Hepatitis E in industrialized countries is mainly associated with genotype 3 hepatitis E virus (HEV) and normally causes a sporadic self-limiting disease in immunocompetent individuals. Unlike genotype 3, genotypes 1 and 2 circulate in developing countries, produce severe disease and occur in the epidemic form. Hepatitis E occurring in travellers returning from endemic areas in developing countries is not a novel epidemiological occurrence, however the vast majority of cases remain to be genetically studied. The present study describes two cases of severe acute hepatitis E that required hospitalization for 6 and 9 days in two individuals of Indian nationality that had recently migrated to Portugal to work. The retrieved HEV sequences both belonged to genotype 1 and had a high degree of nucleotide sequence identity, clustering with strains isolated in India and Nepal, in 2013 and 2014. Confirmed HEV genotypes of increased pathogenicity like genotype 1 are being introduced into otherwise naïve populations of industrialized countries such as European countries with consequences difficult to predict. As far as we know the present study is the first in Portugal to describe and genetically characterize imported cases of hepatitis E infection caused by HEV genotype 1.
In industrialized countries hepatitis E virus (HEV) infection has usually been associated with travellers to endemic areas but it is now known that locally acquired HEV infection is common in industrialized regions. However, autochthonous HEV infections in these areas contrasts from those in developing countries presenting distinct clinical and epidemiologic profiles.1 Hepatitis E in industrialized countries is mainly associated with genotype 3 and normally cause sporadic self-limiting disease in immunocompetent individuals, but may progress to chronic hepatitis in the immunocompromised patients or be associated with a range of extra-hepatic manifestations.2,3 Genotypes 1 and 2 that circulate in developing countries are normally associated with more severe disease4 and occur in the epidemic form with mortality rates in epidemics ranging from 0.2% to 4.0%.2 For unknown reasons, mortality is higher in infants under 2 years of age and in pregnant women during the third trimester (reaching 10% to 25%), causing fulminant hepatic failure and obstetric complications such as eclampsia or haemorrhage.2
In Portugal HEV genotype 3 is the only genotype detected so far, and closely related isolates have been detected in both animals and humans.5–8 Up until this report no case of HEV genotype 1 infection has ever been documented in Portugal. Here we report the first cases of HEV genotype 1 in Portugal, associated to severe acute hepatitis that required hospitalization in two migrants from India that had recently entered the country. This report highlights the possibility of introduction of exotic HEV genotypes into the Portuguese territory with different pathogenic profiles and with the potential to cause severe disease in more susceptible groups, namely in children and pregnant women.
Case ReportCase 1On 14th January 2016, a 31-year-old Indian man was admitted to the Emergency Unit (EU) of Centro Hospitalar Lisboa Central (CHLC), Lisboa, Portugal, with abdominal pain in the epigastric and right hipochondrium regions associated with coluria, nausea and jaundice. Upon admission he reported he had resided in the North of India until coming to Portugal 6 weeks previously. He had no history of risky sexual behaviour or drug addiction. Abdominal ultrasonography and radiography, as well as electrocardiography were performed and none showed abnormalities. Liver function tests (Table 1) showed a marked elevation of hepatic enzymes (AST and ALT ≥ 400 IU/L) with ALT typically higher than AST, supporting the presumptive diagnosis of viral hepatitis. The patient was transferred to the Infectious Diseases Unit (IDU) on the following day (15th January) where he stayed for 6 days until discharge (20th January). During the stay at the IDU a blood sample was collected (18th January) and submitted to the current diagnostic algorithm of acute viral hepatitis, that focus on HAV, HBV, HCV, EBV and CMV. All the viral serologic markers of acute infection (IgM anti-HAV, AgHBs, IgM anti-HBc, anti-HCV/RNA HCV, IgM anti-VCA, IgM anti-CMV) were negative. He also tested negative for HIV, Fasciola hepática and syphilis. IgM anti-HEV was not performed but HEV RNA was detected in this serum sample by real-time RT-PCR using the generic primers/probe targeting the open reading frame (ORF) 2 region that covers all HEV genotypes.9 Patient management comprised supportive measures that included hypolipidemic diet. Clinical evolution was favourable making a progressive improvement of cutaneous/sclerotic jaundice and subsequent discharge a week after admission.
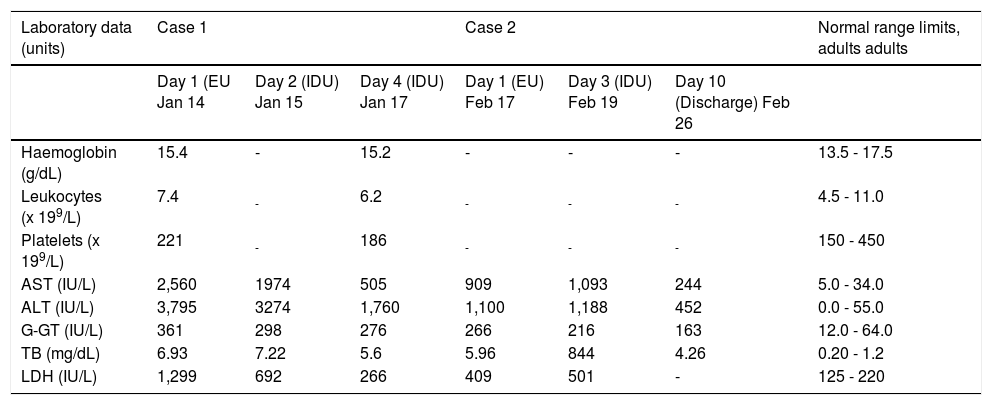
Laboratory findings of the two Indian patients (case 1 and 2) with acute hepatitis E.
| Laboratory data (units) | Case 1 | Case 2 | Normal range limits, adults adults | ||||
|---|---|---|---|---|---|---|---|
| Day 1 (EU Jan 14 | Day 2 (IDU) Jan 15 | Day 4 (IDU) Jan 17 | Day 1 (EU) Feb 17 | Day 3 (IDU) Feb 19 | Day 10 (Discharge) Feb 26 | ||
| Haemoglobin (g/dL) | 15.4 | - | 15.2 | - | - | - | 13.5 - 17.5 |
| Leukocytes (x 199/L) | 7.4 | - | 6.2 | - | - | - | 4.5 - 11.0 |
| Platelets (x 199/L) | 221 | - | 186 | - | - | - | 150 - 450 |
| AST (IU/L) | 2,560 | 1974 | 505 | 909 | 1,093 | 244 | 5.0 - 34.0 |
| ALT (IU/L) | 3,795 | 3274 | 1,760 | 1,100 | 1,188 | 452 | 0.0 - 55.0 |
| G-GT (IU/L) | 361 | 298 | 276 | 266 | 216 | 163 | 12.0 - 64.0 |
| TB (mg/dL) | 6.93 | 7.22 | 5.6 | 5.96 | 844 | 4.26 | 0.20 - 1.2 |
| LDH (IU/L) | 1,299 | 692 | 266 | 409 | 501 | - | 125 - 220 |
EU: Emergency Unit, IDU: Infectious Diseases Unit, AST: aspartate aminotransferase. ALT: alanine aminotransferase. G-GT: gamma-glutamyl transpeptidase. TB: total bilirubin. LDH: lactate dehydrogenase. The dashes indicate that the test was not done.
On 17th February 2016, a 50-year-old Indian man was admitted to the EU of CHLC, Lisboa, Portugal, with abdominal pain in the epigastric region, anorexia and asthenia. Upon admission he reported his previous residence as Jalandhar, North of India, until coming to Portugal one month previously. He had no history of drug addiction and was a moderate consumer of alcohol, having been abstinent for the past five days. Abdominal ultrasonography and radiography, as well as electrocardiography were performed and none showed abnormalities. On that day blood was taken for analysis and liver function tests (Table 1) and showed elevated levels of transaminases (≥ 400 IU/ L) with the typically higher profile of ALT compatible with the diagnosis of viral hepatitis. The patient was transferred to the IDU on the 19th February where he stayed for seven days until discharge (26th February). The blood sample collected on 19th February was also submitted to the diagnostic algorithm of acute viral hepatitis but all the viral serologic markers of acute infection were negative. IgM anti-HEV was not performed but serum was found positive for HEV RNA by real-time RT-PCR.9 The patient tested negative for HIV but was positive for Fasciola hepatica (antibody titer 320). Since the patient was non-eosinophilic and showed normal abdominal ecographic patterns a diagnosis of fasiolosis was ruled out. Patient management comprised supportive measures that included hypolipidemic diet. Clinical evolution was favourable and he was discharged a week after admission.
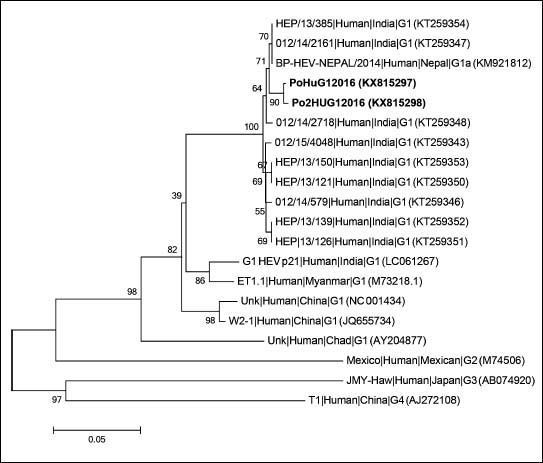
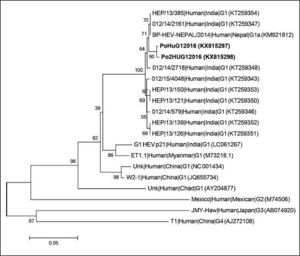
Genotypic characterization of HEV detected in patientsTo characterize the HEV sequences retrieved from the two patients a nested broad-spectrum RT-PCR with amplification within the ORF 1 was used.10 The final 330 bp amplified products were sequenced and compared with HEV reference strains, using a neighbor-joining method based on the Jukes-Cantor model for selected HEV isolates representing genotypes 1–4 and including sequences with highest nucleotide identity, according to nucleotide BLAST (Basic Local Alignment Search Tool). The phylogenetic analysis indicated that the two HEV sequences retrieved from these patients were nearly identical, sharing 99.7% nucleotide sequence identity between them, and clustering with HEV genotype 1 sequences isolated in 2013 and 2014 in Jabalpur District, India, and from an HEV outbreak in Nepal, 2014 (Figure 1) with which they shared 99.0% nucleotide identity. The obtained sequences were deposited in GenBank (PoHuG12016; GenBank accession no. KX815297 and Po2HUG12016; GenBank accession no. KX815298).
Phylogenetic tree based on partial RNA-dependent RNA-polimerase region of open reading frame 1 (330 bp) using neighbor-joining method based on the Jukes-Cantor model and 1000 bootstrap ressamplings. Scale bar indicates substitutions per nucleotide position. Sequences are defined in tree as Strain|Host|Origin|Subgenotype (accession number). Sequences characterized in this study are in bold.
The present report describes two cases of severe acute hepatitis that required hospitalization for 6 and 9 days in two individuals of Indian nationality that had recently migrated to Portugal. HEV genotype 1 was identified as the cause of the acute hepatitis in both patients that most likely acquired the HEV infection in the North of India where they lived, before coming to Portugal. There was no com- mon link between them and they now live in Lisbon in separate accommodations. The sequences of HEV identified in these patients showed a high degree of identity clustering with strains retrieved from India and Nepal, in 2013 and 2014. Hepatitis E occurring in travellers returning from HEV endemic areas is not a novel epidemiological occurrence, however the vast majority of cases remain to be genetically studied. Genetic characterization of imported HEV genotype 1 isolates have been scarcely documented with reports from travellers returning to UK,11 Spain12 and Germany.13,14 HEV genotypes circulating in developing countries such as India are considered a serious public health problem15 and are known to cause an estimated 20.1 million infections with 3.4 million acute cases and 3 000 stillbirths annually worldwide with an estimated 70 000 deaths.16
Over the past few decades there has been growing concerns about population migration flows from low- to high-income countries and its impact on infectious disease emergence. If HEV genotypes of increased pathogenicity like genotype 1 are being introduced in otherwise naïve populations of industrialized countries such as European countries, the consequences of such imported events are difficult to predict. Our findings demonstrate the need to implement and improve strategic HEV surveillance in high-income countries with substantial migration flows.
Abbreviations- •
CHLC: Centro Hospitalar Lisboa Central.
- •
EU: Emergency Unit.
- •
HEV: hepatitis E virus.
- •
IDU: Infectious Diseases Unit.
- •
ORF: open reading frame.
None.