and resource intensive process that significantly limits application to the clinical arena. In vivo mobilization of induced hematopoietic BMSCs, via GCSF, however, has shown clear advantage in patients with corticosteroid naïve as well as corticosteroid refractory alcoholic hepatitis, and in acute on chronic liver failure. In patients with chronically decompensated cirrhosis, GCSF combined with other growth factors improved survival, severity scores, ascites control, nutrition, and quality of life. Patients with compensated cirrhosis seem to derive no benefit with stem cell based therapies, which may not be surprising given their functional hepatic reserve.
It is plausible that patients with advanced cirrhosis have a severe degree of hepatic parenchymal extinction, and the mobilization and hepatic differentiation of BMSCs tip the balance back towards compensation (although other currently unknown mechanisms may also be at play). It is unlikely that this would obviate the need for transplant. Nevertheless, stem cell therapies, especially with GCSF, may help tide over the crisis situations of alcoholic hepatitis and decompensated cirrhosis, and help bridge patients to transplant. This is especially important in patients with alcoholic hepatitis, where no other treatment is effective and transplant is precluded in the acute setting in most centres (i.e. until patients have completed a proscribed period of alcohol abstinence or had time to work with alcohol counsellors, etc.). In patients who do not have transplant prospects, the improvement in quality of life and ascites control is definitely advantageous and in alcoholic hepatitis, with the passage of time, a proportion of these patients could recover without the need for transplantation if treatment allows them to survive the initial phase of their disease.
The early encouraging evidence, safety and ease of administration make GCSF a viable therapy to be explored in difficult clinical situations as alcoholic hepatitis and decompensated cirrhosis. However, the available evidence is limited by small, single centre studies with varied protocols and limited follow-up. Large multicentre, double-blinded studies that can substantiate the indication of benefit from early trials are required in order for GCSF to become accepted as a conventional therapy of alcoholic hepatitis and decompensated liver disease as a bridge to transplant. These studies should be adequately powered to detect meaningful outcomes-transplant free survival, subsequent hepatic decompensations, and quality of life. Moreover, long term data is definitely required both for assessment of durability, as well as to rule out any increase in risk of malignancy after the use of these growth factors. Further clinical trials would hopefully address the current lacunae in evidence of efficacy and safety.
Author contributionsSahaj Rathi: Conceptualization, literature review, and drafting of original manuscript
Trana Hussaini: Critical editing of manuscript
Eric Yoshida: Senior author – Oversight and guidance, critical editing of manuscript
Conflicts of interestNone of the authors have any relevant conflicts of interest.








 GCSF in patients with alcoholic hepatitis.
GCSF in patients with alcoholic hepatitis. 



