Introduction. The treatment of brain dead donors with combined hormonal resuscitation protocols, including methylprednisolone (MP) and triiodothyronine (T3), among others, was developed to increase the viability and function of transplanted organs, primarily heart and lung. Even when it has regarded successful results in term of donors and organs recovery, its effects over specific parameters in organs like the liver are unknown.
Material and methods. Male Sprague-Dawley rats were pretreated with MP (0.34 mg/kg) and/or T3 (0.05 mg/kg) or their vehicles, and then subjected to partial hepatectomy of 70%. Three experimental groups and their respective controls were conformed: a. T3; b. NaOH; c. MP; d. vMP; e. MP+T3 and f. vMP+NaOH. The groups were evaluated at 0, 16, 24, 72 and 120 h post surgery. The effects of this protocol on regeneration, liver mass recovery, liver injury, oxidative stress and liver function were analyzed.
Results. MP+T3 pretreatment does not deleteriously affect liver regeneration after partial hepatectomy, as shown in the curve of total mass recovery, Ki67 staining and mitosis counting, and does not alter liver function. In addition, the treatment modestly decreases oxidative stress and liver injury, as evidenced by transaminases levels, histological analysis and oxidized proteins content.
Conclusion. These preclinical results indicate that MP+T3 is harmless for liver tissue regeneration post hepatectomy and additionally exhibits anti-inflammatory and antioxidant effects; therefore, it would not be contraindicated for the treatment of multiorgan donors in brain death and particularly, if the occurrence of small for size syndrome is suspected.
Success of organ transplantation is dependent on several factors, among them, organ quality. Brain death (BD) is the condition of most donors during transplantation, and is accompanied by hemodyna-mic, hormonal and inflammatory alterations, which can irreversibly damage organ function.1 To minimize the occurrence of these events, several strategies have been developed; one of them, hormonal resuscitation, is becoming more frequently used and has proven to increase the quality of extracted organs, as well as recovery of donors and grafts.2
Hormonal therapy, also known as T4 protocol or hormonal resuscitation protocol, may include the use of thyroid hormones, vasopressin, insulin and corticoids. It was initially applied by Rosendale and Novitzky’s group with successful results,2 particularly in cardiac function;3 however, its effect over specific parameters in other organs, like liver and kidney, is poorly studied.
The incidence of primary graft nonfunction (PNF) is related to ischemia-reperfusion liver injury, organ quality and donor risk index.4 The incidence of PNF in hormonal treated donors has not been published and some authors suggest it may be higher.5 Results have been communicated regarding the role of hormonal therapy and the detrimental response of steatotic liver to ischemia-reperfusion injury in experimental models;5 nevertheless, the effect of such protocols in normal liver response to injury has not been reported.
Organ transplantation is a complex procedure and the potential damage of the organ is due to preservation, cold and warm ischemia, reperfusion, and the surgery per se. It is of the outmost importance for the liver to preserve the proliferation capacity in order to recover tissue damage.6 Furthermore, in the case of BD donors where the liver does not match the receptor size, regeneration is vital to meet the “small for size” syndrome.7
Liver regeneration is an evolutionary conserved process that involves the interaction of liver cells, signaling pathways, paracrine signals, and influence from other organs. In humans, liver regeneration occurs when recovery of lost hepatocytes is needed, for example after liver damage from hepatitis, liver failure, ischemia-reperfusion, partial hepatectomy (PHx) and transplantation, among others.6 One factor that greatly influences outcome after transplantation is liver capacity to regenerate after transplant, which is modified by several conditions, including extension of ischemic injury, graft size, immunosup-pression, steatosis, donor age and viral hepatitis.8
Our aim was to study the effect of the combined administration of triiodothyronine and methylpredni-solone over liver regeneration after partial hepatec-tomy, considering this capacity as an important factor for the outcome after liver transplantation.
Material and MethodsAnimals and treatmentsMale Sprague-Dawley rats (Animal Facility, Faculty of Medicine, University of Chile) weighing 180–200 g, housed on a 12–h light/dark cycle and provided with rat chow and water ad libitum, were used throughout the experiments. Triiodothyronine (T3, 0.05 mg/kg, Sigma-Aldrich, USA) and/or methylprednisolone (MP, 34 mg/kg, Solu-medrol, Pfizer, Switzerland) were administered 2 and 3 h before surgery, respectively. Control animals received an equivalent volume of T3 vehicle (NaOH 0.1 N) and/or MP vehicle (provided by the manufacturer).
Partial hepatectomyAnimals were anesthetized with 2% isoflurane and subjected to partial hepatectomy: briefly, median, right central and left lateral lobes, which constitute 70% of the total liver, were separately ligated, resected and removed under microscope.9 Control (sham surgery) consisted in abdominal wall opening, cavity exposure and closure. Resected liver was weighed and frozen in liquid nitrogen. Recovery was allowed in a controlled environment, buprenorphine hydrochloride 0.01 mg/kg (temgesic, 324 mcg/mL, Essex Pharma, Munchen, Germany) was administered, and daily monitoring including body weight and clinical condition was assessed until harvesting time.
Liver harvesting was performed in anesthetized animals (0.2 mL Zoletil®, Tiletilamine Clorhidrate 50 mg/kg, Zolazepam Clorhidrate 50 mg/kg, Virbac, France). Remnant liver was weighed and blood samples taken, centrifuged, and serum storage at −20 °C. Tissue samples were formalin-fixed and paraffin included or frozen in liquid nitrogen and storage at -80°C for further determinations.
Experimental animal protocols and procedures complied with the Guide for the Care and Use of Laboratory Animals and approved by local authorities (Faculty of Medicine, University of Chile, CBA No. 0274 FMUCH).
Liver proliferation parametersRestoration of liver mass after PHx was determined as percentage of liver mass recovered and calculated using the ratio of liver weight/body weight of the animal after PHx. Weight of remnant liver after PHx (29.06% of total weight) was estimated with 4 animals euthanized for this purpose.
Mitotic figures were counted in HE stained slides, in 30 high power fields (HPF, 400X), and expressed as the number of mitosis per HPF.
Immunohistochemistry studiesStaining for Ki-67 and NF-κΒ was performed after deparaffination, rehydration, and antigen retrieval in citrate buffer pH 6.1 (10 mM Sodium Citrate, 0.05% Tween-20) for 30 min at 95 °C, endogenous biotin was blocked with Biotin Blocking System solution (Dako, CA, USA).
The anti-Ki67 staining was performed according to manufacturer’s instructions (Dako, CA, USA). Positive Ki67 nuclei of hepatocytes were counted under light microscope in 10 adjacent HPF per slide. This parameter was expressed as N° of positive cells per HPF.
NF-κΒ p65 Polyclonal antibody (Thermo Scientific, IL, USA) was used according to the manufacturer’s instruction. A positive control of liver submitted to ischemia reperfusion, with known activation of NF-κΒ10 and a negative control without the primary antibody were included. Analysis was performed under light microscope in 10 adjacent HPF per slide.
Hepatocytes areaHepatocytes area (size in μm2) was assessed with Mi-crometricsTM software in hematoxylin-eosin stained slides, and observed under light microscope at higher magnification (400 x) in at least five adjacent fields.
Liver injury assessment
Aspartate amino transferase (AST) and alanine amino transferase (ALT) activities were measured with commercial kits (Valtek Diagnostics, Santiago, Chile) and expressed as U/L serum.
Liver tissue samples for histology were fixed in formalin, paraffin included, cut and stained with hematoxiline-eosin (HE). Histological evaluation was performed in a blind fashion, recording the parameters liver architecture, necrosis, inflammation and steatosis
Oxidative protein damageOxidized proteins content was determined in frozen tissue, treated with 2, 4 dinitrophenylhydrazine to form a Schiff base. Production of the corresponding hydrazone was measured spectrophotometrically between 350 and 390 nm to determine concentration of carbonyls, and at 280 nm to determine total protein concentration.11 Values were expressed as nmol carbonyls/mg protein.
Liver functionSerum Albumin quantification was carried out with Rat Albumin ELISA Kit (Abcam, Cambridge, USA) following the supplier’s instructions. Values were expressed as mg/dL.
RT-PCR studies for inflammation induced proteins- •
TNF-α and IL-6.
Liver mRNA was extracted with a kit (EZNA® Tissue RNA Kit, Omega Bio-tek, USA) using supplier’s instructions, total extracted RNA was quantified spectrophotometrically (260/280 nm) and purity and integrity confirmed. cDNA was synthesized with Super Script II® system (Invitrogen Life Technologies Inc., CA, USA) with hexanucleotides as random primers (Random Primer). Obtained cDNA was used in chain reaction (PCR) using Go-Taq® System (Promega, Madison, USA), and specific primers for genes 18S (F 5’-GGA CAT CTA AGG GCA TCA CA-3’) TNF (tumor necrosis factor)-α (F 5’-AAA TGG GCT CCC TCT ATC AGT TC-3’), IL (interleukin)-6 (F 5’-TCC TAC CCC AAC TTC CAA TGC TC-3’), in a Biometra TPersonal thermocycler (Goettingen, Germany). Each PCR product (250 bp) was loaded in 0.8% agarose gel. Electrophoresis was performed for 20 min at 80 Volts, visualizing the samples by staining with ethidium bromide. 18 s was used as a control of 350 bp. Bands were analyzed by densitometry using the Scion Image program. Quantification was conducted according to the relationship IL-6/18 s, TNF-α/18s as appropriate and expressed as arbitrary densitometric units (DU).
Statistical analysisStatistical analysis was performed with Graphpad Prism 4 software (CA, USA). Values shown represent the mean ± SD or SEM for the number of separate experiments indicated; one-way ANOVA and the Newman-Keuls test assessed the statistical significance of differences between mean values. P < 0.05 was considered significant.
ResultsRecovery rate of liver mass, estimated as percentage of liver weight to body weight of the animal, was measured 16, 24, 72 and 120 h after the PHx (Figure 1). In control conditions, liver weight (LW) corresponds to 3.8% of rat body weight (BW), ratio indicated with dotted line. In panel A, a continuous increase in the recovery of liver mass through hours was observed in all groups, which reached normal LW/BW ratio after 120 h post PHX. In panels B to E the detail of LW/BW for each time is shown: at 16 hours post PHx (Figure 1B), the group pretreated with MP + T3 showed a higher LW/BW ratio (2.135 ± 0.019%) compared to groups pretreated with T3 and MP, while at 24 h (Figure 1C) no significant differences between groups were observed. At 72 h post PHx (Figure 1D), the group pretreated with T3 alone showed a significantly decreased LW/BW ratio (2.758 ± 0.063%) compared to its vehicle and groups treated with MP alone (3.435 ± 0.180%) or combined treatment MP + T3 (3.313 ± 0.113%).
Liver mass recovery after partial hepatectomy in animals receiving combined hormonal pretreatment. Progression of liver weight recovery, as liver weight to body weight ratio, after partial hepatectomy (A), and detail at 16 (B), 24 (C), 72 (D) and 120 h (E) after PHx. Results are shown in percentage of liver weight to body weight ratio (LW/BW). Dotted line indicates normal LW/ BW = 3.8%. Each bar represents the mean ± SEM (n = 4). The letters on each bar represent significant differences between the groups (p < 0.05, one-factor ANOVA and Newman Keuls test).
At basal conditions and 16h after PHx scarce mi-totic figures were observed. Significant changes in mitosis counting in groups T3 vs. NaOH were observed at 24 (T3, 1.20 ± 0.51; NaOH, 0.2 ± 0.082) and 72h (T3, 1.55 ± 0.47; NaOH, 0.38 ± 0.21) post PHx. At 72h T3 mitotic counting was also increased significantly compared with the combined treatment group (MP + T3, 0.75 ± 0.13) while at 120h MP + T3 mitotic counting (0.3 ± 0.15) was higher than T3 (0.14 ± 0.11) and MP (0.08 ± 0.1). All results are expressed as mean number of mitosis per HPF ± standard deviation.
Figure 2 shows representative pictures of Ki67 IHC (immunohistochemistry) for the treated groups at 24, 72 and 120 h post PHx and the Ki67 number of positive nuclei (indicated by arrows) per HPF, counted in 10 HPF, as mean ± SD corresponding to each group. At 24 h post PHx an increased number of positive nuclei in all groups compared to their controls, was observed; although values found with MP treatment (Figure 2D) were significantly higher than T3 (Figure 2A) and MP+T3 (Figure 2G). At 72 h post PHx, T3 values (Figure 2B) (33.2 ± 7.4) were higher than its control NaOH (12.45 ± 4.15 positive nuclei/field) and MP group (Figure 2E). At 120 h no changes were observed between treatments or with their respective controls (panels C, F, I). Cell size, expressed as μm2, was analyzed at 24, 72 and 120 h post PHx (Figure 3). T3 treatment increased cell size at all times compared to its control group and MP alone. At 24 and 120 h (Figure 3A and 3C) the cell size of the T3 treated groups was also significantly higher than the group receiving MP + T3. The combined treatment also showed an increased cell size compared to its control and MP alone at 72 h (Figure 3B) post PHx (p < 0.05).
Liver Ki67 immunostaining after partial hepatectomy in animals receiving combined hormonal pretreatment. Representative microphotographs of Ki67 positive cells at 24, 72 and 120 h post PHx. Each photograph is representative of nuclei counting in 10 HPF of n = 3 slides per condition (in parenthesis, mean ± SD of nuclei counting, higher magnification of 400x).
Hepatocytes size changes after partial hepatectomy in animals receiving combined hormonal pretreatment. Cell size (µm2) was measured after 24 (A), 72 (B) and 120 h (C) of PHx. Each bar represents mean ± SEM (n = 3 slides) of area estimated in 10 HPF (400X). Letters on each bar represent significant differences between the groups (p < 0.05, one-factor ANOVA and Newman Keuls test).
Activity of serum transaminases ALT and AST was analyzed 16 and 24 h after PHx (Figure 4). T3 significantly decrease ALT levels (66.63 ± 21.48 U/L), compared with its vehicle (368 ± 66.34 U/L) at 16 h, but not at 24 h post PHx (panel A). ALT levels were decrease significantly by the combined pretreatment (248 ± 20.65 U/L) compared with its vehicle (623 ± 47.85 U/L) and T3 alone (537 ± 106.2) 24h after PHx (panel B). No significant changes were observed in AST levels.
Serum transaminases ALT and AST activities after partial hepatectomy in animals receiving combined hormonal pre-treatment. Serum levels of ALT and AST after 16 (A, C) and 24 h (B, D) of hepatectomy. Each bar represents mean ± SEM (n = 4). Letters on each bar represent significant differences between the groups (p < 0.05, one-factor ANOVA and Newman Keuls test).
Figure 5 shows total content of oxidized proteins 16 and 24 h after PHx. At 16h (panel A), only the combined pretreatment group, MP+T3 (7.56 ± 0.79 nmol carbonyl/mg protein) varied significantly from its control vMP+NaOH (13.69 ± 0,849 nmol car-bonyl/mg protein); while at 24 h after PHx (panel B) T3 pretreatment (2.48 ± 061 nmol carbonyl/mg protein) significantly decrease the value of oxidized proteins compared to NaOH (10.79 ± 1.23 nmoles carbonyl/mg protein).
Liver oxidized proteins content after partial hepatectomy in animals receiving combined hormonal pretreatment. Content of carbonylated proteins after 16 (A) and 24 (B) h of PHx. Each bar represents mean ± SEM (n = 4). Letters on each bar represent significant differences between the groups (p < 0.05, one-factor ANOVA and Newman Keuls test).
Liver arquitecture and presence of necrosis, inflammation foci and steatosis were evaluated per group in 3–4 slides, 24 and 120 h post PHx, and expressed as a mean score of 10 HPF, according to a modified Ishak score12 (Table 1). 24 h post PHx all groups have preserved liver architecture, and presence of mild microvesicular steatosis. Necrosis foci were more frequently observed in NaOH compared with T3, and were absent in MP group, while in combined vs. control treatment the presence of necrosis was similar. Mild to moderate presence of inflammatory foci was observed in T3 and NaOH, while the treatment with MP alone or combined with T3 presented isolated foci of inflammatory infiltrate. At 120h the pretreated groups (T3, MP and MP + T3) have normal liver architecture, without necrosis or inflammatory infiltrate. Persistent, mild micro-vesicular steatosis and slight disorganization, with isolated ballooned hepatocytes was observed in MP and vMP+NaOH groups.
Necrosis, inflammation and steatosis score. The values correspond to the average histological analysis of 3–4 slides 3–4. Reference values: 0 = absent, 1 = mild, 2 = moderate, 3 = severe (modified from Ishak, reference 11).
| Hours after PHx | Treatment | Necrosis | Inflammation | Steatosis |
|---|---|---|---|---|
| 24 | T3 | 0 | 1 | 1.3 |
| NaOH | 2 | 1.5 | 1 | |
| 1 | MP | 0 | 0 | 1 |
| vMP | 1 | 0.2 | 0.5 | |
| MP + T3 | 1 | 0.25 | 1 | |
| vMP + NaOH | 1.25 | 1 | 0.25 | |
| 120 | T3 | 0 | 0 | 0 |
| NaOH | 0 | 0 | 0 | |
| MP | 0 | 0 | 1 | |
| vMP | 0.5 | 0 | 0 | |
| MP + T3 | 0 | 0 | 0 | |
| vMP + NaOH | 1.25 | 1 | 0.25 |
Liver function was not compromised in any of the groups after PHx, as determined by concentration of serum albumin analyzed at 16 and 24 h, which values were within the normal range of 3.8 to 4.8 mg of albumin per dL serum (data not shown).
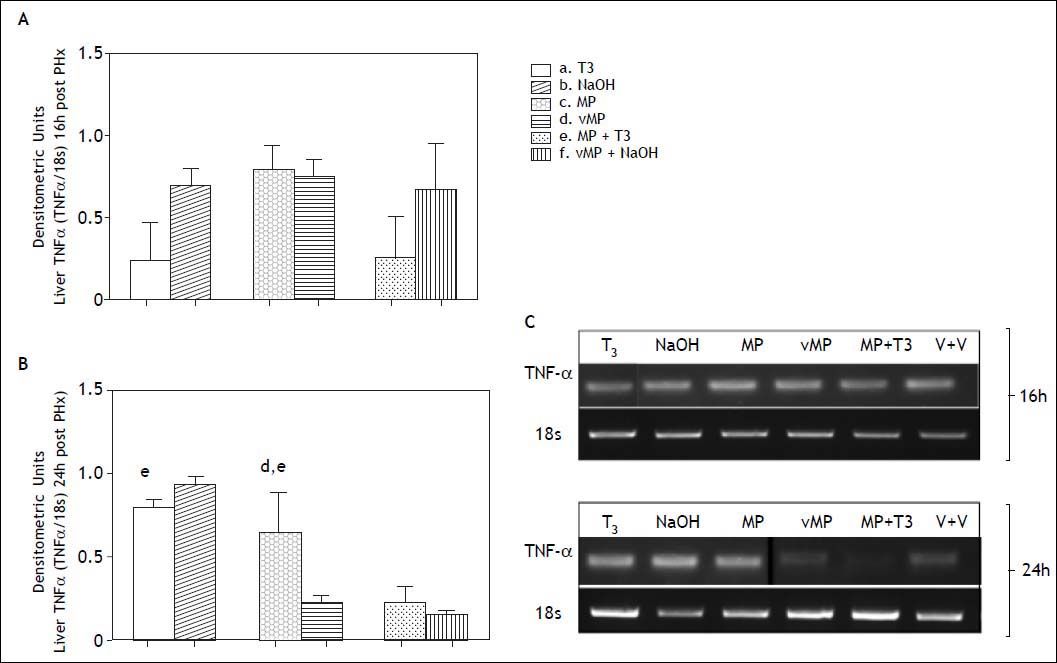
TNF-α expression at 16h was not significantly different in pretreated groups compared with their respective controls (Figure 6, panels A and C). At 24 h post PHx (Figure 6, panels B and C) the combined treatment decreased TNF-α expression (0.23 ± 0,098 DU) compared to T3 (0.8 ± 0.044 DU) and MP (0.65 ± 0.24 DU).
Liver TNF-α expression by RT-PCR after partial hepatectomy in animals receiving combined hormonal pretreatment. Each bar represents the mean ± SEM (n = 4) densitometric units at 16 (A) and 24 (B) h after PHx. The letters on each bar represent significant differences between groups (p < 0.05, one-factor ANOVA and Newman Keuls test). Photographs in (C) show representative bands in agarose gels.
Expression level of IL-6 as densitometric units of IL-6/18s at 16 and 24 h post PHX for each group did not have significant differences between pretreated and control groups or pretreated groups compared between them (data not shown).
NF-κΒ nuclear staining was negative in all groups (Figure 7, panels A to F), showing not significant nuclear translocation at 24 h after partial hepatectomy in any of the groups or controls. The presence of low to moderate cytoplasmic staining is also present in the negative control, therefore, is not specific of cytoplasmic presence of the nuclear factor. NF-κΒ nuclear staining was also performed at 16h with similar results (data not shown).
Liver NF- κB immunostaining after partial hepatec-tomy in animals receiving combined hormonal pretreatment. Representative microphotographs of NF- kB stained slides at 24 h post PHx are shown (panels A to F). Each photograph is representative observation of 5 HPF of n = 3 slides per condition. Positive (panel G) and negative (panel H), are included.
In this experimental study, we tested the effect of a combined hormonal pretreatment composed of me-thylprednisolone and triiodothyronine (MP+T3) over healthy liver regeneration capacity after partial hepatectomy (PHx).
The changes observed in terms of liver mass recovery showed that the treatments, either applied separately or combined, may have an effect in the recovery speed rate, but do not affect the final result, since all groups reached basal values of LW/ BW after 120h of partial hepatectomy (Figure 1). The statistical significance observed between the combined treatment compared with each drug alone at 16h may not be of biological relevance, since no differences were observed compared with the combined vehicle (Figure 1B). At 72h the group treated with T3 had a lower LW/BW index, which may suggest a delay in the process of recovery (Figure 1D); however, all groups reached normal values after 120 h of PHx (Figure 1E). This result was confirmed by Ki67 immunostaining, which showed differences in earlier, but no later times in terms of positive nuclei per field (Figure 2). Mitosis counting showed differences compared to Ki67 results, and no return to basal conditions was observed in any of the groups at 120h; however, the lower number obtained at this point suggest that the return to rest conditions may occurred shortly later. Regardless the differences in the proliferation process progression, hormonal therapy MP+T3, either applied combined or not, does not compromise liver regeneration. The evaluation of earlier points may be of interest to determine if a differential entrance in the cell cycle exists or modifications of the pathways involved in the priming phase occur.
Cell size was significantly increased at all times by T3 pretreatment (Figure 3), which is consistent with literature reports, where T3 induces direct hy-perplasia and hypertrophy.13,14 This factor should be considered within the liver mass increase achieved by the treatment with T3 alone.
Weight recovery (Figure 1), mitosis count, positive Ki67 nuclei (Figure 2) and hepatocytes size (Figure 3) coincide with normal parameters found in the liver regeneration process in healthy animals.15
Hormonal treatment affects normal regeneration: exogenous supplementation of T3 acts as primary mitogen.13,16,17 T3 (at doses of 0.2 mg/kg) stimulates proliferation by accelerating the induction of cyclin D1,17 and also triggers Cdk-2 expression through Küpffer cell-dependent TNF-α secretion, JNK phos-phorylation and AP-1 activation.18 Exogenously applied prior to PHx, T3 shows the same patterns of acceleration of hepatic regeneration.19 The evidence suggests T3 stimulates DNA synthesis by different pathways that ultimately converge with those involved post PHx.20–21
Administration of a high dose of MP prior PHx decreases proliferation of hepatocytes and reduces liver regeneration in adult rats after PHx;22 however, the administration of corticoids pre or post surgery may inhibit overproduction of inflammatory cytoki-nes such as TNF-α, and reduce surgical stress.23,24 Other authors have shown that, in low doses, MP does not change liver mass recovery after PHx,25 and may even act as a factor facilitating the recovery of the impaired liver regeneration after radio-frequency ablation.26 Our results confirm steroids may act as anti inflammatory but not anti proliferating drugs at low doses.
The comparison of both drugs used, T3 and MP, in terms of mass recovery after PHx, has not been done before, the results found suggest a balance of individual effects with the dosage used, which may have its explanation in the equilibrium of cytokines production needed for liver proliferation, and the differential effect of the drugs over these parameters.
As for injury parameters, T3 and MP+T3 treatments decrease ALT serum levels and oxidized proteins content early after PHx (Figures 4 and 5). The protective effect of T3 has been seen in ischemia-re-perfusion liver injury, where it diminishes transami-nases and up to 91% of the total content of oxidized proteins.27 MP treatment in neuronal tissue has shown a protective action, inhibiting lipid peroxidation;28 which may occur in our model. The effects observed with both drugs combined do not suggest addition, synergy or potentiation.
Histological analysis confirms the previous results (Table 1): T3, MP and the combined treatment decrease inflammation and necrosis foci. Microvesicu-lar steatosis was evident in all groups, which is concordant with the transient accumulation of lipid in hepatocytes, characteristic of early regeneration, which may represent an energy source that supports the growth.29 Additionally, no deleterious effects were found in liver function.
TNF-α levels were decreased at 24h after PHx by the combined treatment, while T3 and MP alone groups exhibited higher levels of the cytokine. This may be related with the observation of less inflammatory foci observed in tissue slides, although, it should be interpreted with caution, since TNF-α is also required for the progression of proliferation and activates protective pathways.30 This event will require further research to elucidate the role of MP + T3–triggered TNF-α in the proliferative response.
The expression levels of IL-6 were not significantly different in pretreated and not treated animals, this may be due to an earlier activation of these genes, necessary to trigger the proliferative cycle, characteristically found between 60 minutes and 8 h post PHx.31
The transcription factor NF-kB is essential for liver proliferation. Mice with hepatocyte-specific disruption of the inhibitory complex subunit NEMO (NF-κB essential modifier) have higher mortality after 50% hepatectomy and impaired liver regenera-tion.32 Although NF-κB is related to liver inflammation and carcinogenesis, its activation, induced by TNF-α, is also important in the priming phase of liver regeneration, up to 12 h after PHx, which may explain why we have not observed NF-κB nuclear staining at 16 and 24h after PHx.33 At these points, hormonal treatment did not increase NF-κB nuclear translocation. We hypothesized that this transcription factor dual role in liver proliferation may be related to the time frame of activation, the cell in which it is activated and the level of activation, in a similar way described for free radicals.34 In a different system, gut ischemia-reperfusion, a dual role for NF-κB has also been described.35 Additional studies are needed to test this hypothesis.
In conclusion, we have found preclinical evidence that supports the clinical use of MP+T3 as part of hormonal resuscitation protocols administered to multiorgan donors with healthy livers, and particularly when occurrence of small for size syndrome is suspected. Even when it is argued that T3 is not beneficial in this scenario,36 the protection against injury and oxidative stress we report, which might be related to anti-inflammatory properties and dose dependent, is a factor to be considered when evaluating the indication of such protocols.
Financial SupportSupported by Fondecyt Grants N° 11090240 and 1130274.