Background and rationale. We aimed to provide novel information to better understand the molecular mechanisms underlying gallstones formation and explore the potential protein markers for gallstones progression. The gallbladder tissues were collected from 20 patients with cholesterol gallstone and 10 liver transplant donors from November 2010 to April 2011. The proteomics were compared between gallstone patients and controls by two-dimensional gel electrophoresis (2-DE). The differentially expressed proteins were identified and validated by western blotting and real-time PCR.
Results. Total 19 protein spots were found to be different between two groups and 11 proteins were identified, among which 4 ones (such as Peroxiredoxin 3/Prdx3) were down-regulated and 7 (such as Tropomyosin 4/TPM4, Transgelin/SM22, Transthyretin/TTR) were up-regulated in gallstone group. Results of western blotting and RT-PCR were consistent with the 2-DE results. Conclusion. The differentially expressed proteins of TTR, TPM4, SM22 and Prdx3 may play key roles in gallstone formation and may be markers for gallstone progression.
Cholesterol gallstones are formed by accretion of the bile components in the gallbladder. The presence of cholesterol gallstones can result in gallstone ileus, acute cholecystitis and the other serious conditions such as ascending cholangitis or pancreatitis.1–3 There is an increasing trend for developing cholesterol gallstones in women and people near or above 40 years.4 The prevalence of cholesterol gallstones was found to be due to comprehensive factors such as inherited body chemistry, body weight, and low calorie diet.5,6
Although advances have been made in the prevention of gallstones formation, the morbidity and mortality caused by gallstones are high all over the world.7 Many studies have focused on the pathogenesis of the gallstones formation. It was reported that premarin increased the bile by enhancing hepatic lipoprotein uptake and inhibiting bile acid synthesis.8 The enhanced hepatic secretion of cholesterol in people with obesity contributed to the development of precipitates in bile.5 However, the understanding of the mechanism underlying gallstones formation is far from being clear. Currently, advances in proteomics research proved a novel perspective to understand the molecular mechanism of gallstones. Comparative proteomics is a branch of proteomics research,9–11 which focused on screening differential expression protein between two tissue samples in different conditions. The two-dimensional gel electrophoresis (2-DE) technique has been increasingly used in proteome analysis for its ability to separate a large amount of proteins at a time.12 In the present study, we applied 2-DE and mass spectrometry (MS) techniques to compare the proteomics in gallbladder tissue of cholesterol gallstone patients and normal ones. In this paper, we aimed to improve the understanding of the mechanism of gallstone formation and explore the potential biomarkers for gallstone treatment.
Material and MethodsSubjectsFrom November 2010 to April 2011, total 20 patients with cholesterol gallstone received elective cholecystectomy in General Surgery Department of Huashan Hospital affiliated Fudan University. All the cases were confirmed to have the calculi cholesterol content of > 70% by B-ultrasound and pathological examination. The median age of the patients (including 10 males and 8 females) were 52 years old, ranging from 36 to 69 years. Total 20 gallbladder wall samples were collected from the patients with cholesterol gallstone during cholecystectomy and 10 normal gallbladder tissues were collected form 10 liver transplant donors (including 6 males and 2 females; median age 32 years, ranging from 26 to 48 years).
The study was approved by the hospital ethics committee and all the subjects or their parents signed the informed consent before this study.
Samples preparationIn Petri dishes with 0.9% normal saline, all the gallbladder tissues were grinded into powder in liquid nitrogen. Then the samples were lysed and subjected to ultrasound pyrolysis for 6 to 10 times and ice cracking for 3~4 h. After centrifugation at 15,000 r/min for 60 min at 4 °C, the supernatant were collected and stored at 70 °C for further studies.
Two-dimensional Gel electrophoresisProteins were processed with 2D Clean-up kit (Amersham Pharmacia Biotech Company, United States) and mixed based on the source of patients and normal controls. 2-DE was performed according to a previous method with slight modification.13 After rehydration (8 M urea, 2% CHAPS, 0.5% Pharmalyte, 18 mM DTT, add DTT before use), total 250 μL samples from patients and control group were added into 13 cm pH3-10NL IPG strip tank. Hydration and isoelectric focusing were performed at 20 °C with the maximum current at 50 μA/strip and voltage at 30V 13h (hydration), 100V 1h, 200V 1h, 500V 1h, 1,000V 1h, 1,000-8,000V gradient 0.5h, 8,000V 4h). After isoelectric focusing, IPG strips were put into the equilibrium liquid I (6 M urea, 50 mM Tris, 30% (v/v) glycero, 2% SDS, a small amount of bromophenol blue, add 1% DTT before use) and then equilibrium liquid II (add 2.5% iodoacetamide before use) each for at least 15 min. The equilibrated gel strip was placed on the top of 12.5% SDS-PAGE gel. The SDS-PAGE electrophoresis was carried out at constant voltage 40V/gel and then 100V/gel for 30 min until the bromophenol blue reached the bottom of the gel.
Image acquisition and analysisThe separated proteins were analyzed by silver staining. The 2-DE images were visualized by Image Scanner image scanner scans. The image background subtraction, spot detection, erase, protein spots matching and differentially expressed proteins were analyzed by 2D Image Master Analysis Software.
Spot identification by mass spectrometryThe protein spots were excised from the gels and analyzed by 4700 MALDI-TOF/TOF Proteomics Analyzer. Proteins in gel plugs were digested with two trypsins: [M + H] + 842.51 and [M + H] + 2,211.10 as internal standards. Analyzer (TOF/TOF) with automatic retrieval software system was applied to obtain mass spectra against NCBI database. The matching score was set to 65 points or greater.
Western blot analysisWestern blot analysis was performed as previously described.14 Proteins (50 μL) of gallbladder tissues from patients and control were separated by 10% SDS-PAGE electrophoresis and then transferred to PVDF membrane. After sealed in 5% skim milk at room temperature for 2h, the membranes were incubated with primary antibodies against TPM4 (mouse anti-Human TPM4 monoclonal antibody, 1:1,000; Creative biomart, Shirley, US), sm22 (rabbit anti-human SM22 monoclonal antibody, 1:1,000; Abcam, Cambridge, MA) and TTR (mouse antihuman TTR monoclonal antibody; 1:500; Creative biomart, Shirley, US) at 4 °C overnight and then incubated with horseradish peroxidase (HRP)-conjugated second antibodies (Kang-Chen Biotech, Shanghai, China). GAPDH was used as an internal control.
Real-time quantitative PCRTotal RNA was isolated from gallbladder tissues of patients and control using TRIzol® reagent (Invitrogen, San Diego, CA) according to the manufacturer’s instructions. The cDNA was synthesized by reverse-transcription reaction according to the previous method14 and then RT-PCR was performed with the cDNA productions as templates. Primers for TTR, SM22, Prdx3 and GAPDH were designed by Primer 3 software (Table 1). The amplification program was performed as follows: 95 °C for 5 min, 30 cycles of 95 °C for 30 sec, 65 °C for 30 sec, 72 °C for 2.5 min and then 72 °C for 10 min. The RT-PCR analysis was performed for different proteins in triplicates and the target DNA fragments were detected by 1% agarose gel electrophoresis.
Primers in RT-PCR.
| Genes | Primer sequence (5’- 3’) |
|---|---|
| TTR | |
| Sense | GGGGGTACCATGGCTTCTCATCGTCTGG |
| Anti-sense | CCCGAGCTCATTCCTTGGGATTGGTG |
| SM22 | |
| Sense | AGAGGGCTAGCCCTGAGC |
| Anti-sense | CTTCTTCAATGGGCTTTTGC |
| Prdx3 | |
| Sense | TACATATGCCTGCTGTCACCCAGCACG |
| Anti-sense | CTCTTCCAGGTAGTCATCCTAGGTAG |
| GAPDH (internal control) | |
| Sense | GAAGGTGAAGGTCGGAGTC |
| Anti-sense | GAAGATGGTGATGGGATTTC |
The small model bile and comprehensive model bile were prepared according to the method published in the previous literatures. Nucleation times were determined as previously described.15 Briefly, the model bile was ultra-centrifugated at 45,000 rpm for 30 min at 37 °C to remove crystal debris with 0.22 μm filter membrane. Then the retinol binding protein (RBP), 500 μL human serum albumin (ALB as control), or 500 μL TTR at 100 μg/mL were added in the model bile samples. The mixtures were placed in constant temperature incubator at 37 °C for 30 min. Then the time zero for nucleation time was determined arbitrarily. The refraction of cholesterol crystallization was observed with polarizing microscope every 24 h for continuous 21 days. The interval between time zero and the first detection of the formation of cholesterol crystal precipitate was defined as the nucleation time (NT). Additionally, the nucleation activity (NT test/NT control) were calculated.
Statistical analysisResults were displayed as mean ± standard deviation (¯X ± SD). All the data were analyzed by SPSS11.0 software. Differences were analyzed by bilateral Student’s t test. P < 0.05 was considered to be significantly different.
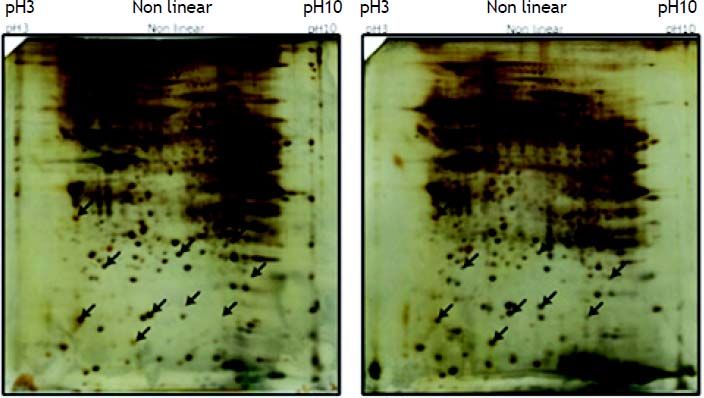
ResultsProtein expression profiles of gallbladder Tissues in cholesterol gallstones patient group and controlsThe 2DE images of the separated proteins were shown in figure 1. The distribution of protein spots in patient and control was basically accordant. After background subtraction, the average matching points of the protein expression profiles of cholesterol gallstones patients and controls were 589 ± 57 and 537 ± 58, respectively by ImageMaster software analysis and the average matching rate was 87.56 and 78.89%. Compared the 2DE images of patient and control group, the matching rate of protein spots was 65.56% with 436 ± 34 spots matched.
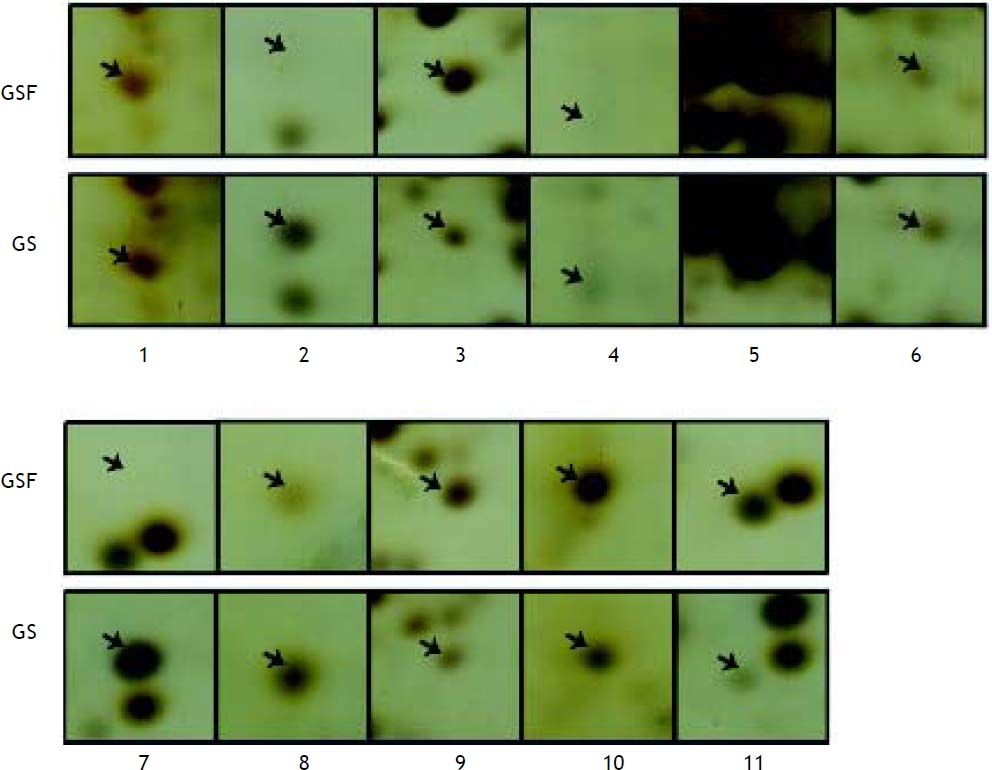
Differential analysis by student’s t test revealed that 19 protein spots showed quantitative changes compared the 2D images from patient and control group (Figure 2). After MS analysis, 11 proteins were successfully identified, among which 4 ones (such as Prdx3) were down-regulated and 7 ones (such as TTR, TPM4, SM22) were up-regulated (Table 2).
Differentially expressed protein by mass spectrometry.
| Spot No. | Protein description | Accession No. | Theoretical Mr (Da)/pI | Protein Score | GS |
|---|---|---|---|---|---|
| 1 | Tropomyosin 4(TPM4) | gi|4507651 | 28504.5/4.67 | 204 | up |
| 2 | Transgelin variant (SM22) | gi|62897565 | 20875.6/8.69 | 120 | up |
| 3 | Chain A, Transthyretin(TTR) | gi|443295 | 13752.9 /5.35 | 144 | up |
| 4 | Chain B, Cyanomet Rhb1.1 | gi|2982014 | 15903.3/7.13 | 299 | up |
| 5 | Regulatory myosin light chain long version | gi|33338062 | 19880.4/4.8 | 243 | down |
| 6 | Peroxiredoxin 3 isoform b (Prdx-3) | gi|32483377 | 25822.3/7.04 | 171 | down |
| 7 | Chain A, The structure of holo type human Cu, Zn superoxide dismutase | gi|31615344 | 15794.9/5.7 | 133 | up |
| 8 | Haptoglobin related protein precursor | gi|3337391 | 38786.6/6.67 | 80 | up |
| 9 | Transgelin variant | gi|62897565 | 20875.6/8.69 | 128 | up |
| 10 | Hypothetical protein | gi|71413112 | 64957.2/6.21 | 69 | down |
| 11 | T cell receptor alpha chain | gi|853649 | 3445.8/10.02 | 65 | down |
In order to confirm the results above, we performed western blotting and RT-PCR analysis. Three identified proteins including TTR, SM22, TPM4 were randomly selected for western blotting analysis. As shown in figure 3, the expression index of TTR, SM22 in cholesterol gallstone patients were significantly higher than those in normal control group, which was consistent with the results of proteomic analysis. In addition, we performed RT-PCR method to test SM22, Prdx3 and TTR mRNA expression levels. After normalized by the expression value of GAPDH, the mRNA expression levels of SM22 and TTR were higher in patients group compared with controls, while the expression of Prdx3 was lower in cholesterol gallstone patient group (Figure 4). All these suggested that either protein level or the mRNA level of TTR were significantly up-regulated in cholesterol gallstone patients.
In the Small model bile system, the mean nucleation time of TTR and ALB were 14.5 ± 1.29 18.0 ± 0.82 d, and the difference was statistically significant (P < 0.01). The nucleation activity of TTR in Small model bile system was 0.81. In comprehensive model bile system, the mean nucleation time of TTR (13.5 ± 0.58 d) was significantly shorter than ALB (18.5 ± 1.29 d) (P < 0.01). The nucleation activity of TTR in comprehensive model bile system was 0.73.
DiscussionGallstone is one of the most common digestive disease with a prevalence of 10-15% in adults.16 Gallbladder, a cystic organ, is composed of mucous membrane, smooth muscle, fibrous connective tissue and outer layer serosa tissues, which has functions in storing and concentrating bile.17 The dysfunction of gallbladder is closely related with the cholesterol gallstones. Gallbladder emptying dysfunction makes gallbladder bile stay longer in the gallbladder, thus result in nucleation and crystallization and eventually lead to the stone formation.18 Additionally, the changes of bile components (lipids, proteins, etc.) can affect functional changes of the gallbladder wall. Thus, in this paper, we collected the gallbladder tissues from cholesterol gallstone patients and normal ones, and explored the changes in the proteome by 2-DE and MS technique.
Our results revealed that the protein expression profiles of patient and control group showed high repeatability in high-abundance proteinogram, while 11 protein spots were found to have different expression values, which suggested that the different abundance proteins may be closely related with cholesterol gallstone formation. The differentially expressed proteins were identified by MS and were verified by western blotting and RT-PCR analysis.
Transthyretin (TTR), served as a kind of synthetic glycoprotein, mainly exists in liver cells and partly in the choroid plexus of the brain or retinal photosensitive tissue.19 The main physiological function for TTR is to transport thyroid hormone and vitamin A, and TTR has thymus hormone activity, which can improve the body’s immunity by promoting maturation of lymphocytes.20 Studies have shown that the elevated levels of serum TTR is closely related to diabetes.21 Refai, et al.22 indicated that the TTR tetramer converting into polymer induced β-cell dysfunction and leaded to the diabetes development. Sundsten, et al.23 used SDS-PAGE and SELDI-TOF MS technologies to analyze the proteome in the serum of diabetic patients, results showed that the level of TTR was up-regulated in patients with diabetes in comparison with normal controls, and the pathogenesis of diabetes was related with cholesterol stones formation. Diabetes can cause the complications of lipodystrophy, visceral autonomic nervous system dysfunction, and microvascular lesions, and induce the imbalanced ratio of cholesterol, bile acids and phospholipids in bile, which may increase the risk of stone formation. Besides, the data of Nucleation time test in our study showed that TTR accelerated the nucleation time of cholesterol crystallization in the model bile system in vitro. In our paper, TTR expression was significantly up-regulated in gallbladder tissues from cholesterol gallstone patients compared with controls. Although the direct relationship between TTR level in gallbladder tissues and cholesterol stones has not been reported, TTR may be a biomarker for cholesterol gallstone.
Tropomyosin is a two-stranded helical structure protein, which is an important regulatory proteins in muscle contraction process.24 In mammals, tropomyosin has more than 40 isoforms encoded by 4 genes including TPM1, TPM2, TPM3 and TPM4. Recently, Elif Kaga, et al.25 applied proteomics technology to analyze the protein expression profiles in atherosclerotic arterial tissues, and found that peroxiredoxin1, peroxiredoxin2, tropomyosin were differentially expressed in patients with atherosclerosis. Evidence showed that the progressive atherosclerosis had close association with hyperlipidemia. It is reported that hyperlipidemia is characterized by abnormalities in cholesterol and bile acid metabolism, which is the primary risk factor for suffering gallstone disease. So we hypothesized that tropomyosin might play a key role in gallstone formation.
Transgelin, also named as SM22 for the molecular mass of 22,000, was first discovered by LeesMille et al in chicken gastric smooth muscle. Transgelin is a critical structure protein and plays a key role in cytoskeletal contraction regulation, through combining with actin.26 Recent evidence showed that the transgelin and peroxiredoxin3 (prdx3) were decreased in patients with degenerative aortic stenosis compared with normal controls and the significantly changed proteins were considered to be involved in the pathogenesis of degenerative aortic stenosis.27 Dweck, et al.28 considered that the degenerative aortic valve stenosis is closely related to hyperlipidemia and cholesterol deposition. Therefore, in this paper, the differentially expressed proteins of SM22 and Prdx3 may affect lipid metabolism, thus contributing to the formation of gallstones.
Furthermore, Xie, et al. found that the mutation of Prdx3 gene increased the risk of obesity.29 Studies conducted by Eric, et al. have shown that inflammatory cytokines selectively reduced antioxidant genes (such as Prdx3) in visceral adipose tissues and resulted in the increased lipid and protein carbonylation.30 In this study, Prdx3 was down-regulated in cholesterol gallstone group, so we further hypothesized that the reduction of Prdx3 caused excessive lipids secreted by gallbladder wall and finally, led to the occurrence of gallstones in patients.
LimitationsAlthough we found some significant protein associated with gallstones formation, our paper remained some limitations. First, despite of the advantages of 2-DE technique in reproducibility and sensitivity, 2-DE method cannot detect hydrophobic protein, acidic protein and alkaline protein with extreme PH and some low-abundance proteins. So it is far from being able to capture all intracellular proteins. Second, MS analysis requires necessary purification prior to sample testing, and therefore cannot achieve high-throughput analysis of “all” of the proteins. Besides, the sample size in this paper was small and large scale of studies should be conducted to verify our findings.
ConclusionIn summary, a proteomic approach was carried out on gallbladder tissues of gallstones patients and normal controls. Total 11 proteins were found to be differentially expressed in gallbladder tissues of gallstones group in comparison with controls. The differentially expressed proteins of TTR, SM22, Prdx3, TPM4 may be involved in the lipid and cholesterol metabolism and play key roles in the progression of cholesterol stones formation. The proteomics provided new protein targets associated with the complex event of cholesterol stones formation. However, the significant meaning for these specific proteins should be verified in the future.
Conflicts of Interest and Source of FundingNone declared.