Hepatocellular carcinoma (HCC) is one of the main indications for orthotopic liver transplantation (OLT). In Brazil, selection criteria for HCC is an expanded version of the Milan Criteria (MC), the so-called "Brazilian Milan Criteria" (BMC). Our aims were to evaluate post-OLT outcomes in patients with HCC and analyze the BMC performance.
Materials and MethodsWe conducted a multicenter, retrospective cohort study, analyzing medical records of 1,059 liver transplant recipients with HCC. Tumor was staged according to MC and BMC and correlated with overall survival (OS) and disease-free survival (DFS). We compared the ability of MC and BMC to predict OS and DFS using Delta C-statistic.
ResultsPost-OLT OS were 63% in five years and HCC recurrence was observed in 8% of patients. At diagnosis, 85% of patients were within MC. Patients within MC at diagnosis and in the explant showed a higher OS and DFS than patients outside MC and within BMC and patients outside both criteria (p < 0.001). Patients outside MC in the explant had an increased risk of tumor recurrence (HR: 3.78; p < 0.001) and poor survival (HR:1.77; p = 0.003). The BMC presented a lower performance than MC in properly classifying patients regarding recurrence risk.
ConclusionsIn a large Brazilian cohort of HCC patients submitted to liver transplantation, we observed satisfactory overall survival and recurrence rates. However, patients transplanted within the Brazilian expanded criteria had lower OS and DFS when compared to patients within MC, which may generate future discussions regarding the criteria currently used.
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer death in the world [1]. Orthotopic liver transplantation (OLT) is the treatment of choice for patients with early-stage HCC and impaired liver function. OLT has the advantage of allowing a curative treatment for both the tumor and the underlying liver disease [2]. More restrictive selection criteria for OLT were adopted since Mazzafero et al. published the Milan Criteria (MC) [3]. Thus, better survival and lower recurrence rates were observed, leading to 5-year survival rates of 70 - 85%, in recent series [4,5].
Since the first liver transplantation was performed in Brazil in 1968 [6], progress in this area is remarkable, and in the last two decades, there has been an exponential growth in the number of transplantations. Brazil has become the largest public transplant system in the world, and it currently occupies the second position worldwide in absolute numbers of liver transplants performed per year [7]. As in other countries, HCC corresponds to one of the main indications for OLT in Brazil [8]. Nevertheless, Brazil still occupies the 28th position in the number of donors per million population, which leads to a longer waiting time in the transplant list, and a higher risk of dropout and death in HCC patients [7]. Thus, it is vital to select those patients who would benefit the most from the transplant.
Since 2006, a Model for End-Stage Liver Disease (MELD) [9] based organ allocation system was adopted in Brazil, and additional points were attributed to HCC patients who met established criteria [10]. The selection criteria adopted for HCC are based on the Milan Criteria. A Brazilian expanded version of the original MC, so-called “Brazilian Milan Criteria” (BMC), takes into consideration only nodules with a radiological diagnosis of HCC larger or equal 2 cm. Patients with HCC are eligible for “HCC exception score” if they present one nodule from 2 to 5 cm or up to 3 nodules between 2 and 3 cm, plus any number of nodules less than 2 cm [10,11].
In Brazil, as in all Latin America, there are few studies evaluating the results of OLT in patients with HCC. In the context of different landscapes and somewhat different selection criteria, local studies assessing the current situation of liver transplantation in HCC patients and their prognostic factors are essential for a better definition of a national selection criteria for liver transplantation.
The present manuscript reports a large multicenter study in Brazil, which evaluated the results of liver transplantation in patients with HCC in the MELD era. Besides assessing the demographic and clinical characteristics and outcomes of HCC patients who underwent OLT, the performance of the “Brazilian Milan Criteria” was also evaluated.
2MATERIAL AND METHODS2.1Study DesignWe conducted a national, multicenter, retrospective cohort study with data from 13 transplant centers after the introduction of a MELD-based organ allocation system in Brazil.
HCC diagnosis was based on the American Association for the Study of Liver Diseases (AASLD) diagnostic criteria [12]. Medical records of 1,368 recipients with HCC transplanted from July/2006 through to July/2015 were compiled. Patients were excluded from the study for the following reasons: incidental HCC diagnosis in explant (n = 122); incomplete tumor or patient data (n = 151); unconfirmed HCC diagnosis in the explant (n = 14); mixed tumor (hepatocholangiocarcinoma) diagnosis (n = 14) and patients who underwent living-donor liver transplantation (n = 8).
Each transplant center received an electronic questionnaire to be filled out with demographic, clinical, laboratory, radiological, and pathological data, which were obtained by reviewing medical records. The following variables were evaluated: age at transplantation, gender, etiology of chronic liver disease, Child-Pugh [13] and MELD9 scores at the moment of inclusion in the transplant list, if the list inclusion was after "downstaging", radiological HCC staging at diagnosis, median time on waiting list, HCC treatment before OLT, HCC treatment response based on mRECIST criteria [14], pre-OLT serum alpha-fetoprotein (AFP) levels, tumor features in the explant and post-OLT HCC recurrence.
Based on the imaging studies at HCC diagnosis and explant data, the tumor was staged according to the MC [3] and to the BMC. Patients with the following number of HCC nodules were considered within BMC: ≤ 3 nodules between 2 and 3 cm or 1 nodule between 2 and 5 cm, plus any number of nodules ≥ 1 cm and < 2 cm. Patients were later classified as 1) Within MC 2) Outside MC, but within BMC; 3) Outside both criteria (OC).
The diagnosis of post-OLT HCC recurrence was based on the following criteria: 1) Image evaluation showing a lesion with typical vascular findings, compatible with intra or extrahepatic HCC recurrence; 2) Biopsy or result of the pathological assessment surgical specimen with histopathological diagnosis of HCC in intra or extrahepatic lesions that appeared after transplantation.
The study protocol conformed to the ethical guidelines of the 2008 Declaration of Helsinki, and it was approved by the Institutional Review Board of the University of São Paulo School of Medicine (number: 164.120).
2.2Statistical analysisContinuous variables were expressed as mean ± standard deviation or median (range), while qualitative variables were expressed as frequency (percentage). The primary endpoints were overall survival (OS) and disease-free survival (DFS). For OS and DFS, the date of liver transplantation was considered as zero time, and the events of interest were death due to any cause and post-OLT HCC recurrence. Alive patients, patients lost to follow-up, and those who died within 30 days of transplantation were included in the descriptive results but censored for the survival analysis. The objective of excluding patients who died in the immediate postoperative period was to analyze more precisely the role of tumor recurrence in the post-transplant outcomes. Survival curves were presented using the Kaplan-Meier [15] and compared using the log-rank test. Median survival times and their 95% confidence intervals (CI) are also reported. Univariate cox regression analysis [16] was fitted, considering different centers as strata. The proportional hazards hypothesis was tested through the Schoenfeld residuals [17]. For all statistical analyses, a p-value < 0.05 was considered statistically significant.
Furthermore, we performed a comparison between the MC and the BMC using the C-statistic over five years [18]. Each patient was classified at time t as a case if the event of interest (death or recurrence) was observed before time t, or as control otherwise. For each group, we estimated the probability (p) of the event of interest at time t for both criteria using the survival models described above. We estimated the C-statistic for BMC and MC. Each C-statistic is defined as the probability that patients classified as case or control will have the case-patient outside the criterion, while the control patient will be within the criterion. Then, we compared BMC and MC, calculating the 95% bootstrap confidence interval for Delta C = CMC – CBMC. Positive values of Delta C indicate that MC had a higher predictive ability than BMC.
The data were analyzed by using the statistical program R, version 3.3.2, Vienna, Austria [19]. The statistical methods of this study were reviewed by a statistician not masked (DMA).
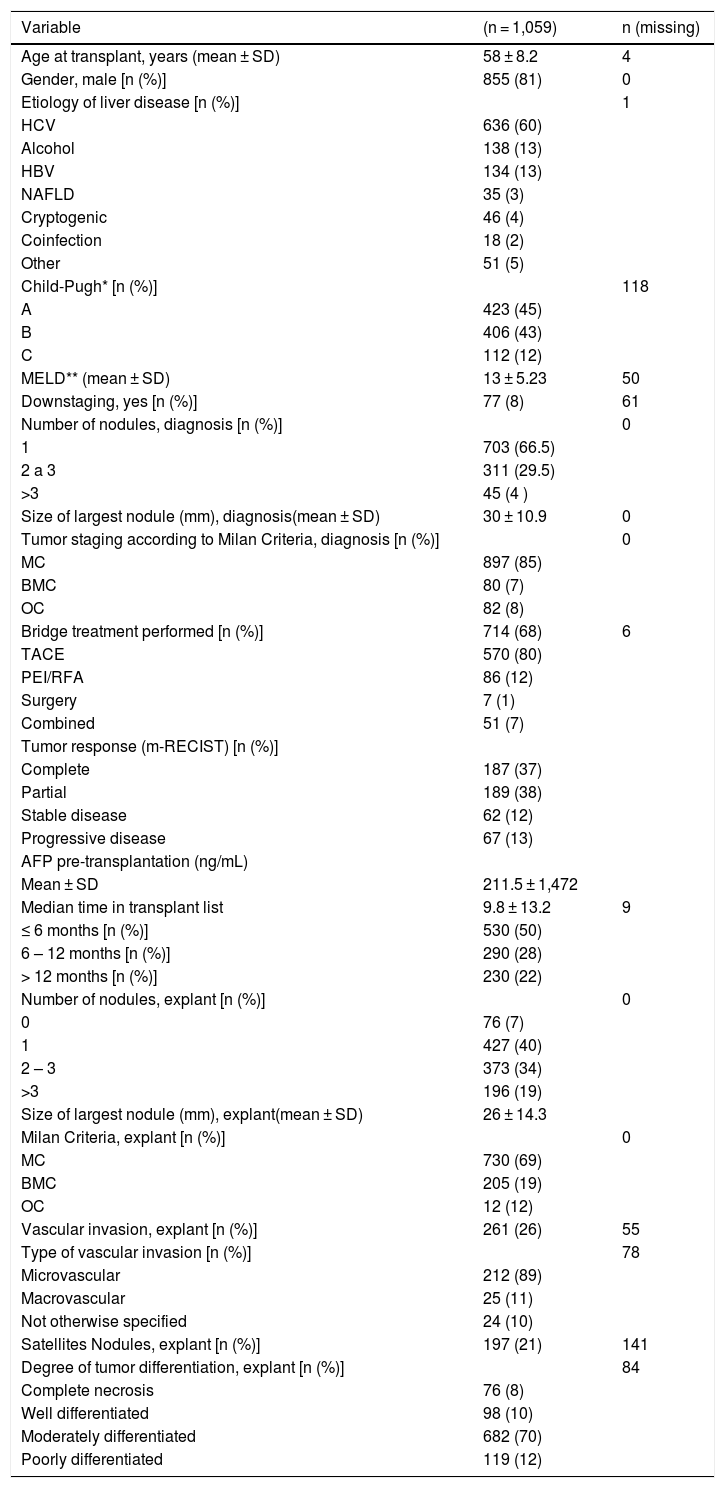
3RESULTS3.1Clinical and demographic characteristicsAfter applying the inclusion and exclusion criteria, 1,059 patients were eligible for the final analysis. The clinical, laboratory, radiological, and pathological characteristics of patients included in the study are summarized in Table 1. The majority of patients were males (81%; 855/1,059), with a median age of 58 years at transplant time. The etiology of liver disease was hepatitis C virus (HCV) in 60% of the patients. Seventy-seven patients (8%; 77 / 998) were included in the transplant list after successful preoperative “downstaging” to the BMC.
Clinical features of patients with hepatocellular carcinoma undergoing liver transplantation.
| Variable | (n = 1,059) | n (missing) |
|---|---|---|
| Age at transplant, years (mean ± SD) | 58 ± 8.2 | 4 |
| Gender, male [n (%)] | 855 (81) | 0 |
| Etiology of liver disease [n (%)] | 1 | |
| HCV | 636 (60) | |
| Alcohol | 138 (13) | |
| HBV | 134 (13) | |
| NAFLD | 35 (3) | |
| Cryptogenic | 46 (4) | |
| Coinfection | 18 (2) | |
| Other | 51 (5) | |
| Child-Pugh* [n (%)] | 118 | |
| A | 423 (45) | |
| B | 406 (43) | |
| C | 112 (12) | |
| MELD** (mean ± SD) | 13 ± 5.23 | 50 |
| Downstaging, yes [n (%)] | 77 (8) | 61 |
| Number of nodules, diagnosis [n (%)] | 0 | |
| 1 | 703 (66.5) | |
| 2 a 3 | 311 (29.5) | |
| >3 | 45 (4 ) | |
| Size of largest nodule (mm), diagnosis(mean ± SD) | 30 ± 10.9 | 0 |
| Tumor staging according to Milan Criteria, diagnosis [n (%)] | 0 | |
| MC | 897 (85) | |
| BMC | 80 (7) | |
| OC | 82 (8) | |
| Bridge treatment performed [n (%)] | 714 (68) | 6 |
| TACE | 570 (80) | |
| PEI/RFA | 86 (12) | |
| Surgery | 7 (1) | |
| Combined | 51 (7) | |
| Tumor response (m-RECIST) [n (%)] | ||
| Complete | 187 (37) | |
| Partial | 189 (38) | |
| Stable disease | 62 (12) | |
| Progressive disease | 67 (13) | |
| AFP pre-transplantation (ng/mL) | ||
| Mean ± SD | 211.5 ± 1,472 | |
| Median time in transplant list | 9.8 ± 13.2 | 9 |
| ≤ 6 months [n (%)] | 530 (50) | |
| 6 – 12 months [n (%)] | 290 (28) | |
| > 12 months [n (%)] | 230 (22) | |
| Number of nodules, explant [n (%)] | 0 | |
| 0 | 76 (7) | |
| 1 | 427 (40) | |
| 2 – 3 | 373 (34) | |
| >3 | 196 (19) | |
| Size of largest nodule (mm), explant(mean ± SD) | 26 ± 14.3 | |
| Milan Criteria, explant [n (%)] | 0 | |
| MC | 730 (69) | |
| BMC | 205 (19) | |
| OC | 12 (12) | |
| Vascular invasion, explant [n (%)] | 261 (26) | 55 |
| Type of vascular invasion [n (%)] | 78 | |
| Microvascular | 212 (89) | |
| Macrovascular | 25 (11) | |
| Not otherwise specified | 24 (10) | |
| Satellites Nodules, explant [n (%)] | 197 (21) | 141 |
| Degree of tumor differentiation, explant [n (%)] | 84 | |
| Complete necrosis | 76 (8) | |
| Well differentiated | 98 (10) | |
| Moderately differentiated | 682 (70) | |
| Poorly differentiated | 119 (12) |
HCV: Hepatitis C virus; HBV: Hepatitis B virus; NAFLD: Non-alcoholic fatty liver disease; MELD: Model For End-Stage Liver Disease; MC: Milan Criteria; BMC: outside MC, but within Brazilian Milan Criteria; OC: Outside both criteria; AFP: alpha-fetoprotein; TACE: Transarterial Chemoembolization; PEI: Percutaneous Ethanol Injection; RFA: Radiofrequency ablation; SD: standard deviation.
*Child-Pugh at the moment of inclusion on transplant list;
**MELD at the moment of inclusion on transplant list.
Upon diagnosis, radiological studies revealed that most patients had uninodular HCC (66.5%; 703/1,059), with a mean size of the largest tumor of 30 ± 10.9 mm. The majority of patients (85%; 897/1,059) were within MC, 7% outside MC but within BMC and 8% were outside both criteria. The vast majority (73%) of OC patients at diagnosis had gone through successful preoperative “downstaging” and was later on included in the transplant list. In 17% OC patients were transplanted without reference to “downstaging” before LT and in 9.8% no data were provided regarding “downstaging”.
Median time in the OLT waitlist was 9.8 months, and in 49.5% (520/1,050) of patients, this time was longer than six months. During the waiting list period, bridging therapy was performed in 68% (714/1,053) of patients, and the most frequent bridging treatment modality (80%; 570/714) was trans-arterial chemoembolization (TACE).
In explant analysis, differing from radiological diagnosis, only 40% (427/1,059) of patients had one nodule, whereas 19% (199/1,059) had multifocal HCC (> 3 nodules). The average size of the largest tumor was 26 ± 14.3 mm and HCC was moderately differentiated in 70% (682 /975) of cases. Vascular invasion was present in 26% (261/1,004) of patients. In the explant, 69% (730/1,059) of cases were within MC, 19% (222/1,059) outside MC, but within BMC and 12% (103/1,059) were outside both criteria.
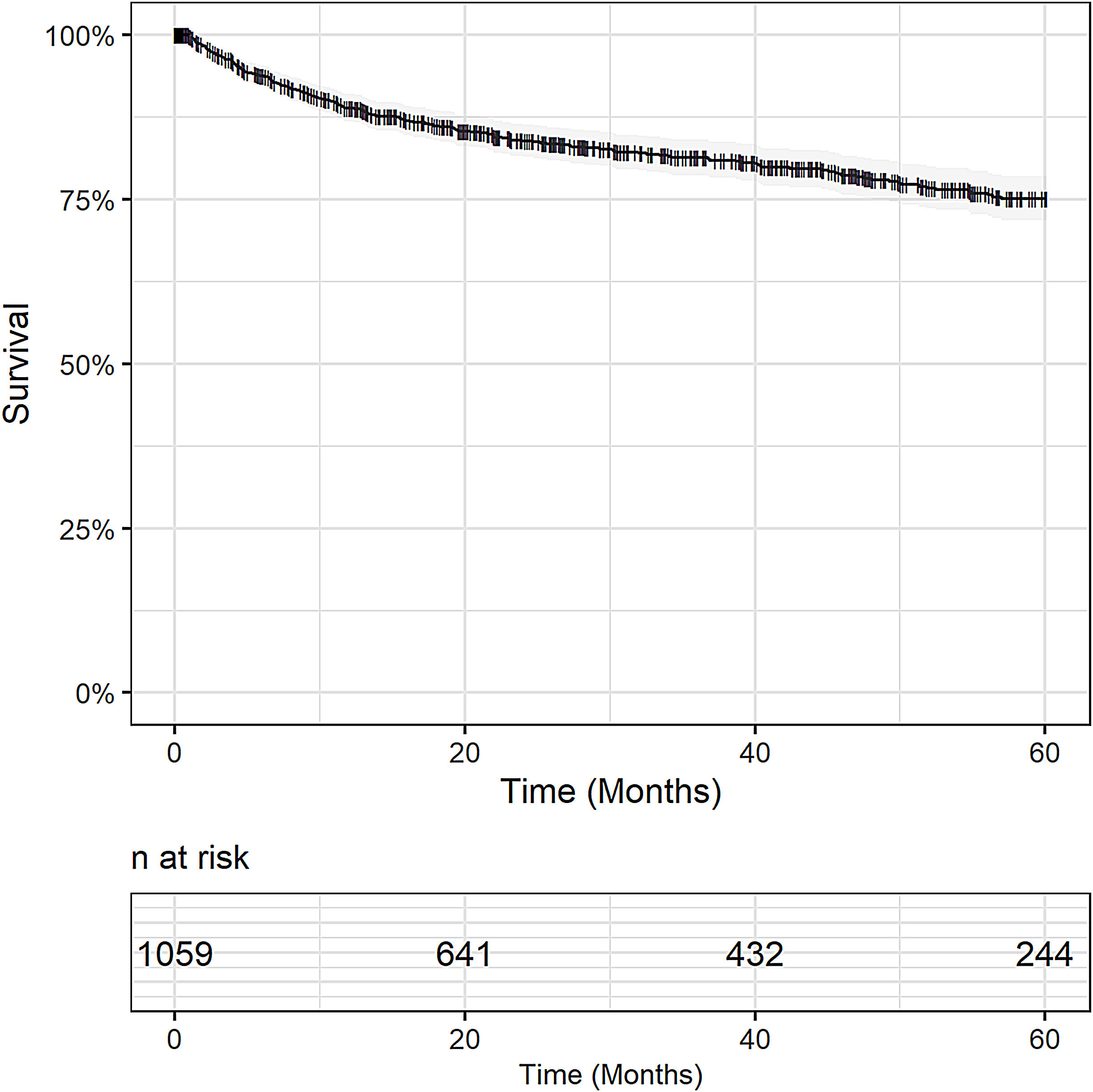
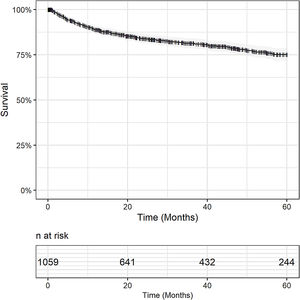
3.2Overall and disease-free survivalMedian follow-up period was 28 months. Overall survival was 79% in one year (95% CI 76.7 - 81.5), 72.5% (95% CI 69.7 – 75.3) in 3 years and 63% (95% CI 58.8 – 66.0) in 5 years. Censoring patients who died within 30 days after surgery, OS was 89% in one year (95% CI 87 – 91), 81% in 3 years (95% CI 79 – 84) and 75% in 5 years (95% CI 72 – 79). Fig. 1.
At the time of censoring, 311 (29.4%; 311/1,059) patients had died, and for 114 patients (36.6%; 114/311) death occurred within the first 30 days after transplantation. In 19% (59/311) of cases, the cause of death was related to post-OLT HCC recurrence.
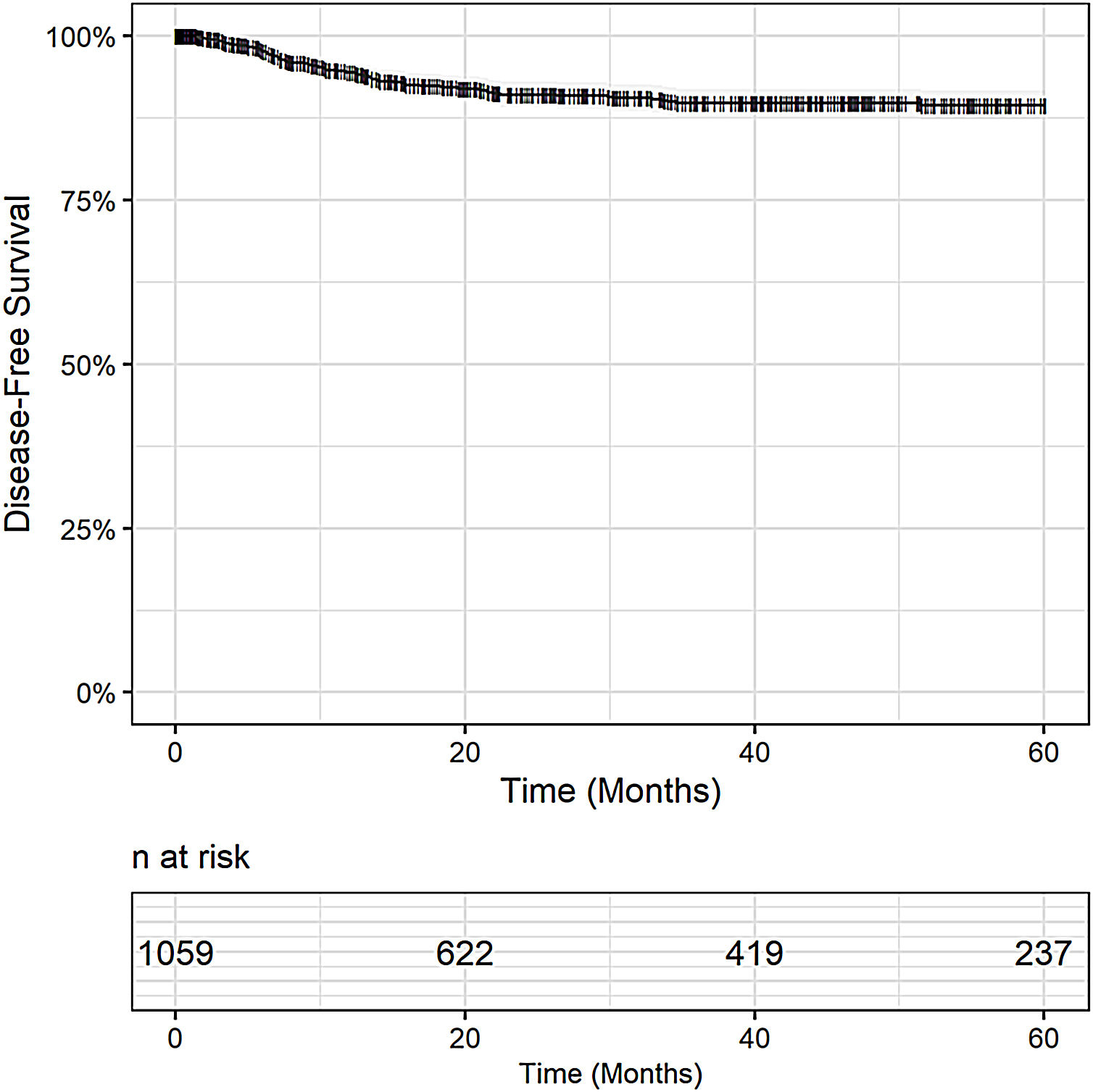
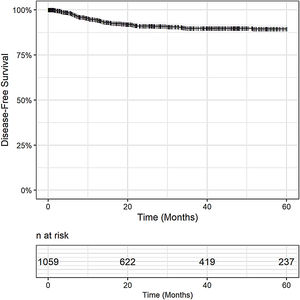
Post-OLT HCC recurrence was observed in 8% (79/1,059) of patients. The DFS was 94% in 1 year (95% CI 93 - 96), 90% in 3 years (95% CI 88 - 92), and 89% in 5 years (95% CI 87 - 92), Fig. 2. Post-OLT HCC recurrence had a major impact on survival. Post-recurrence survival was 34% in 1 year (95% CI: 24.4 - 46.2), 19% in 3 years (95% CI: 11 - 31) and 13% in 5 years (7 – 27).
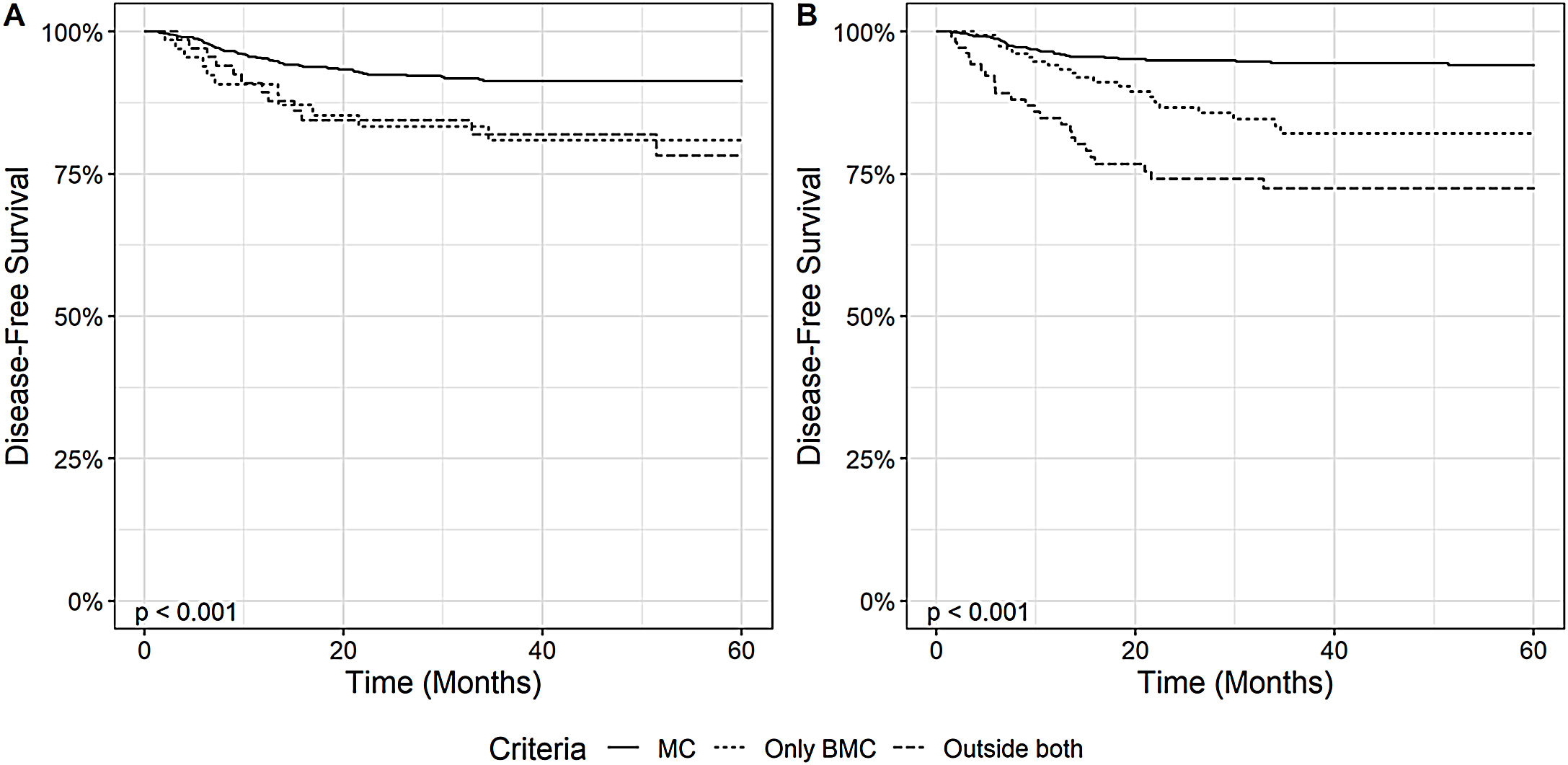
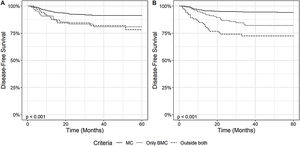
3.3Impact of Milan Criteria and Brazilian Milan Criteria on overall and disease-free survivalThe impact of HCC staging according to MC and BMC on the disease-free survival was assessed, as depicted in Fig. 3. Patients within MC at diagnosis showed a higher DFS than patients outside MC and within BMC, and patients OC (p < 0.001). When we analyzed the explant data, we observed similar results. Patients within MC had a 1-year, 3-year and 5-year DFS, respectively, of 96% (95% CI: 95 - 98), 94% (95% CI: 93 - 96) and 94% (95% CI: 92 – 96), while patients outside MC, but within BMC had DFS of 94% (95% CI: 90 - 98), 82% (95% CI: 75 - 89), and 82% (95% CI: 75 - 89) and OC patients 85% (95% CI: 78 - 92), 72% (95% CI: 64 - 82) and 72% (95% CI: 64 – 82) (p < 0.001).
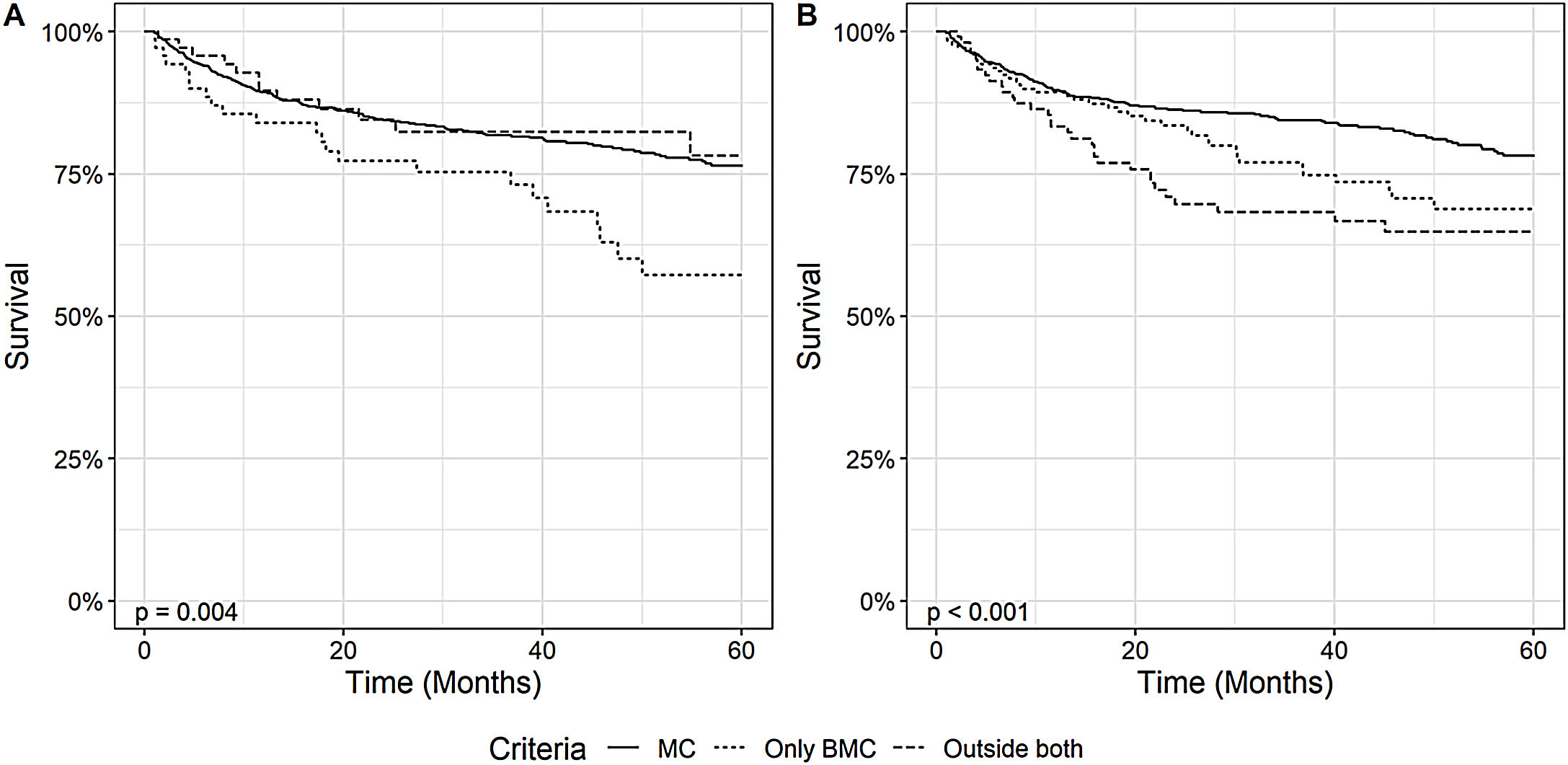
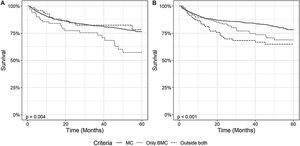
Regarding post-OLT survival, patients within MC also had a higher overall survival than patients within BMC, both when analyzing the diagnosis and explant data, as demonstrated in Fig. 4. Patients within MC in the explant had a survival rate of 90% (95% CI: 87 - 92) in 1 year, 84% (95% CI: 82 - 87) in 3 years and 78% (95% CI: 74 - 82) in 5 years, significantly higher than patients outside MC but within BMC who had, respectively, 1-year, 3-year and 5-year OS of 89% (95% CI: 85 - 94), 77% (95% CI: 70 - 84) and 69% (95% CI: 60 - 78) or OC patients who had an OS, respectively, of 83% (95% CI: 76 - 91), 68% (95% CI: 59 - 79) and 65% (95% CI: 55 - 76) (p = 0.003). We also performed the survival analysis including patients who died within the first 30 days after transplantation. The results obtained were similar, demonstrating a higher survival of patients within MC comparing to patients outside MC and inside BMC. This results are available as a supplement to the article.
We also analyzed the impact of the Milan Criteria on post-OLT HCC recurrence and survival, through univariate Cox regression. Patients outside MC at explant had a higher risk of post-OLT HCC recurrence when compared to patients within MC (HR: 3.78 95% CI 2.41 – 5.93, p < 0,001). When we analyzed the prognostic factors according to the BMC, patients outside MC and within BMC (HR: 2.8, 95% CI 1.63– 4.82) and patients OC (HR: 5.48, 95% CI 3.24 – 9.24) had a higher risk of tumor recurrence when compared to patients within MC. We also demonstrated that being within MC on liver explant were prognostic factors associated with better survival (Table 2).
Impact of Milan Criteria on post-liver transplant hepatocellular carcinoma recurrence and survival by Simple Cox Regression.
| Variable | Group | Simple Cox Regression | |
|---|---|---|---|
| HR (CI 95%) | p value | ||
| Recurrence | |||
| MC, explant | No/Yes | 3.78 (2.41 – 5.93) | < 0.001 |
| MC, explant | Outside MC and within BMC/ MC | 2.8 (1.63 – 4.82) | < 0.001 |
| OC/ MC | 5.48 (3.24 – 9.24) | < 0.001 | |
| Overall Survival | |||
| MC, explant | No/Yes | 1.64 (1.22 – 2.21) | 0.001 |
| MC, explant | Outside MC and within BMC/ MC | 1.46 (1.01 – 2.1) | 0.003 |
| OC/ MC | 1.93 (1.3 – 2.85) | 0.001 | |
HR: hazard ratio; CI: confidence interval; MC: Milan Criteria; BMC: Brazilian Milan Criteria; OC: Outside both criteria
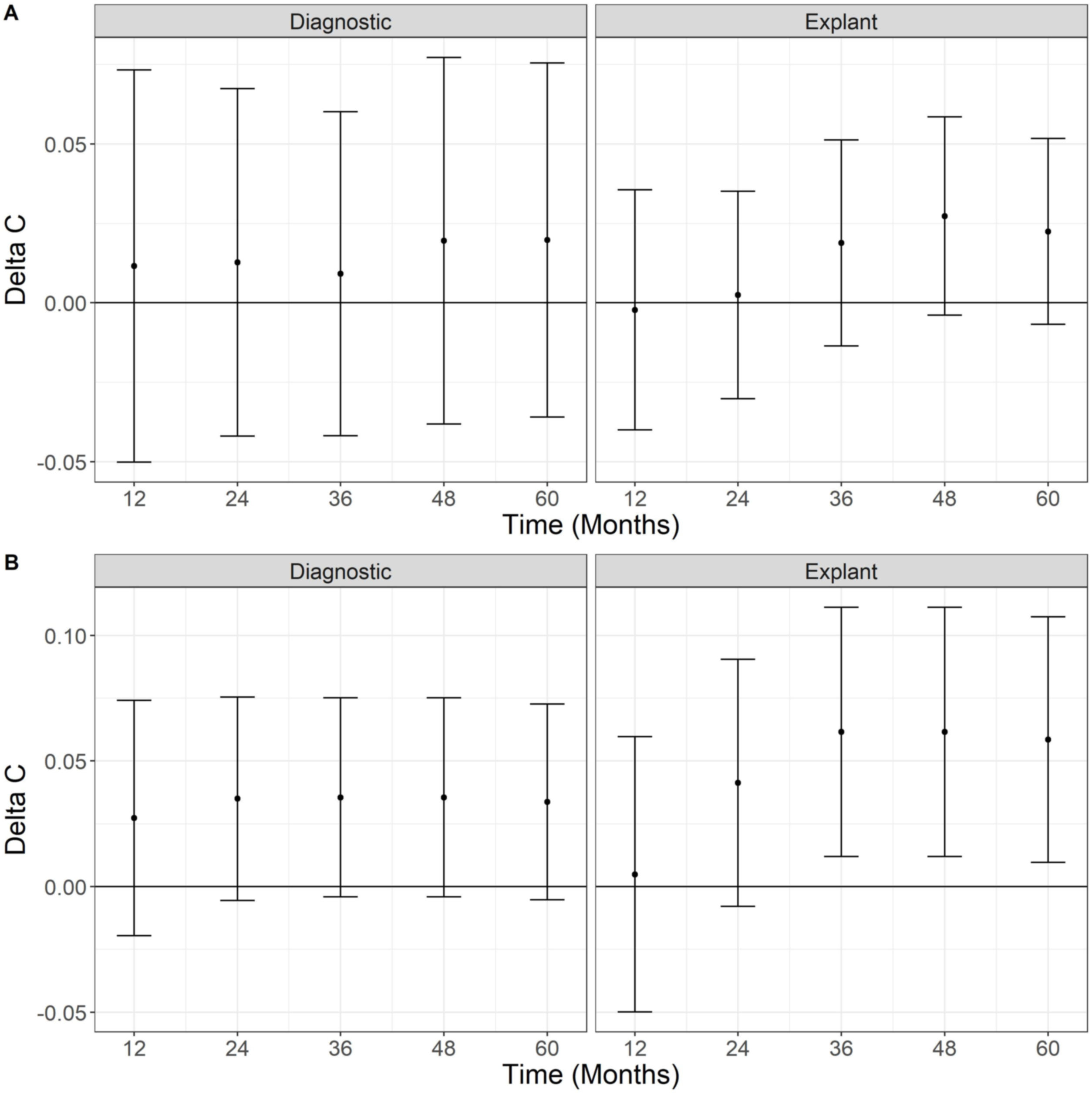
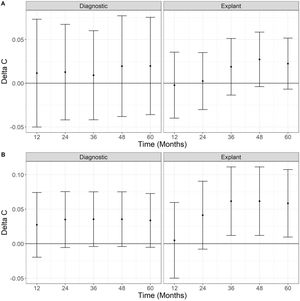
We compared the MC and the BMC using the Delta C-Statistic. Fig. 5 displays Delta C-statistic for OS and DFS at diagnosis and on explant over five years. No statistically significant differences of the predictive ability between BMC and CM were found, even though Delta C-Statistic is positive for OS and recurrence. On the other hand, using explant data, the Delta C-statistic was positive for all scenarios indicating that MC in the explant has a higher predictive ability for overall death and recurrence than BMC, with values that are statistically greater than zero (95% confidence does not include zero) for recurrence at 36, 48 and 60 months.
4DISCUSSIONThis study was designed to assess the performance of the Brazilian expanded version of Milan Criteria, called “Brazilian Milan Criteria”. We demonstrated that patients transplanted outside MC and within BMC had lower OS and DFS when compared to patients within MC.
The MC are still the most widely used criteria worldwide. It is the recommended criteria for HCC patient’s selection for OLT in the main guidelines and it is the basis for comparison with other suggested criteria [20–24]. Notwithstanding, due to improvement in OLT outcomes, MC have been considered too restrictive in these last few decades. Several studies have been carried out to identify the patients who, despite being outside MC, would present an acceptable post-LT survival, similar to those patients transplanted within MC. Several transplant centers have adopted an "expanded criteria" for the selection of HCC patients for LT [25–28].
In Brazil, the BMC was adopted early on by decision of a state expert committee. The decision not to consider nodules smaller than 2 cm as HCC was made taking into account the difficulties in the diagnosis of these small nodules at that time with the available imaging methods, associated with the low sensitivity of the biopsy for these small lesions [10]. However, with the evolution of knowledge and the higher quality of imaging studies currently available, this diagnostic criterion should be review by the transplant committee.
In the present study post-LT OS was 63% at 5 years. This survival is somewhat lower than that observed in Brazil for liver transplant recipients in general. According to data from the Brazilian Association of Organ Transplantation (ABTO), the OS of liver transplant recipients (HCC and non-HCC) in Brazil between 2010 and 2017 was 68% in 5 years [7]. Nonetheless, the survival rate found in our study is similar to that observed in the USA and Europe, where patients transplanted for primary malignant liver tumor have lower OS when compared to those transplanted for other causes [29–32]]. In the European Liver Transplantation Registry (ELTR) of 2018, which analyzed data from 132,466 patients, a 5-year survival of 64% was observed in transplanted patients with primary malignant liver tumors. In Latin America, a multicenter study involving 327 HCC transplant recipients presented the survival rate of 62.7% at 5 years [33]].
In the present work 36.6% of deaths occurred within the first 30 days after liver transplantation, demonstrating the great impact of the early postoperative period on survival. When we censored these patients, the 5-year OS was 75%. These results may reflect the fact that it is a large study involving several transplant groups, with different levels of experience in performing liver transplantation, also demonstrating the heterogeneity of transplantation services in Brazil in terms of human, financial and structural resources. Other studies have demonstrated similar results. In the ELTR, 20% of the deaths occurred in the first 30 days post-transplant and 38% in the first 6 months [32].
Post-OLT HCC recurrence occurred in 8% of patients in our study and had a major impact on prognosis, with a post-recurrence survival of only 13% in 5 years. Tumor recurrence was responsible for 19% of the deaths, corresponding to the second cause of death. These results are similar to that observed in other studies5. 32-34. In a systematic review that included 1,021 cases of post-transplant HCC relapse, the mean relapse rate was 16% and the median post-recurrence survival was 13 months [34]. Tumor recurrence was the cause of death in 9% of patients who underwent LT in Europe [32]. Such facts highlights the significant impact of HCC recurrence on survival and the importance of the evaluation of the factors related to a higher risk of recurrence in the selection of patients with HCC for OLT.
In the present study, the impact of the Milan Criteria in post-transplant outcomes was confirmed. Most of our patients were within the MC at diagnosis and in the explant and patients who were within MC in these assessments had higher DFS and OS when compared to patients outside MC. Patients within MC had a 5-year DFS of 94%, while patients outside MC, but within BMC and patients OC had DFS, respectively, of 82% and 72% in 5 years. Likewise those within MC in the explant had a OS of 78% significantly higher than patients outside MC, but within BMC or patients OC, who had, respectively, 69% and 65% survival in 5 years.
Several authors have also demonstrated the impact of the MC in post-OLT survival [35,36]. In the study by Donat et al., patients within MC had a 70.1% 5-year survival, compared with 47.1% in patients outside MC36]. A meta-analysis published by Mazzafero et al. that included 17,780 patients also confirmed the role of the MC as an independent prognostic factor for post-OLT outcomes, being able to identify patients with a favorable evolution and potential of achieving a 5-year survival rate of at least 70%, similar to patients transplanted without HCC[35].
In addition, being outside MC was a factor associated with a higher risk of HCC recurrence and worse survival. In a meta-analysis that included 1,198 patients, tumor staging outside MC in the explant showed a significant correlation with the risk of HCC recurrence [37].
We also demonstrated that BMC is inferior to MC to predict post LT tumor recurrence based on C-statistic. As the Milan Criteria are considered the gold standard in selection criteria for transplantation in the context of HCC, we expect that a new selection criterion would present better performance than MC in the ability to select patients adequately for liver transplantation. This evaluation was somewhat limited by the fact that BMC and MC are binary criteria instead of a continuum of probabilities. Therefore, the MC and the BMC ignore a broad spectrum of patients among those who meet and do not meet these criteria. Other selection criteria, such as Up-to-Seven criteria, created from a mathematical model, called “Metroticket Calculator,” do not have this limitation [38].
Our study had some limitations. Since it was a retrospective and multicenter study we had a great heterogeneity regarding the quality and availability of data provided by the centers. In the design of our study we considered for survival analysis death from all causes and not specifically death related to HCC and patients were censored at the time of death. We opted for this design since it was not possible to accurately assess the cause of death in all patients. In addition, it was not possible to perform a review of the imaging examinations and the explants. Despite of this, it was the largest multicenter study conducted in Brazil analyzing HCC transplanted patients, which makes this study of great relevance.
5CONCLUSIONSIn this large Brazilian multicenter study that evaluated the outcomes of HCC patients submitted to liver transplantation, we observed satisfactory survival and recurrence rates, comparable to those found in other countries. However, patients transplanted with Brazilian expanded criteria had lower OS and DFS when compared to patients within MC and showed a lower performance than MC in the ability to select patients adequately for liver transplantation. In view of the shortage of organs in many countries, the findings of the present study may lead to further reflection on the use of an expanded criteria always taking into consideration the heterogeneous organ donation patterns and transplant list dropout and mortality rates in patients with and without HCC.ABBREVIATIONSAASLD American Association for the Study of Liver Diseases Alpha-fetoprotein Brazilian Association of Organ Transplantation Brazilian Milan Criteria Disease-free survival European Liver Transplantation Registry Hepatocellular carcinoma Hepatitis B virus Hepatitis C virus Milan criteria Model for End-Stage Liver Disease Non-alcoholic fatty liver disease Outside both criteria Orthotopic liver transplantation Overall survival Percutaneous Ethanol Injection Radiofrequency ablation Standard deviation Transarterial Chemoembolization.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICT OF INTERESTNothing to disclose.
Brazilian HCC Study Group: Adriano Cláudio Pereira de Moraes, André Luís Conde Watanabe, Carlos Eduardo Sandoli Baia, Débora Raquel Benedita Terrabuio, Fernanda Branco, Gabriela Foinquinos, Gustavo de Sousa Arantes Ferreira, Leila Maria Moreira Beltrão Pereira, Luciana Oba Onishi Kikuchi, Márcio Dias de Almeida, Maria Lucia Zanotelli, Mariana Bina Possato, Elaine Cristina de Ataide, Paulo Everton Garcia Costa, Paulo Reichert, Paulo Roberto Fontes, Sylene Coutinho Rampche de Carvalho.