Hepatorenal syndrome is complication of advanced cirrhosis characterized by renal failure, changes in systemic blood pressure, and increased activity of endogenous vasoactive systems. Renal failure is due to severe renal vasoconstriction developing in the late stages of cirrhosis. The pathogenesis of hepatorenal syndrome is the result of an extreme underfilling of the arterial circulation secondary to an arterial vasodilation located in the splanchnic circulation. This underfilling triggers a compensatory response with activation of vasoconstrictor systems. The diagnosis of hepatorenal syndrome is based on established diagnostic criteria aimed at excluding nonfunctional causes of renal failure. The prognosis of patients with hepatorenal syndrome is very poor. Liver transplantation is the best option in selected patients, but it is not always applicable due to the short survival expectancy and donor shortage. Pharmacological therapies based on the use of vasoconstrictor drugs (terlipressin, midodrine, octreotide or noradrenline) are the most promising in aims of successfully offering a bridge to liver transplantation. Prevention of hepatorenal syndrome with albumin infusion is recommended in patients with spontaneous bacterial peritonitis and with pentoxifylline in patients with acute alcoholic hepatitis.
Hepatorenal syndrome (HRS) is the development of functional renal failure in patients with advanced chronic liver disease, liver failure, and portal hypertension in the absence of any identifiable renal pathology. Patients with HRS show a severe disturbance in their systemic hemodynamics with low arterial pressure and high cardiac output. The splanchnic circulation in patients with HRS is subjected to the effects of circulating vasodilators arising from the endothelium that cause significant arterial vasodilation in this vascular bed with resulting activation of endogenous vasoconstrictor systems. In several nonsplanchnic vascular beds there is marked vasoconstriction, which in the kidney results in low glomerular filtration rate (GFR). Although HRS occurs predominantly in advanced cirrhosis, it may also develop in other chronic liver diseases associated with severe liver failure and portal hypertension, such as alcoholic hepatitis, or in acute liver failure.1
With the advent of liver transplantation, HRS is now relatively uncommon with a reported incidence of about 7-10% among hospitalized patients with cirrhosis and ascites.2 Nonetheless the probability of developing HRS in patients with cirrhosis and ascites is near 20% at one year and increases to 40% at 5 years.2 Patients with ascites and marked sodium and water retention as well as those with marked arterial hypotension have the highest risk of developing HRS. Two types of HRS are observed in clinical practice. Type 1 HRS is an acute form with a very poor prognosis. Type 2 HRS develops slowly over weeks; these patients usually have diuretic-resistant ascites and have a slightly better prognosis compared with those with type 1 HRS.
There are several mechanisms that play a contributory role in pathogenesis of HRS, including extrarenal and intrarenal factors, abnormalities in systemic hemodynamics, and the diseased liver causing portal hypertension and hepatic failure. This review will describe the pathogenesis, clinical features, diagnostic approach and treatment of HRS in cirrhosis.
PathophysiologyThe pathophysiologic hallmark of HRS is vasoconstriction of the renal circulation. The mechanisms underlying renal vasoconstriction are complex and include changes in systemic arterial circulation, portal hypertension, activation of vasoconstrictor factors, and suppression of vasodilator factors acting on the renal circulation (Table I). A common pathway for these derangements is the development of an intense splanchnic arterial vasodilation, likely due to the production of local vasodilators like nitric oxide and adenosine, which trigger an important compensatory response by activating vasoconstrictor and antinatriuretic systems such as the renin-angiotensinaldosterone system (RAAS), the sympathetic nervous system (SNS) and arginine vasopressin (AVP) accounting for sodium and water retention as well as renal vasoconstriction3(Figure 1).
Vasoactive factors involved in the regulation of renal perfusion in cirrhosis and the pathogenesis of hepatorenal syndrome.
| Vasodilators |
| Prostacyclin |
| Prostaglandin E2 |
| Nitric oxide |
| Atrial natriuretic peptide |
| Kallikrein-kinin system |
| Vasoconstrictors |
| Angiotensin II |
| Norepinephrine |
| Neuropeptide Y |
| Endothelin-1 |
| Adenosine |
| Thromboxane A2 |
| Cysteinyl leukotrienes |
| F2-isoprostanes |
The regulation of renal circulation in cirrhosis depends on the interaction between vasoconstrictor and vasodilator factors acting on the renal vasculature (Table I). In the early stages of cirrhosis, for example, renal blood flow can be kept within normal limits due to the effect of local vasodilators, which antagonize the renal vascular effect of the systemic vasoconstrictors. Whenever there is stimulation of the endogenous vasoconstrictors, there is also an activation of renal vasodilators (prostaglandins, nitric oxide and natriuretic peptides) in order to maintain renal perfusion and GFR. The renal production of prostaglandins and the circulating levels of natriuretic peptides are increased in patients with cirrhosis and ascites without HRS. However HRS will ensue when circulating vasoconstrictors overcome the effect of renal vasodilators, leading to renal vasoconstriction and reduction in GFR. In some cases a precipitating cause of circulatory dysfunction such as spontaneous bacterial peritonitis (SBP) leads to worsening of renal vasoconstriction. Once vasoconstriction develops, intrarenal mechanisms perpetuate HRS due to development of intrarenal vicious circles in which hypoperfusion leads to an imbalance in intrarenal vasoactive systems that in turn cause more vasoconstriction.
Systemic vasoconstrictor factorsThe RAAS, SNS, and AVP are overactivated in most cirrhotic patients with ascites due to a reduced effective arterial blood volume; consequence of the marked splanchnic arterial vasodilation.4-6 AVP is mainly responsible for increased water reabsorption in the renal tubules and the subsequent development of dilutional hyponatremia in advanced cirrhosis. The circulating levels of endothelin- 1, an endothelial-derived peptide with potent vasoconstrictor effect, are also increased in patients with HRS.7 Increased levels are due to an enhanced release of the peptide from the hepatic and splanchnic circulation. Endothelin also induces vasoconstriction of the renal circulation and may play a role in the pathogenesis of HRS. The major natriuretic hormones; atrial natriuretic peptide and brain natriuretic peptide, are increased in patients with cirrhosis and ascites.8 These peptides by inducing renal vasodilation and natriuresis may act as a homeostatic response to counteract the effects of RAAS and SNS on the renal circulation.
Intrarenal factorsMetabolites of arachidonic acid are important intrarenal factors that regulate renal perfusion in cirrhosis. Arachidonic acid is metabolized in the kidney through three separate pathways. The most important of these three pathways is dependent on cyclooxygenases which give rise to prostaglandins (PGs). Patients with cirrhosis and ascites without HRS have an increased renal production of PGs when compared with controls and cirrhotics without ascites.9 The renal PGs cause renal vasodilation and by doing so protect the kidney from vasoconstrictors. In fact, PGs synthesis inhibition with non-steroidal antinflammatory agents may cause renal failure in cirrhotics with ascites.10 With disease progression renal production of PGs is reduced and the imbalance between the vasoconstrictor systems and PGs may result in severe renal vasoconstriction and HRS. Other intrarenal factors that may play a role in the regulation of renal perfusion in cirrhosis and pathogenesis of HRS are nitric oxide, intrarenal kallikrein-kinin and renin-angiotensin systems and adenosine (Table I).
The arterial vasodilation theoryThe theory that better explains the relationship between changes in the renal circulation; activation of vasoconstrictor mechanisms, and presence of marked disturbances in systemic hemodynamics is the arterial vasodilation theory3(Figure 1). This theory proposes that renal hypoperfusion represents the extreme manifestation of underfilling of the arterial circulation secondary to a marked vasodilation of the splanchnic vascular bed. Arterial underfilling is clinically manifested by arterial hypotension. This event leads to a baroreceptor-mediated activation RAAS and SNS with vasoconstriction not only in the renal circulation but also in other vascular beds. In consequence, the splanchnic area would “escape” the effect of vasoconstrictors due to an enhanced local production of vasodilator factors. In the early stages of cirrhosis, renal perfusion initially would be maintained within normal limits despite activation of RAAS and SNS due to increased levels of renal vasodilators. However, with progression of disease, renal perfusion could not be maintained because of extreme arterial underfilling causing maximal activation of vasoconstrictor systems and decreased activity of renal vasodilators. At this critical point HRS would ensue.
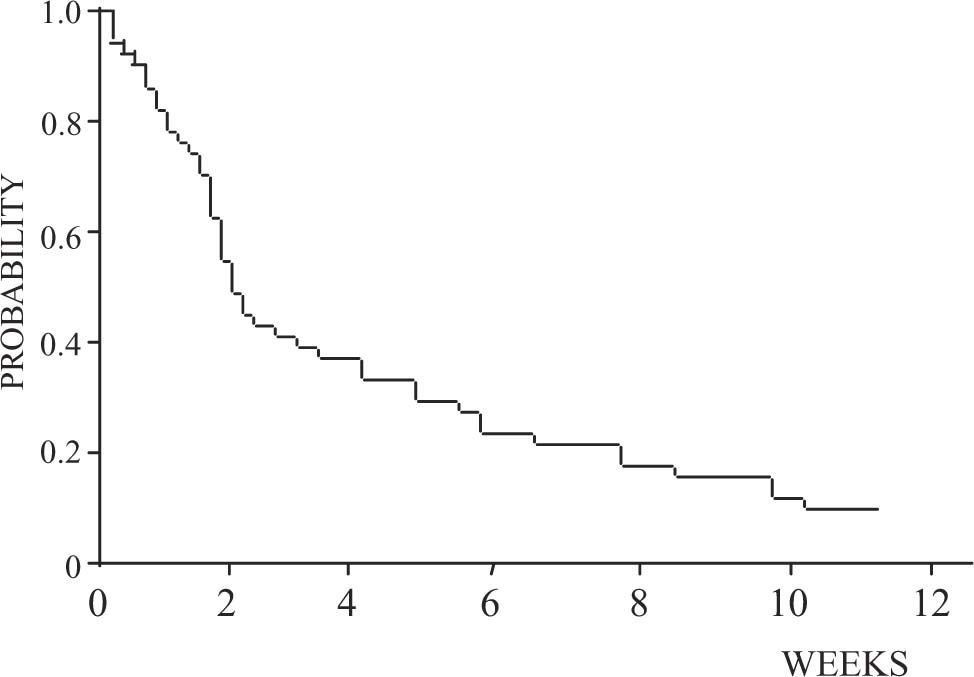
Clinical and laboratory findingsThere are no specific clinical findings in HRS. The majority of patients have features of advanced liver disease with hyperbilirubinemia, elevated prothrombin time, low platelets, hepatic encephalopathy, low albumin, and large ascites. Low arterial blood pressure and elevated heart rate and cardiac output are present in most patients. Renal failure in HRS is often associated with oliguria, urinary sodium retention, and spontaneous dilutional hyponatremia. Two types of HRS have been described1,11(Table II). Type 1 HRS is characterized by a rapid and progressive impairment of renal function as defined by a doubling of the initial serum creatinine to a level higher than 2.5 mg/dL or a 50% reduction of the initial 24-hr creatinine clearance to a level lower than 20 mL/min in less than 2 weeks. Serum creatinine levels in patients with HRS are usually lower than values observed in patients with acute renal failure without liver disease due to a reduced muscle mass and low endogenous production of creatinine in cirrhosis. In some patients, this type of HRS develops spontaneously without any identifiable precipitating factor, whereas in others it can occur in close association with systemic bacterial infections, in particular SBP or acute alcoholic hepatitis. SBP is a common precipitating cause of type 1 HRS.12 It occurs in approximately one-third of cases with SBP despite a rapid resolution of the infection with intravenous antibiotics. In addition, large-volume paracentesis without plasma expansion may precipitate type 1 HRS. Although gastrointestinal bleeding is considered to be a major precipitating factor of HRS, the development of renal failure after this complication is uncommon in patients with cirrhosis and occurs almost exclusively in patients who develop hypovolemic shock, and in most cases is associated with ischemic hepatitis, which suggests that renal failure in patients with gastrointestinal bleeding is probably related to the development of acute tubular necrosis.13 Without treatment, the median survival time of patients with type 1 HRS is less than 2 weeks and practically all patients die within 8-10 weeks after the onset of renal failure (Figure 2).
Clinical types of hepatorenal syndrome.
| Type 1. Rapid and progressive impairment of renal function as defined by a doubling of the initial serum creatinine to a level higher than 2.5 mg/dL or a 50% reduction of the initial 24-hour creatinine clearance to a level lower than 20 mL/min in less than 2 weeks. |
| Type 2. Impairment in renal function (serum creatinine > 1.5 mg/dL) that does not meet the criteria of type 1. |
In contrast to type 1 HRS, type 2 HRS is characterized by more subtle course with serum creatinine levels around 1.5-2.0 mg/dL. The main clinical consequence of type 2 HRS is diuretic-resistant ascites. As expected, survival is longer in this group of patients than in those with type 1 HRS (median survival of 5-6 months), but is shorter than that of patients with ascites without renal failure.
Predictive factors associated with a greater risk of developing HRS in cirrhotic patients with ascites are listed in table III. The most easily recognized are severe urinary sodium retention, (<10 mEq/day), spontaneous dilutional hyponatremia (serum sodium < 130 mEq/L), and low mean arterial blood pressure (< 80 mmHg).2 Interestingly, neither the degree of liver failure, as assessed by classic parameters of liver function (serum bilirubin, albumin, and prothrombin time) or the Child-Pugh classification, correlate with the risk of developing HRS.
Parameters associated with a higher risk of hepatorenal syndrome development in nonazotemic cirrhotic patients with ascites.
| Slightly increased BUN and/or serum creatinine levels** |
| Dilutional hyponatremia |
| Reduced free water excretion after water load |
| Low urinary sodium excretion |
| Arterial hypotension |
| High plasma renin activity |
| High plasma norepinephrine concentration |
| Previous episodes of ascites |
| Absence of hepatomegaly |
| Poor nutritional status |
| Moderately increased renal vascular resistive index |
Due to the lack of specific diagnostic tests to distinguish between HRS and other causes of renal failure that may occur in cirrhosis, the diagnosis of HRS is a diagnosis of exclusion based on several criteria described in table IV.1 Low GFR is defined as serum creatinine greater than 1.5 mg/dL or 24-hr creatinine clearance lower than 40 mL/min, without diuretic therapy. Other criteria include the absence of clinical conditions that predispose to the development of acute renal failure, no improvement of renal function following diuretic withdrawal and plasma expansion, no proteinuria, and a normal renal ultrasound. Most cases of HRS have urine sodium below 10 mEq/L and urine osmolality above plasma osmolality because of a preserved tubular function. Nevertheless, a minority of patients may have higher urine sodium and low urine osmolality, similar to values found in acute tubular necrosis. Conversely, some cirrhotic patients with acute tubular necrosis may have low urine sodium and high urine osmolality. For these reasons, urinary indices are not considered major criteria for diagnosis of HRS.1
Diagnostic criteria of hepatorenal syndrome
| Major criteria* |
| 1. Low glomerular filtration rate, as indicated by serum creatinine greater than 1.5 mg/dL or 24-hr creatinine clearance lower than 40 mL/min. |
| 2. Absence of shock, ongoing bacterial infection, fluid losses and current treatment with nephrotoxic drugs. |
| 3. No sustained improvement in renal function (decrease in serum creatinine to 1.5 mg/dL or less or increase in creatinine clearance to 40 mL/min or more) following diuretic withdrawal and expansion of plasma volume with 1.5 L of a plasma expander. |
| 4. Proteinuria lower than 500 mg/day and no ultrasonographic evidence of obstructive uropathy or parenchymal renal disease. |
| Additional criteria |
| 1. Urine volume lower than 500 mL/day. |
| 2. Urine sodium lower than 10 mEq/L. |
| 3. Urine osmolality greater than plasma osmolality. |
| 4. Urine red blood cells less than 50 per high power field. |
| 5. Serum sodium concentration lower than 130 mEq/L. |
Other causes of renal failure, particularly prerenal failure secondary to volume depletion, acute tubular necrosis, drug-induced nephrotoxicity, and glomerulonephritis should be excluded before the diagnosis of HRS is made. Causes that may predispose to prerenal failure such as volume depletion due vomiting or diarrhea, or renal fluid losses due to excessive diuretic therapy are common in cirrhotic patients and should be sought after. In prerenal failure due to volume depletion, renal function improves rapidly after the intravenous administration of fluids, whereas no improvement occurs in patients with HRS. Shock before the development of renal failure in a cirrhotic patient precludes the diagnosis of HRS, and usually indicates acute tubular necrosis. Likewise, the diagnosis of HRS should not be made in patients with recent or current treatment with nephrotoxic drugs, mainly non-steroidal antinflammatory drugs or aminoglycosides. Proteinuria (> 500 mg/d) and/or ultrasonographic abnormalities in the kidneys indicate organic renal disease or obstructive uropathy.
ManagementGeneral measuresHRS develops in the setting of advanced liver disease and in some others in the setting of acute liver failure. In either case patients are very unstable and require hospitalization, preferrably in an intensive care unit for those with type 1 HRS. Continous monitoring of vital signs, fluid intake, daily weights and urinary output should be performed. Central line access with central venous pressure measurement is helpful in assesing volume status, particularly when intravenous fluid challenge of a plasma expander is administered to rule out renal failure due to intravascular volume depletion (Table IV). Adequate measures to ensure proper nutrition with a low salt diet ae extermely important as these patients are frequently malnourished. Since the majority of patients have ascites, diagnostic paracentesis must be performed to rule out SBP. In patients with tense ascites, a therapeutic tap of 5-liters associated with albumin infusion may aid in providing comfort.
The most accepted therapies for HRS include the use splanchnic vasoconstrictors, portosystemic shunts, and liver transplantation (Figure 3).
Pharmacologic therapyA variety of phamacologic interventions have been used to treat HRS. The use of renal vasodilators like dopamine and prostaglandin analogues was abandoned due side effects and lack of substantial data confirming adequate benefit.14 Other drugs such as endothelin blockers (BQ123) and N-acetylcysteine seem to be promising, but larger uncontrolled as well as controlled studies are needed to confirm their role in the therapy of HRS.14 Systemic vasoconstrictors with plasma expansion seems to be the best therapy now that several uncontrolled studies have confirmed a beneficial role in HRS. Vasoconstrictors are used because the initial event in the pathogenesis of HRS is an arterial splanchnic vasodilation causing activation of endogenous vasoconstrictors systems.
Vasoconstrictors used for HRS include vasopressin analogues (ornipressin and terlipressin), somatostatin analogues (octreotide), and catecholamines (midodrine and noradrenaline). Vasopressin analogues have a marked vasoconstrictor effect in the splanchnic circulation and have been used for many years in the management of acute variceal bleeding in cirrhotic patients. The most studied vasopressin analogue in HRS is terlipressin. The administration of terlipressin and albumin is associated with a significant improvement of GFR and normalization of serum creatinine in approximately 2/3 of patients with cirrhosis and HRS.15-22 Although one of the initial concerns about using terlipressin was the development of ischemia (heart or extremities), this has not been the case. There is a low incidence of ischemic side-effects (approximately less than 5%) as demonstrated by several studies that now pool more than 150 patients.15-22 The administration of midodrine, an alpha-adrenergic agonist in association with octreotide, an inhibitor of the release of glucagon and other vasodilator peptides, and albumin also improves renal function in cirrhotic patients with HRS although information about this therapeutic approach is still very limited.23 Finally, the administration of noradreline in association with intravenous albumin resulted in a significant improvement of renal function in a small group of cirrhotic patients with type 1 HRS.24 In all studies recurrence of HRS after stopping therapy in patients showing a normalization of serum creatinine was uncommon.
One of the primary goals of phamacological therapy is that of succesfully reversing renal failure so that suitable liver transplant candidates can undergo transplantation with less morbidity and have similar survival to patients without HRS. Non-transplant candidates may also benefit from such therapy by reducing morbidity and mortality. In two studies, patients that responded to therapy of HRS (decrease of creatinine to < 1.5 mg/dL) with terlipressin and albumin had an increased survival compared to those that did not respond to this therapy.16,17 The recommended doses and duration of vasoconstrictor therapy are summarized in table V.
Recommendations for using vasoconstrictors in type 1 hepatorenal syndrome.
| 1. Duration of therapy: between 5-15 days |
| 2. Goal of therapy: reduction of serum creatinine below 1.5 mg/dL. |
| 3. Recommended drugs and doses are: |
| A.Terlipressin 0.5 mg intravenously every 4 hours; can increase dose in a stepwise fashion (i.e. every 2-3 days) to 1 mg/4h and then up to 2 mg/4h in cases showing no decrease in creatinine. |
| B.Midodrine 7.5 mg orally three times daily with an increase to 12.5 mg three times daily if needed and octreotide 100 μg subcutaneously three times daily with an increase to 200 μg three times daily if needed. |
| C.Noradrelanine 0.5-3 mg/hr continuos intravenous infusion. |
| 4. Concomitant intravenous albumin infusion (1 g/kg on the first day, followed by 20-40 g/day) should be administered to all patients. |
| 5. These drugs should be avoided in patients with cardiac diseases, peripheral vascular disease, and/or cerebrovascular disease, due to the potential risk of ischemic events. |
TIPS is a non-surgical method of portal decompression used as an alternative therapy for cirrhotic patients bleeding from esophageal or gastric varices that are refractory to endoscopic and medical treatment. TIPS by reducing portal pressure returns some of the volume of blood pooled in the splanchnic circulation to the systemic circulation. This event supresses RAAS and SNS activity and ameliorates their vasoconstrictor effect on the renal circulation. Small studies indicate that TIPS may improve renal function and GFR as well as reduce the activity of RAAS and SNS in cirrhotics with type 1 HRS. However, effects on renal function and the clinical course of patients after TIPS insertion is variable; some have a delayed response and others actually do worse. Experience from a large series of cirrhotic patients undergoing TIPS for refractory ascites indicate that those with hepatic encephalopathy, liver failure and severe coagulopathy are prone to more complications.25 Although uncontrolled studies suggest that TIPS improves prognosis in patients with type 1 and 2 HRS,26 the impact of this therapy on patient survival remains to be assessed.
DialysisSmall and uncontrolled studies using hemodialysis and peritoneal dialysis suggest that both are ineffective because of a high incidence severe side effects, including arterial hypotension, coagulopathy and gastrointestinal bleeding and increased mortality. In some centers, hemodialysis is used to treat patients with HRS awaiting for liver transplantation. The effectiveness of dialysis in this setting has not been appropriately studied. Continuous arterio- venous or veno-venous hemofiltration have also been used but their efficacy remains to be determined. The beneficial effect of an extracorporeal albumin dialysis system (MARS), which consists of a modified dialysis method that enables the selective removal of albumin bound substances that accumulate in liver failure by the use of an albumin containing dialysate has been recently reported in patients with HRS.27 Although promising, these results require further evaluation in order to consider dialysis as a therapy, or more importantly as a bridge to liver transplantation in patients with HRS.
Liver transplantationLiver transplantation is the best treatment for selected patients with HRS, as it offers a cure to both the diseased liver and the circulatory and renal dysfunction. The longterm outcome of cirrhotic patients with HRS treated by liver transplantation is usually good, although the presence of HRS is associated with increased morbidity and early mortality after transplantation.28 After transplantation, a further impairment in renal function may be observed in some patients that will require hemodialysis during their postoperative stage. Nonetheless only 5% will need long-term hemodialysis. Because cyclosporine and FK506 treatment may contribute to this degree of renal impairment postoperatively, other drugs such as azathioprine, steroids, IL-2 receptor antagonists or anti-lymphocyte agents should preferably be used until diuresis and improvement of renal function is observed, usually in 48 to 72 hours after transplantation. After this initial impairment, GFR starts to improve and reaches an average of 40 to 50 mL/min by the sixth week after the surgery. Aside from renal impairment patients develop more complications, spend more days in the intensive care unit and in the hospital, and have a higher in-hospital mortality rate than patients without HRS. Therefore succesfull pharmacological therapy of HRS before transplantation may prevent and decrease the high incidence of complications these patients experience after the surgery. Despite this increased morbidity, long-term survival of transplanted patients with HRS is not markedly different from that of cirrhotic patients without HRS; the probability of survival after 2-3 years of transplantation is approximately 60-65%28,29(Figure 4).
Survival rates of patients with and without HRS following liver transplantation. (From Gonwa TA, Morris CA, Goldstein RM, et al. Long-term survival and renal function following liver transplantation in patients with and without hepatorenal syndrome-experience in 300 patients. Transplantation 1991; 51:428-430, with permission).
HRS can be prevented in two clinical settings. First, in patients with SBP the administration of albumin (1.5 g/kg at diagnosis of infection and 1 g/kg 48 hours later) prevents the circulatory dysfunction and subsequent development of HRS.30 The incidence of HRS in patients with SBP receiving albumin together with antibiotic therapy is of 10%, compared with an incidence of 30% in patients not receiving albumin. Most importantly, the administration of albumin improves survival in these patients. Second, in patients with acute alcoholic hepatitis the administration of pentoxifylline, an inhibitor of tumor necrosis factor, (400 mg tid orally for 28 days) also prevents the development of HRS and improves survival in this setting.31