Background. Combination therapy with terlipressin and albumin substitution is considered a widely accepted treatment regimen for patients with hepatorenal syndrome (HRS). However, only half of the patients respond to treatment and to date albumin substitution and terlipressin therapy are among the most expensive medical treatments available for patients with liver diseases. Thus, we aimed to identify clinical and etiological parameters to predict treatment response and overall mortality in patients with HRS.
Material and methods. We retrospectively evaluated 21 patients, 13 male/8 female, aged 43-72 years with HRS. Four patients were transplanted after following combination treatment. Terlipressin was administered by continuous intravenous perfusion (2–6 mg/d) and albumin drips (50 mg) were given daily. Treatment response was defined by a decrease in serum creatinine level to < 1.5 mg/dL or by a > 50% reduction of the baseline concentration.
Results. 57% of the patients responded to treatment, which was associated with improved survival at day 60, compared to non-responders. However, the overall mortality was not different between the two groups. Median age of 63 years was a significant negative predictor for therapy response. High baseline urinary sodium levels were of prognostic value for survival. The Model of End stage Liver Disease score (MELD score) did not correlate with therapy response.
Conclusion. In conclusion high age is a predictor of non-response. Low urinary sodium before treatment is associated with poor survival. Terli-pressin and albumin co-treatment is associated with increased two-months survival rate. This seemingly moderate extension in survival rate can, however, be decisive for obtaining liver transplantation.
Hepatorenal syndrome (HRS) represents one of the most serious and life-threatening complications of end-stage liver disease. HRS is defined by a rapid progressive decline in renal function in the absence of other triggers such as obstruction or parenchymal renal disease, bacterial infection, circulatory shock, severe hypovolemia, or nephrotoxic drugs.1 HRS does not respond to two days of diuretic withdrawal and adequate plasma volume expansion with albumin.1–6 HRS Type 1 (HRS1) is characterized by a precipitous decline in renal function with increased serum creatinine (> 2.5 mg/dL; > 220 µmol/L) or a 50% decrease in initial 24-h creatinine clearance within two weeks. HRS Type 2 (HRS2) is a more gradual, chronic decline in renal function with a modest increase in serum creatinine (> 1.5 mg/dL;132 µmol/L) and has a more favourable prognosis of 70% three-months survival, compared to HRS1.2
The key components of the profound circulatory dysfunction associated with HRS in cirrhosis are significant splanchnic vasodilatation and reduced effective arterial blood volume7 which results in activation of neurohormonal compensatory mechanisms.8,9 This activation can ultimately lead to intensive renal vasoconstriction with critical decrease in renal blood flow and profoundly reduced glomerular filtration rate.10 Current therapeutic approaches focus on increasing renal perfusion by counteracting the splanchnic vasodilatation with vasoconstrictors and by increasing the total plasma volume.
Various types of vasoconstrictors have been applied including vasopressin derivates (terlipressin and ornipressin), somatostatin analogs (octreotide) and alpha-1 adrenergic receptor agonists (midodrine, norepinephrine).11 While terlipressin (triglycyl-lysine-vasopressin) is the most common vasoconstrictor in Europe and Asia for treatment of HRS1, the so-called triple therapy, a combination of octreotide, midodrine and albumin is the prevalent treatment of HRS in the USA.7 The synthetic vasopressin analogon terlipressin, which acts via the vasopressin V1 receptor as a systemic vasoconstrictor and is routinely applied in combination with albumin, effectively increases arterial blood volume and renal blood flow.12 Both strategies have been shown to reverse the effects of HRS, but to date, there is no established clinical evidence that either treatment was preferential. We followed the current guidelines from the European Association for the Study of Liver Disease that recommended the administration of terlipressin in combination with albumin as the first line therapy.13
Terlipressin has been proven in clinical studies to be beneficial in reversing the deleterious effects of HRS.13–21 Due to high cost and serious adverse effects in some patients it is necessary to evaluate predictors for therapy response. Although various predictors have been observed in clinical studies (e.g. mean arterial pressure,21 baseline serum creatine,22 MELD score,22,23 baseline bilirubin21) accurate predictors for response and survival are still under debate.
In the present study we checked some of the previously reported predictors with regard to their predictive power for our patient cohort, but also evaluated other possible predictive factors such as age and baseline urinary concentration. This procedure might be instrumental in the identification of cases with high chance for response and/or useful to estimate survival.
Material and MethodsStudy populationThe present study group comprised 21 patients, 13 male and 8 female, with a median age of 58 years (43–72) meeting the criteria of HRS proposed by the International Ascites Club.4,5 The predominant etiologies of cirrhosis were alcoholic (n = 12) and nonalcoholic steatohepatitis (NASH) (n = 5). There were single cases of virus-induced, drug-induced, biliary cirrhosis as well as one cirrhotic patient with unknown etiology. The median MELD score was 25 (10-33). Further relevant clinical baseline parameters are summarized in table 1.
Baseline characteristics of study population: demographic, etiological and clinical parameters, outcome of the terlipressin plus albumin treatment.
| Median | Minimum | Maximum | |
|---|---|---|---|
| Age (years) | 58 | 43 | 72 |
| Sex (male/female) | 13 / 8 | ||
| Height (m) | 1.70 | 1.46 | 1.98 |
| Bodyweight (kg) | 78 | 45 | 121 |
| BMI (kg/m2) | 24 | 17 | 46 |
| Etiology of cirrhosis (alcohol/NASH/virus/toxic/biliary/others) | 12/5/1/1/1/1 | - | - |
| Arterial hypertension (yes/no) | 8/13 | - | - |
| Diabetes mellitus | 9/12 | - | - |
| Follow-up (d) | 103 | 10 | 472 |
| Response to therapy (yes/no) | 12/9 | - | - |
| Death (yes/no) | 16/5 | - | - |
| Intensive care unit (yes/no) | 7/14 | - | - |
| Creatinine (mg/dL) | 2.67 | 1.62 | 6.33 |
| GFR (mL/min/1.73 m2) | 22 | 10 | 46 |
| Serum albumin (g/dL) | 2.9 | 2.0 | 6.6 |
| Serum total protein (g/dL) | 6.45 | 2.40 | 7.20 |
| Urine sodium (mmol/L) | 10 | 2 | 54 |
| AST (U/L) | 64 | 21 | 353 |
| ALT (U/L) | 44 | 19 | 149 |
| AP (U/L) | 158 | 82 | 510 |
| GGT (U/L) | 168 | 37 | 621 |
| GLDH (U/L) | 4 | 1 | 41 |
| LDH (U/L) | 247 | 132 | 472 |
| Bilirubin (mg/dL) | 3.0 | 0.8 | 35.1 |
| INR | 1.34 | 0.95 | 1.90 |
| MELD | 25 | 10 | 33 |
| Serum sodium (mmol/L) | 135 | 125 | 141 |
Terlipressin was routinely administered as a continuous intravenous infusion at a dose of 2 to 6 mg/ day. We followed a goal-directed regimen adjusted by renal function recovery of the individual patient. Therapy response was defined as decrease in serum creatinine level to ≤ 1.5 mg/dL or by a ≥ 50% reduction of the baseline concentration. Therefore, terlipressin doses were modified slightly according to changes in relevant parameters such as serum creatinine. Diagnosis of cirrhosis was biopsy proven or relied on clinical, ultrasonic and endoscopic data. Severity of liver function impairment was assessed by MELD score.
All procedures adhered to the Declaration of Helsinki (updated 2008) and were approved by the local ethics committee of the University Hospital Essen.
Statistical analysisNumerical values of the determined parameters are given as median values and range. Evaluation of predictors for response to therapy or prognosis of survival was performed by univariate analyses employing Mann-Whitney U tests. Survival curves were generated using the Kaplan-Meier method with censoring for orthotopic liver transplantation (OLT). Statistical analyses were done using Graph Pad Prism sofware (GraphPad 4.0 software, San Diego, CA, USA) or SPSS software release version 19 (SPSS, Chicago, IL, USA).
ResultsPatients' baseline characteristicsAll patients suffered from severe liver failure, as indicated by the high median MELD score, and severe renal impairment with low glomerular filtration rates and high serum creatinine levels. The median follow up time was 104 days. There was no significant gender-specific difference in age (Figure 1A) and body mass index (BMI) (Figure 1B), although the range was considerably wider for females. The median BMI of the whole study population was 24 (17-46). The MELD score was not distributed symmetrically around the median of 25 but rather resembled a bimodal distribution of lower scores clustering around 17 and higher scores around 30 (Figure 1C).
Baseline characteristics of the study cohort. Comparison of age (A) and BMI (B) distribution between male and female did not exhibit significant differences, though the BMI range was greater in females. The median BMI value of the study population was 24 (17–46). MELD distribution in the patient cohort (C).
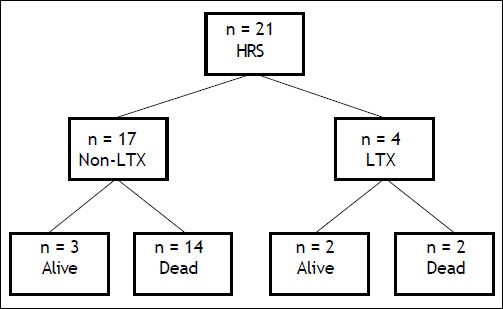
Fifty seven percent of the patients did respond to therapy, as indicated by creatinine decrease (Figure 2). The proportion of responders to non-responders was similar to what has been reported in previous studies.21 Notably, survival of responders at day 60 was improved compared to that of non-responders (60% vs. 45%). However, the overall mortality was not significantly different between the two groups (9/12 responders, 7/9 non-responders deceased). Four patients received OLT of whom 2 died, while 14 of the 17 non-transplanted patients died (Figure 3).
Serum creatinine levels during terlipressin plus albumin treatment. Responders are shown as solid line and non-responders as dashed line. 43% (n = 9) were non-responders, 57% (n = 12) of the patients responded to therapy. Significantly lower serum creatinine values were observed in responders at days 5, 8 and 11. For calculation of significances Mann-Whitney-test was applied (*p<0.05).
Schematic depiction of 400d outcome. In the present study 4 of the 21 patients received liver transplantation, with 2 deaths during the observation period (50% survival). Of the 17 non-transplanted patients 3 stayed alive (18.8% survival). HRS: hepatorenal syndrome. LTX: liver transplantation.
Several baseline variables were analysed for prediction of response to treatment or of survival of HRS patients. Clearly, median age of 63 years was associated with non-response (p < 0.05) (Figure 4A). Figure 4C provides a graphical summary of 6 relevant clinical parameters (AST, ALT, AP, γGT, GLDH and LDH) regarding their response to therapy. None of them revealed prognostic power. Interestingly, initial γGT and AP activities were higher in non-responders. However, statistical analysis revealed no statistically significant differences in yGT and AP activities between the groups (p = 0.07; 0.16 respectively). Also bilirubin showed no prognostic value in our cohort (p = 0.0508). Notably, baseline creatinine did not exhibit a predictive quality (p = 0.483) as shown in figure 4E.
Predictive value of various parameters for response to treatment. A. Older patients showed significantly lower response to treatment. Patients who responded to treatment had a median age of 52 years whereas non-responders had a median age of 63 years. B. High initial urinary sodium concentration was a significant predictor (p < 0.05) of survival. C. Various serum markers of liver damage and function (AST, ALT, AP, GGT, GLDH, and LDH) did not exhibit significant predictive value for response to terlipressin plus albumin therapy. D. The MELD score did not show predictive power for response to treatment. E. Serum creatinine before treatment was also no predictor for response to treatment. For calculation of significances Mann-Whitneytest was applied.
The MELD model is well established to estimate 3-month mortality in patients with liver cirrhosis.24 Therefore we checked whether the initial MELD does predict response to therapy. There was no significant difference in the initial MELD score between responders and non-responders (Figure 4D).
Does urine sodium concentration before treatment predict survival in HRS patients?Cirrhosis results in splanchnic arterial vasodilatation, which is associated with sodium retention and activation of the neurohormonal system. Therefore, we investigated whether urine sodium before therapy serves as a prognostic factor for survival or therapy response, using spot urine for concentration measurement. Remarkably, a significantly lower mortality rate was found among patients who exhibited high urinary sodium levels prior to treatment (Figure 4B).
Does the treatment influence short-term and long-term survival in patients with HRS?The only curative treatment in HRS is OLT. However, the survival of these patients strongly depends on the waiting time before OLT. If HRS is successfully reversed prior to OLT, patient survival is similar to non-HRS-patients.25 The overall probability for survival was independent of the terlipressin and albumin treatment. However, the 60-day survival chance was higher for treatment responders (60-day survival 60% vs. non-responders 45% (Figures 5A-5B).
Overall survival and survival in response to treatment. A. Cumulative survival over 400 days. B. Responders and non-responders did not show significant differences in overall survival. However, patients with response to treatment showed a slightly higher survival from day 60 to 90 and therefore had a higher probability to get an organ assigned for liver transplantation. For calculation of significances Log-Rank-test was applied.
HRS1 has traditionally been considered to be a harbinger of death and OLT the only viable hope for survival for afflicted individuals.16,26,27 Although OLT is considered the best treatment for suitable candidates and should always be the management option of first choice, significant progress has been made in bridging the time between the occurrence of HRS1 and the potentially curative OLT.
Systemic treatment with vasoconstrictors, such as terlipressin, midodrine, octreotide in combination with albumin significantly reduces the mortality rate in individuals awaiting OLT in times of organ shortage.28 Additionally, the presence of HRS1 has been reported to be associated with increased morbidity and early mortality after transplantation,29 although there appears to be some ambiguity in the quantification of survival advantages. Sanyal, et al.16 and Martín-Llahí, et al.17 observed no improvement in overall survival, however, in a more recent systematic review a prolongation of 15-day survival in patients with HRS1 was reported.30 The results of our study confirm a missing effect on overall survival. But when patients responded to the treatment, their 60-day survival is 15% higher compared to that of non-responders. This seemingly marginal bridging advantage represents a 15% higher chance to obtain a curative OLT. Our findings may be limited by the relatively moderate cohort size and the critical situation present in most patients with HRS1. It is, however, important enough to warrant more detailed analyses of a larger number of patients to identify more exactly, which patients might profit from a terlipressin and albumin co-treatment.
The administration of terlipressin was done by continuous intravenous infusion,31 and albumin drips (50 mg) were given daily. This procedure has recently been favoured over the application of boluses, as it appeared to be better tolerated by the patients and seemed to require lower concentrations for the same efficacy.32
Several studies reported sporadic adverse side effects to be associated with terlipressin application.33–38 However, a recent meta-analysis of randomized, controlled trials by Sagi, et al. comprising 223 patients with HRS1 supported the overall positive safety profile of terlipressin. In only 6.8% of the patients severe side effects occurred.39 It is noteworthy to state that none of the major possible side effects occurred in our cohort despite the severity of the clinical condition as indicated by the high mean MELD score. A similar lack of adverse effects was also reported by Alessandria, et al.18 and by Uriz, et al.40
Another interesting observation of our study was that high urinary sodium levels before treatment were associated with a decreased mortality rate. This observation is surprising at first sight, as other clinical parameters have gained priority in characterizing end stage liver diseases. Among these are MELD score, serum creatinine and bilirubin levels. For example, Alessandria, et al.23 reported that only the clinical type of HRS and the MELD score exhibited prognostic value. Patients with HRS1 had a very poor prognosis that was almost independent of MELD score (median survival 1 month). Boyer, et al.22 reported a predictive value for MELD score and baseline creatinine in univariate analysis on HRS reversal and survival chances. Though, in multivariate analysis only serum creatinine remained as independent predictor.22 In part the results presented here are in line with these findings, as neither serum bilirubin nor MELD constituted independent predictors of survival. However, we did not observe the predictive value of creatinine in our study population as indicated by a p value of 0.483. Further analyses of larger cohorts may resolve, wether creatinine or maybe additional novel markers of kidney injury play a prognostic role in this setting. Interestingly, high baseline urinary sodium concentration was the single factor with prognostic relevance in this patient cohort.
Actually it has long been known that the most uniform urinary finding of HRS patients is a strikingly low sodium concentration41 combined with high urinary osmolarity due to preserved tubular function and activation of tubular reabsorption of sodium.42 However, no conclusions concerning predictive value have been based on these clinical data. Our results suggest urine sodium as a prognostic factor for survival in HRS. This finding is novel and possibly important, as it involves an easily accessible clinical parameter and re-incorporates well known urinary characteristics of HRS patients. Therefore its predictive power could be tested with little effort in further studies comprising larger patient cohorts.
A possible rationale for the positive influence of high initial urinary sodium levels could be the following. As arterial vasodilation, especially in the splanchnic bed, leads to a decrease in the effective arterial blood volume with subsequent activation of renal sodium-retentive mechanisms and intrarenal arterial vasoconstriction, a high pre-treatment urinary sodium level could be counteractive to the resulting sodium depletion. Alternatively, higher levels of urinary sodium could reflect a less progressed deleterious HRS cascade.
In the present study we could identify patients' age as the only significant factor determining response to treatment. Median age of non-responders was 63, while responders had a median age of 52, suggesting a cutoff for responsiveness around an age of 60y. This overruling importance of patient age has been mentioned before. Indeed, Testro, et al.19 report two predictors of renal function improvement on terlipressin treatment: type 1 HRS and age. In their study, the mean age of the 59.4% responders to therapy was 51.7 ± 10.5 years, whereas the mean age of the non-responders was 56.9 ± 9.1 years. Both, percentage of responders and mean age of responders are comparable to the present study.
In summary, combined therapy with terlipressin and albumin gives a small survival advantage for responders of about 2 months after start of treatment, while overall survival is not improved. The increased survival chance at day 60 is important, because it is linked to an increased chance to obtain OLT. High age as negative predictor for response has been confirmed and high urinary sodium as a new positive predictor for survival has been identified.
It is evident that the present data have to be viewed in the light of a limited patient cohort, which could introduce a bias towards individual pathologies. Therefore, more direct large scale comparisons of the efficacy of the different strategies and the predictive power of the parameters identified in the present study are highly desirable and necessary.
Abbreviations- •
HRS1: hepatorenal syndrome type 1.
- •
HRS2: hepatorenal syndrome type 2.
- •
MELD score: Model of End stage Liver Disease score.
- •
OLT: orthotopic liver transplantation.
- •
BMI: body mass index.
- •
AST: aspartate amino transferase.
- •
ALT: alanine amino transferase.
- •
AP: alkaline phosphatase.
- •
γGT: gamma glutamyl transferase.
- •
GLDH: glutamate dehydrogenase.
- •
LDH: lactate dehydrogenase.
The authors declare no conflict of interest.
Financial SupportThis work was supported by the Deutsche Forschungsgemeinschaft (DFG grants CA 267/6-1 & CA 267/8-1) and the Wilhelm Laupitz Foundation to A.C.