Background. Topical hypothermia (TH) and ischemic preconditioning (IPC) are used to decrease I/R injury. The efficacy of isolated or combined use of TH and IPC in the liver regarding inflammation and cytoprotection in early ischemia/reperfusion (I/R) injury needs to be evaluated.
Material and methods. Wistar rats underwent 70% liver ischemia for 90 min followed by 120 min of reperfusion. Livers of animals allocated in the sham, normothermic ischemia (NI), IPC, TH, and TH+IPC groups were collected for molecular analyses by ELISA and Western blot, aiming to compare proinflammatory, anti-inflammatory, and antioxidant profiles.
Results. Compared with NI, TH presented decreased tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and IL-12 concentrations and increased IL-10 levels. TH animals displayed lower inducible nitric oxide synthase (iNOS) and higher endothelial nitric oxide synthase (eNOS) expressions. NAD(P)H-quinone oxidoreductase-1(NQO1) expression was also lower with TH. Isolated IPC and NI were similar regarding all these markers. TH+IPC was associated with decreased IL-12 concentration and reduced iNOS and NQO1 expressions, similarly to isolated TH. Expression of Kelch-like ECH-associated protein (Keap)-1 was increased and expression of nuclear and cytosolic nuclear erythroid 2-related factor 2 (Nrf2) was decreased with TH+IPC vs. NI.
Conclusion. TH was the most effective method of protection against early I/R injury. Isolated IPC entailed triggering of second-line antioxidant defense enzymes. Combined TH+IPC seemed to confer no additional advantage over isolated TH in relation to the inflammatory process, but had the advantage of completely avoid second-line antioxidant defense enzymes.
The reversibility of ischemia-related liver dysfunction depends on the cause, intensity, and duration of the ischemic process.1,2 Paradoxically, even though reperfusion is essential for protecting the liver against irreversible dysfunction, it is exactly during this process that most liver injury occurs.3,4 Hepatic ischemia/reperfusion (I/R) injury results from normothermic ischemia (NI) associated with surgical and clinical scenarios such as liver resection, vascular procedures, or hypovolemic shock. In liver transplantation, in which the liver is submitted to both NI and hypothermic ischemia (during storage in a preservation solution), I/R injury accounts for 10-30% of primary graft dysfunction.2,4,5
Various mechanisms play a role in I/R.2,4,6 During reperfusion, endothelial cell edema, vasoconstriction, leucocyte adhesion, and platelet aggregation in hepatic sinusoids cause an uneven decrease in microvascular blood flow, producing scattered hypoxic regions.2,4 Kupffer cells (KC) and neutrophils are thus activated and secrete inflammatory mediators and reactive oxygen species (ROS), increasing tissue damage.7,8 Inflammatory mediators related to I/R include, among several others, tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and IL-12, while IL-10 acts as an anti-inflammatory cytokine.3,8,9 Nitric oxide (NO), in addition to inducing vasodilation, produces reactive nitrogen species (RNS), contributing to oxidative stress.10,11 An imbalance between endothelial nitric oxide synthase (eNOS) and inducible nitric oxide synthase (iNOS) promotes I/R injury.10–12 If I/R injury is not soon halted, the liver will unleash the production of second-line antioxidant enzymes, including NAD(P)H quinone oxidoreductase-1 (NQO1), by regulating signaling molecules such as the nuclear erythroid 2-related factor 2 (Nrf2).13–16 Activated Nrf2 is uncoupled from a cytosolic complex with Kelch-like ECH-associated protein (Keap)-1, and translocates to the nucleus, in order to affect I/R.6,15,17
Some therapeutic measures have been developed with the aim of protecting the liver from I/R injury, such as induction of topical hypothermia (TH) and ischemic preconditioning (IPC), which consists in the use of brief periods of ischemia interspersed with reperfusion intervals.18–21
Our group has previously evaluated the efficacy of TH in isolation or associated with IPC (TH+IPC) to ameliorate early stage I/R injury in rats submitted to 90-min 70% liver ischemia followed by 120 min reperfusion.22 In that study, all animals presented bile flow blockade during ischemia; however, bile flow was completely restored at 45 min of reperfusion in the groups submitted to TH alone and TH + IPC, but not in the animals submitted to isolated IPC or maintained in NI. This suggests that TH itself might ameliorate the I/R injury, with the possibility of an additive protective role IPC when the two measures were combined. The present study aimed to evaluate the behavior of pro- and anti-inflammatory cytokines, as well as oxidative stress and second-line antioxidant markers, in livers of the animals analyzed in that previous experiment, helping to define at the molecular level the mode of action and efficacy of each protective method.22
Material and MethodsSurgical procedureThis study evaluated liver samples obtained following euthanasia from animals (n = 32) submitted to I/R injury by Grezzana Filho, et al.22 Male Wistar rats, weighing between 200-250 g, underwent a 90 min 70% liver ischemia, including left and median lobes by clamping the hepatic artery and the portal vein and preserving the patency of bile ducts, followed by 120 min of reperfusion. Five groups were studied:
- •
Sham (n = 4).
- •
NI (n = 7).
- •
IPC (n = 7).
- •
TH (n = 7), and
- •
TH + IPC (n = 7).
IPC consisted of consecutive 10-min periods of ischemia and reperfusion before the ischemic insult. TH was induced by the superfusion of cooled saline at 26 °C onto the ischemic lobes. All animals were euthanized immediately after the end of the experiment. Samples were immediately stored in liquid nitrogen and kept at −80°C.
Protein concentration assessmentProtein concentration in tissue homogenates was assessed in accordance with the technique described by Bradford.23 Samples were analyzed by spectrophotometry at 595 nm and the obtained values were expressed in mg/ mL. These values were employed for the calculations of ELISA and Western blot assays. For each method, specific homogenates were prepared.
Enzyme linked immune sorbent assay (ELISA)ELISA was used to assess concentrations of IL-1β (eBioscience 88-6010, San Diego, CA, USA), TNF-α (eBioscience 88-7340, San Diego, CA, USA), IL-6 (BioSource KRC0061, Camarillo, CA, USA), IL-12p70 (BioSource KRC2371, Camarillo, CA, USA), and IL-10 (BioSource KRC0102, Camarillo, CA, USA).
The liver tissue samples were homogenized in a PBS buffer containing a protease inhibitor mix/cocktail (Sigma P8340, Saint Louis, MO, USA) in ice and centrifuged at 4,000 rpm for 10 min at 4 °C. The supernatant was collected for determining the concentration of the studied markers.
All analyses were done according to manufacturer instructions. Absorbance was measured by a spectrophotometer at 450 nm. Color intensity was directly proportional to cytokine concentration in the samples. Results were expressed in pg/mg of protein.
Cytoplasm and nuclear extracts preparationCytoplasm extracts for determining eNOS, iNOS, Nrf2, Keap1 and NQO1 protein expressions, as well as nuclear extracts for ascertaining nuclear Nrf2 protein expressions, all by Western blot, were prepared from liver tissue fragments using a protocol adapted from Sadowiski & Gilman.24 Briefly, liver samples were homogenized in ice with 500 μL hypotonic buffer A (1M HEPES pH 7.9, 0.5M NaF, 100 mM Na3VO4, 100 mM glycerophosphate Na, 0.5M EDTA-Na pH 7.5, 100 mM EGTA pH 7.5 and 1mM DTT). Nonidet P-40 and protease inhibitor were added to this solution. Samples were centrifuged at 1,000 rpm during 10 min at 4°C. The supernatant was removed and stored at -80°C for Western blotting of the cytoplasmic extract. The nuclear pellet present in the liver sediment fraction was resuspended with 50 μL of hypotonic buffer B (1M HEPES pH 7.9, 0.5M NaF, 100mM Na3VO4, 4M NaCl, 100 mM glycerophosphate Na, 0.5M EDTANa pH 7.5, 100 mM EGTA pH 7.5 and 1 mM DTT) and vortexed at 5 min intervals during 30 min. Nuclear extracts were then centrifuged at 12,000 rpm during 20 min at 4 °C and the supernatant was stored at −80 °C until the accomplishment of the Western blot assay.
Western blotSamples containing 100 μg of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (9-12% acrylamide) and transferred to polyvinylidene fluoride membranes. The membranes were then blocked with 5% nonfat dry milk in PBS containing 0.05% Tween 20 (PBS-T) for 1 h at room temperature and probed overnight at 4°C with polyclonal anti-eNOS (SC 8311/ 130 kDa), anti-Keap1 (SC 33569/ 70 kDa), antiNrf2 (SC 30915/ 57 kDa), monoclonal anti-iNOS (SC 7271/ 120 kDa), anti-NQO1 (SC 376023/ 31 kDa) and anti-ß-actin (SC 8432/ 43 kDa) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:200-1:1,000 dilution with PBS-T in 5% nonfat dry milk. After the membranes were washed with PBS-T and incubated for 1 h at room temperature with secondary HRP-conjugated antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:5,000-1:10,000 dilution with PBS-T in 5% nonfat dry milk. Protein detection was performed via chemiluminescence using a commercial ECL kit (WBKLS0050 Millipore Co., Billerica, MA, USA). The density of the specific bands was quantified with an L-Pix Chemi Molecular Imaging densitometer. Results were expressed in arbitrary units (au).
Liver histologySamples of liver tissue from every studied animal were formalin-fixed and embedded in paraffin, and five milimeter sections were obtained and stained with hematoxylin and eosin. Slides were evaluated under light microscopy with a 400 x magnification. A semi-quantitative assessment of the liver histopathology alteration was done according to the scale developed by Suzuki, et al., which assigns a value from 0 to 4 to observed morphologic alterations in relation to sinusoidal congestion, neutrophil infiltration and hepatocellular necrosis.25 The sum of these values was calculated for each animal, and the scores were presented as a mean of each group.
The histologic evaluation was performed by one expert in Pathology, blinded in regards to the experimental group in which every single animal was included.
StatisticsThe calculation of the necessary sample size was based on the preliminary analysis of the behavior of the pro-inflammatory molecules TNF-α and IL-1β evaluated by the ELISA assay. We concluded that the number of 32 animals, previously used in the study by Grezzana Filho, et al. conferred a statistical power of 80% at 5% level significance to the present study.22
Normality of data distribution was evaluated using the Shapiro-Wilk test. Because distributions were parametric in the comparison between groups, ANOVA was used followed by the Tukey post-hoc test. P < 0.05 was considered statistically significant.
EthicsThe study was approved by the Ethics Committee at Hospital de Clínicas de Porto Alegre, which follows the Council for International Organization of Medical Sciences (CIOMS) ethical code for animal experimentation.
ResultsIn this study, the NI group represented the unprotected liver against I/R injury, while sham animals were employed as normal controls. Animals submitted to the protective methods IPC, TH and TH + IPC were compared with both these groups.
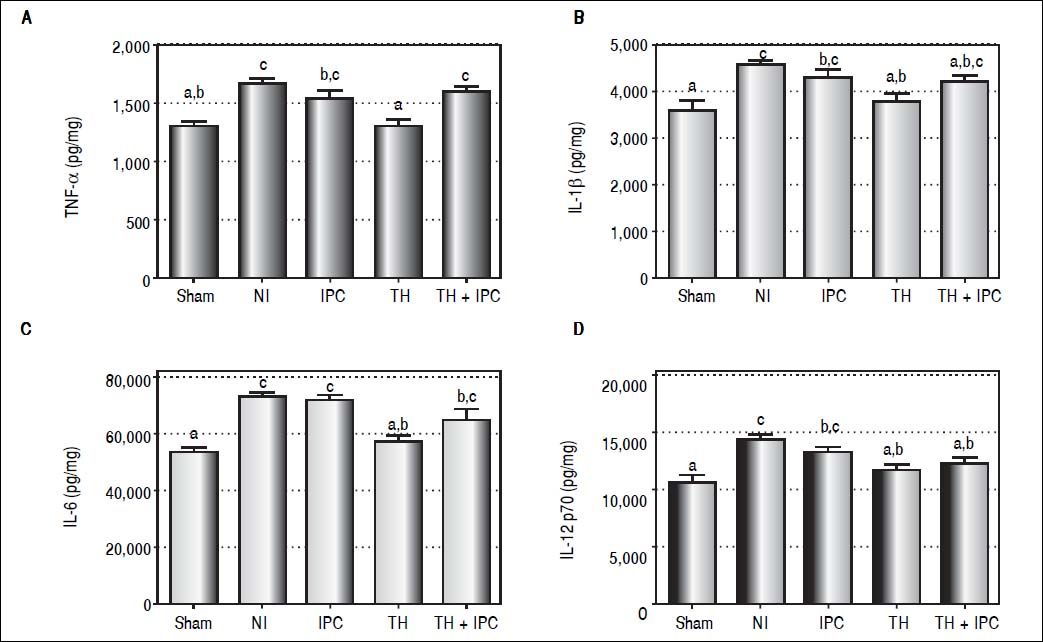
Effect of protective methods on inflammatory cytokine behaviorWe sought to determine the effects of each protective method on early I/R injury in the liver reflected by the concentration of inflammatory molecules (Figures 1A-1D). Significant differences were observed among the groups for the concentrations of all inflammatory molecules evaluated: TNF-α (P < 0.001), IL-1β (P = 0.001), IL-6 (P < 0.001), IL-12 (P < 0.001). The concentrations of all these molecules were decreased in the TH group in comparison to NI: TNF-α (P < 0.001), IL-1β (P = 0.005), IL-6 (P = 0.001) and IL-12 (P = 0.002), with values similar to those of the sham group. The IPC and TH + IPC groups were similar to NI for TNF-α (P = 0.416 and P = 0.873, respectively), IL-1β (P = 0.659 and P = 0.419 respectively), IL-6 (P = 0.997 and P = 0.149 respectively). IL-12 concentration was decreased in the TH + IPC group in comparison to the NI group (P = 0.016), with values comparable to the sham group, while the IPC group presented IL-12 concentrations similar to those of NI (P = 0.411).
Liver expression of TNF-α (A), IL-1β (B), IL-6 (C) and IL-12 p70 (D) after reperfusion (by ELISA assay). Data expressed as mean ± SEM. Different letters indicate a significant difference observed in the means of groups (P < 0.05). NI: normothermic ischemia. IPC: ischemic preconditioning. TH: topical hypothermia.
We evaluated the role of anti-inflammatory cytokine IL-10 in the protection against I/R injury. Results are described in figure 2. Liver concentrations of IL-10 were significantly different among the groups (P < 0.001), with the TH group presenting higher IL-10 concentrations vs. the NI group (P = 0.015). In the isolated IPC and TH + IPC groups, IL-10 levels were similar to those of the NI group (P = 0.988 and P = 0.981 respectively).
Effect of protective methods on nitric oxide synthase isoformsIn addition to inflammation markers, we investigated the expression of nitric oxide synthases with each protective method (Figures 3A-3C). eNOS expression differed significantly among the groups (P = 0.006), with the TH group displaying higher expression in comparison with both the NI (P = 0.037) and TH + IPC (P = 0.006) groups. Regarding iNOS expression, significant differences were also observed among the groups (P < 0.001). The sham group presented the lowest expression compared to NI (P < 0.001), IPC (P = 0.013), and TH + IPC (P = 0.029), while TH and TH + IPC presented a lower expression in comparison with both NI (P = 0.001 and P < 0.001, respectively) and isolated IPC (P = 0.034 and P = 0.013 respectively).
Liver expression of nitric oxide synthase isoforms. A. Western blot analysis. B. eNOS expression (densitometry analysis). C. iNOS expression (densitometry analysis). Data expressed as mean ± SEM. Different letters indicate a significant difference observed in the means of groups (P < 0.05). NI: normothermic ischemia. IPC: ischemic preconditioning. TH: topical hypothermia.
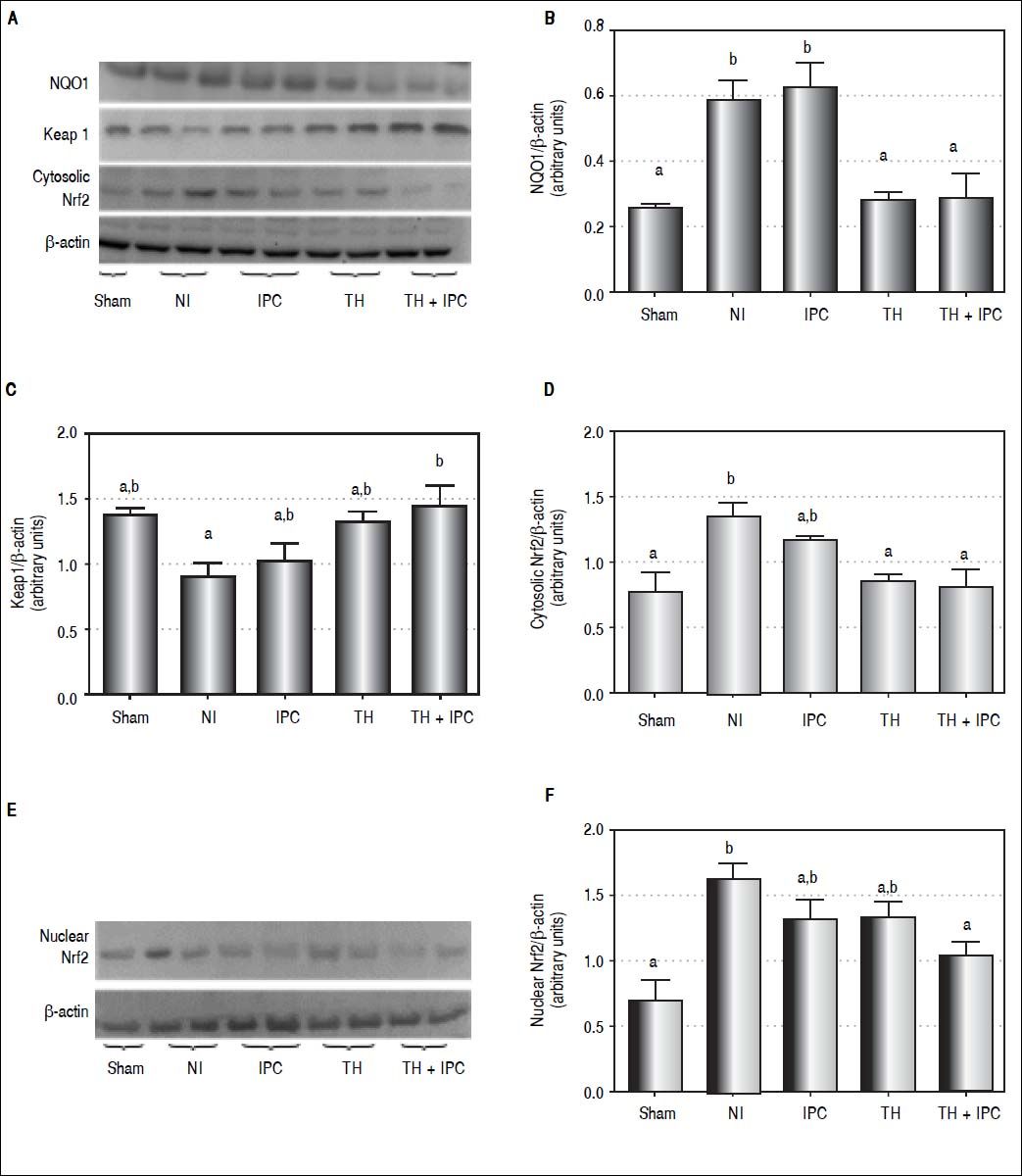
We tried to identify which protective method activated second-line antioxidant defenses. For that, the expression of NQO1 protein and the behavior of the Keap1-Nrf2 complex, including cytosolic and nuclear Nrf2 expression, were analyzed (Figures 4A-4F). We observed differences among groups in the expression of NQO1 (P = 0.001), Keap1 (P = 0.027), and cytosolic (P = 0.006) and nuclear (P = 0.005) Nrf2. NQO1 expression was similar in the IPC and NI (P = 0.991) groups, and was higher in both these groups as compared to the sham (P = 0.022 and P = 0.042, respectively), TH (P = 0.009 and P = 0.020, respectively) and TH + IPC (P = 0.010 and P = 0.022, respectively) groups. Keap1 protein expression in TH + IPC group was higher than in NI (P = 0.035). The NI group displayed the highest cytosolic Nrf2 expression, with values similar to those observed in the isolated IPC group (P = 0.722), and the highest nuclear Nrf2 expression, which was higher than that recorded in the sham (P = 0.004) and TH + IPC (P = 0.029) groups.
Liver expression of NQO1, Keap1 and Nrf2 (cytosolic and nuclear). A. Western blot analysis. B. NQO1 expression (densitometry analysis). C. Keap1 expression (densitometry analysis). D. Cytosolic Nrf2 expression (densitometry analysis). E. Western blot analysis. F. Nuclear Nrf2 (densitometry analysis). Data expressed as mean ± SEM. Different letters indicate a significant difference observed in the means of groups (P < 0.05). NI: normothermic ischemia. IPC: ischemic preconditioning. TH: topical hypothermia.
The histopathology effect of the I/R injury after a 2-h reperfusion period was evaluated (Figures 5A-5F). The overall comparison among all the groups showed significant differences in relation to the histopathology score (P = 0.011). Regarding the differences between the specific groups, a significant difference was observed between the NI and sham groups (P = 0.012). The remaining groups presented only negligible alterations.
Liver histology of the groups under study and comparison by the histopathological score. Slide images refer to sham (A), NI (B), IPC (C), TH (D), TH+IPC (E) groups. The NI group presented sinusoidal congestion, neutrophil infiltration and hepatocellular necrosis, alterations not found in the remaining groups. Magnifications: 400X. 5f- Comparison by the histopathological score. Data expressed as mean ± SEM. Different letters indicate a significant difference observed in the means of groups (P < 0.05). NI: normothermic ischemia. IPC: ischemic preconditioning. TH: topical hypothermia.
The mechanisms by which TH and IPC operate in I/R injury are not completely clear, but they are thought to play a crucial role in relieving inflammation and production of ROS and RNS following reperfusion.18,19,21,26 The association of TH + IPC has been used in cardiac and retinal sur-geries.27–29 Regarding the liver, our group was the first to report on the effectiveness of this synergistic approach to prevent early P/R injury in a rat model.22 We analyzed the protective effect of isolated or combined TH and IPC on bile flow and on the behavior of first-line antioxidant enzymes, comparing these treatment groups with sham and NI groups. We observed that in animals submitted to TH and TH + IPC, bile flow was restored during reperfusion after bile flow blockade during ischemia. The present study was designed to continue that initial investigation. For that, we focused on the molecular mechanisms and effectiveness of TH and IPC methods used alone or in combination for protecting against hepatic I/R injury, considering pro- and anti-inflammatory molecules as well as markers associated with the second-line antioxidant defense.22
Hepatic I/R injury can be classified into two stages in relation to reperfusion: an early phase, corresponding to the first 6 h after the start of reperfusion, and a late phase that lasts 48 h.5,12 In early I/R, activated KC secrete pro-inflammatory mediators, such as TNF-α, IL-1β, and IL-6, which intensify cell injury.3,7 In this study, the TH group showed decreased hepatic concentrations of TNF-α and IL-1β in comparison with the NI group, and the concentration of these molecules in the TH group was similar to that found in sham animals.
In the presence of I/R injury, TNF-α and IL-1β have similar activity, exerting a stimulatory effect on the expression of other inflammatory cytokines and chemokines, regulating free radical production, and helping neutrophil recruitment and adhesion to sinusoidal endothelial cells.30 The present findings agree with those obtained by other groups, showing that the use of hypothermia in hepatic I/R injury reduces inflammation by lowering TNF-α and IL-1β production.18,30,31 Mahmoud, et al. observed that inhibition of TNF-α induces decreased inflammation and oxidative stress, possibly providing a useful therapeutic approach to prevent I/R injury.8 In the present study, TNF-α and IL-1β concentrations were similar in IPC and NI animals. Koneru, et al. did not observe advantages in using IPC during surgical procedures to decrease TNF-α concentrations.32
In this study, hepatic IL-6 concentrations were decreased in TH and sham groups in comparison with NI, while in the IPC group IL-6 concentrations were not different from NI. IL-6 is an important marker of tissue damage and inflammation, and decreasing its expression minimizes I/R injury.33 The present findings suggest that TH, and not IPC, has a protective role against hepatic inflammation. Qi, et al. using an animal model of hepatocellular carcinoma, reported that the use of IPC induced decreases in TNF-α and IL-1β serum levels, associated with increased levels of IL-6 and higher signal transduction and activation of transcription 3 (STAT3) expression.18 They proposed that the beneficial effects of IPC might be cancelled out by the activation of the IL-6/ STAT3 pathway, thus accelerating carcinogenesis. Other studies, however, propose that increased IL-6 concentrations lead to STAT3 activation, inducing the expression of mitogenic and anti-apoptotic proteins capable of protecting against I/R injury and contributing to hepatocellular regeneration.34,35
In the present study, TH, alone or associated with IPC, led to diminished hepatic IL-12 concentration, with levels similar to those of the sham group. IL-12 is a pro-inflammatory mediator involved in hepatic I/R injury, and animal models have revealed that exogenous application of this cytokine led to KC activation, expression of cell adhesion molecules, and neutrophil accumulation in liver parenchyma. Conversely, mice treated with neutralizing antibodies for IL-12 presented decreased TNF-α and interferon-γ expression, causing reduced neutrophil recruitment to liver parenchyma.9,36 In the present study, the IPC group presented the highest concentrations of IL-12, suggesting that IPC does not protect against the action of the pro-inflammatory mediators that worsen hepatic I/R injury.
In turn, IL-10 (previously called cytokine synthesis inhibitory factor) plays an important anti-inflammatory role against I/R injury. In this study, not only was TH responsible for decreasing the concentration of inflammatory cytokines, it also caused increased secretion of IL-10 in comparison with the NI group. IL-10 levels were similar in livers of IPC and NI animals. This finding differs from the report by Serafín, et al. who observed a protective effect of IPC in livers of animals with fatty liver disease, associated with increased levels of IL-10 and reduced inflammation.37 Some studies have reported blocked nuclear factor-κβ activity as a result of IL-10 secretion in I/R injury, and thus the expression of inflammatory mediators, protecting liver against cell damage.3,7,38
NO plays a key role in the regulation of liver blood flow in normal conditions, but the increased production of NO during I/R injury aggravates hepatic tissue damage owing to free radical production.10,12 NO is synthesized through the action of NOS. eNOS leads to the production of physiological amounts of NO, while iNOS prompts copious and lasting NO synthesis, thus explaining iNOS involvement in several pathologic conditions.10,11 The present study showed higher eNOS expression in the TH group compared to NI. NO synthesis attributable to the action of eNOS has a protective effect against I/R injury, modulating vasodilation and inhibiting the production of inflammatory cytokines and free radicals by KC.12,39,40 In this study, higher iNOS expression was also observed in the IPC group as compared to the TH alone or TH+IPC group, but similar to the NI group. iNOS derived NO may be toxic or protective, depending on the type of aggression, on NO levels, and on the duration of iNOS expression.4,11 Our findings indicate that TH provided protection against I/R injury in liver by promoting an increase in the levels of eNOS, while IPC, on the contrary, led to increased expression of iNOS, which may aggravate hepatocellular damage.
ROS and RNS are generated along the entire period of I/R injury, but the detrimental effect of oxidant agents associated with a decrease in antioxidant defenses produces oxidative stress.2,4 The antioxidant enzymes superoxide dismutase (SOD), catalase and glutathione peroxidase are classified as first-line antioxidant defenses against I/R injury.41 Grezzana Filho, et al. showed that TH induced increased levels of SOD in comparison with NI, IPC and TH + IPC.22 The increased levels of SOD induced by TH are capable of abolishing the damage caused by I/R injury, precluding the use of second-line antioxidant defenses.
Second-line antioxidant defense enzymes are activated whenever those in the first line fail.17,41 Second-line enzymes synthesis is mediated through activation of the Nrf2-antioxidant response element signaling pathway.6,13 In normal conditions, Nrf2 is located in cytoplasm, associated with its repressor Keap1, both forming an inactive complex. Under stress condition, inducers such as ROS oxidize Keap1 cysteine residue, leading to dissociation of the inhibitory complex.15,17 Nrf2 uncouples from Keap1, and translocates to the nucleus in order to promote the gene transcription of second-line antioxidant enzymes, such as NQO1.13–15 However, during a short time span before entering the nucleus, activated Nrf2 accumulates in cytoplasm.17
In this study, TH was effective in controlling the generation of ROS, protecting against the cellular damage caused by reperfusion. The TH group had the lowest concentrations of inflammatory cytokines and the highest IL-10 levels, as well as a more favorable liver vasoactive response as assessed by iNOS and eNOS expression. These findings indicate lower production of ROS and RNS and an adequate restoration of the cell redox balance with TH. Reduced oxidative stress prevents damage to lipids, proteins, and DNA.2,16 The use of hypothermia requires special care in terms of hemodynamic monitoring, since it can trigger some deleterious effects such as edema, lactate accumulation and intracellular acidosis.21,27 Several methods of hypothermia application have described, including the TH. Yamanaka, et al. studied patients with chronic liver diseases and hepatocellular carcinoma who underwent right-sided segmentectomy under normothermia or TH with temperatures ranging from 20 to 25 °C.42 Those authors showed that the patients submitted to TH presented a reduced blood loss and tolerated a larger ischemia period in comparison with those who underwent the procedure under normothermia. Beneficial effects of a moderate hypothermia have also been reported in studies that evaluated patients suffering from acute liver failure.43
Conversely, NQO1 expression was higher in the IPC group in comparison with the TH and TH + IPC groups, suggesting that IPC failed to control oxidative stress through first-line antioxidant defense enzymes. Shokeir, et al. evaluated Nrf2 and NQO1 expressions in animals submitted to the IPC approach against renal I/R injury.13 The results obtained by those authors are similar to those of our study, with IPC animals presenting higher levels of Nrf2 and NQO1 expression in comparison with the unprotected sample. Some studies, however, report a synergic effect of NQO1 and SOD, which protects cells against the generation of ROS and RNS.16,44 In the present study, TH alone or in combination with IPC produced the lowest expression of Nrf2 in cytoplasm, indicating less Nrf2-Keap1 uncoupling and activation and suggesting that in this group activation of the second-line defense system was unnecessary. Isolated IPC, on the contrary, produced similar cytosolic Nrf2 expression to NI, indicating failure of the first antioxidant defense line. The combination of the two methods, TH and IPC, caused complete inhibition of Keap1/Nrf2 uncoupling, preventing translocation of Nrf2 to the nucleus. This strong inhibition resulted in reduced expression of both NQO1 and nuclear Nrf2. These findings suggest that the association of IPC with TH may contribute an additional protective effect as compared to TH alone.
In this study, findings of significant histopathologic injury occurred only in the NI group in comparison with sham group. Dinant, et al. evaluated the protective effects of cooling by in situ hypothermic perfusion with a Ringer solution at 4°C during 60-min of total vascular exclusion in a porcine liver I/R model.45 Those investigators showed a significant increase of histopathologic injury in the nor-mothermic group, which presented increased liver cell vacuolization, neutrophil infiltration and parenchymal necrosis. The histopathologic alterations were not observed in the animals submitted to hypothermia. The his-topathologic alterations and bad outcome were not observed in the animals submitted to hypothermia. Nieu-wenhuijs, et al. evaluated the bile flow and histologic alterations in an experimental protocol of IPC, using 10-min ischemia and 10-min reperfusion periods followed by a 60-min reperfusion phase.46 Those authors observed that the bile flow levels obtained at reperfusion were dependent on the length of the ischemia period. Significant histopathologic alterations associated to the functional disturbance were absent since, according to the authors, necrosis and apoptosis occur only in the late phases of reperfusion.
One unexpected finding in this study was that isolated IPC was ineffective against I/R injury in the liver. Although most studies present evidences favoring the use of IPC against the I/R injury in the liver, some groups showed that this method offers only minor beneficial effects, or is useless.47,48 Pasupathy, et al. reported that IPC offers a protective effect against I/R injury in two distinct time points: in the early stage, comprising the first 3 h after the organ reperfusion, and in the late stage, between 12 and 24 h. If IPC is applied in this last situation, the protective effect can persist for up to 3 days.49 A putative explanation for our divergent findings is that the 90 min ischemia chosen in the design of this study can be beyond the limits of the protection affordable by IPC. Additionally, other groups obtained similar results to our findings.47,50 Presently, our team is carrying out projects making use of the same experimental model, but applying a longer period of reperfusion, and assessing histologic and liver enzyme alterations in the late phase of reperfusion (24 h).
In conclusion, using an animal model of early I/R injury, we showed that TH alone was more effective than IPC alone to protect the liver against inflammation and oxidative stress, precluding the need to activate second-line antioxidant defenses. Isolated IPC did not have a protective effect as indicated by the markers evaluated in this study.
Abbreviations- •
eNOS: endothelial nitric oxide synthase.
- •
I/R: ischemia/reperfusion.
- •
IL: interleukin.
- •
iNOS: inducible nitric oxide synthase.
- •
IPC: ischemic preconditioning.
- •
KC: Kupffer cells.
- •
Keapl: Kelch-like ECH-associated protein-1.
- •
NI: normothermic ischemia.
- •
NO: nitric oxide.
- •
NQO1: NAD•(P)H quinone oxidoreductase-1.
- •
Nrf2: nuclear erythroid 2-related factor 2.
- •
RNS: reactive nitrogen species.
- •
ROS: reactive oxygen species.
- •
SOD: superoxide dismutase.
- •
TH: topical hypothermia.
- •
TNF-α: tumor necrosis factor-α.
Fundo de Incentivo a Pesquisa e Eventos-Hospital de Clínicas de Porto Alegre (FIPE-HCPA) and Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior (CAPES).