The liver imaging reporting data system (LI-RADS) for hepatocellular carcinoma (HCC) was proposed to standardize and enhance consensus of reporting. However, clinical utility of LI-RADS has not been evaluated in Latin America. We therefore sought to compare LI-RADS categories with histopathology findings in liver transplant (LT) explants in a regional center.
Materials and methodsProspective cohort study conducted between 2012 and 2018 in a single center from Argentina including patients with HCC listed for LT. LI-RADS definitions were applied to magnetic resonance images (MRI) or computed tomography (CT) abdominal scans at time of listing and at final pre-LT reassessment and compared to explant pathology findings; specifically, major nodule (NOD1).
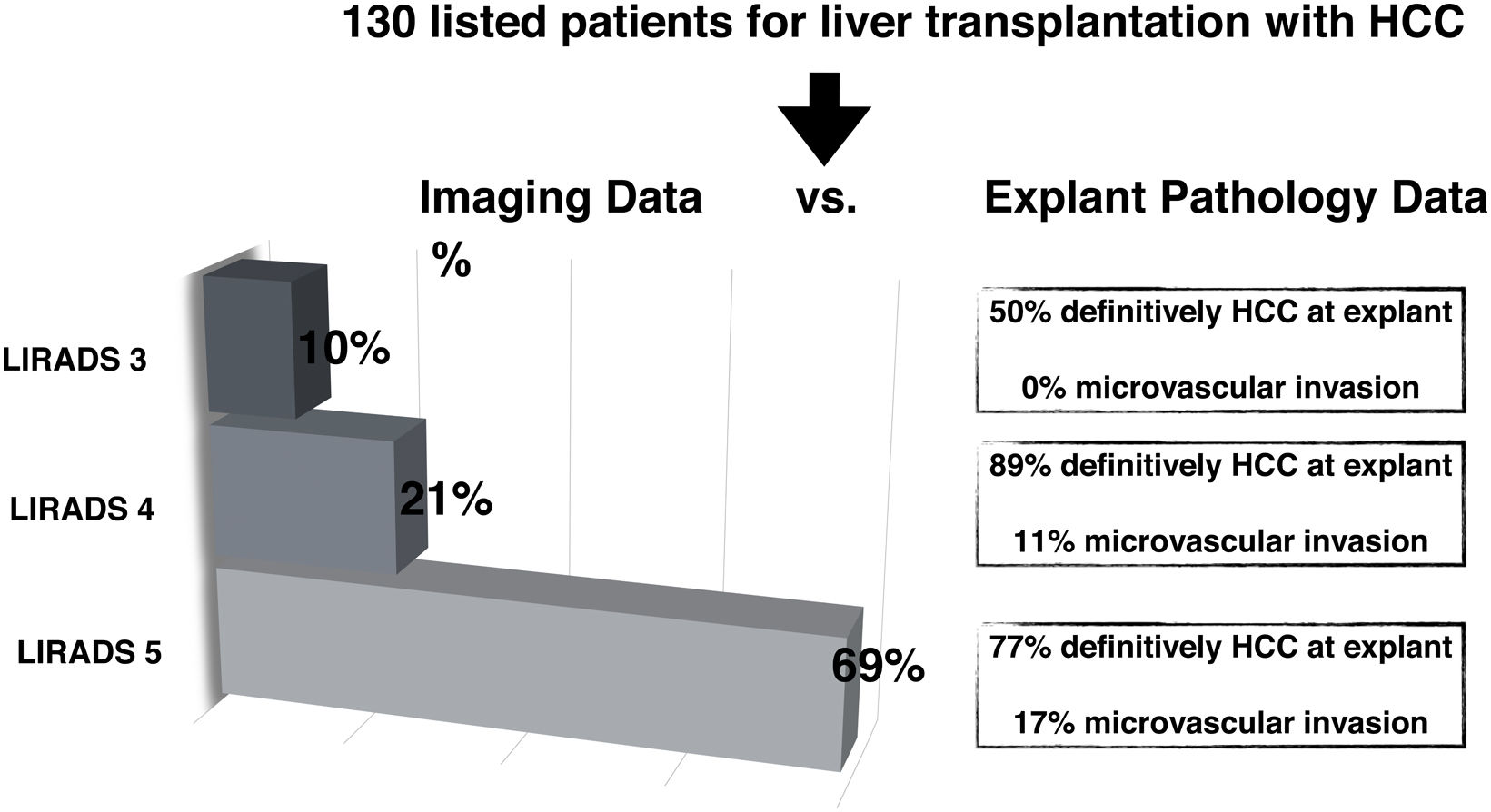
ResultsOf 130 patients with HCC listed for LT (96.1% with cirrhosis and 35.6% with hepatitis C virus infection), 72 underwent LT. Overall, 65% had imaging HCC diagnosis based on MRI (n = 84), 26% with CT (n = 34) and 9% (n = 12) with both methods. Among LT patients with pre-transplant imaging at our institution (n = 42/72), 69% of the NOD1 were LR-5, 21% LR-4 and 10% LR-3. Definite HCC diagnosis was 50% in LR-3 NOD1 (CI 18–90); none presented microvascular invasion. In LR-4 NOD1, HCC was confirmed in 89% (CI 59–98), of which 11% showed microvascular invasion; whereas in LR-5 NOD1 77% (CI 64–87) had confirmed HCC, 17% with microvascular invasion.
ConclusionsLI-RADS was useful to standardize reports; however, no significant differences were observed between LR-4 and LR-5 HCC probability when compared to explant pathology.
Hepatocellular carcinoma (HCC) has become a growing public health problem worldwide. It is the fifth most common cancer and the second cause of cancer-related mortality with 800,000 deaths per year [1]. Most patients have chronic liver disease, cirrhosis or viral hepatitis as underlying etiology [2]. Incidence has been growing globally during the last 25–30 years [1]. Although enormous efforts and progress have been made in HCC treatment, prognosis remains directly associated with disease stage at diagnosis. In early stages, curative therapies are feasible; conversely in advanced stages, prognosis is poor. Despite new first and second line systemic treatment options, with unthinkable outcomes compared to those of ten years ago, HCC mortality is still rising, making it a major public health concern [1].
Use of imaging criteria is crucial for HCC diagnosis. High organ specificity, means non-invasive diagnosis of HCC can be established using three-phase dynamic computed tomography (CT) scan or magnetic resonance imaging (MRI) in patients with cirrhosis, or chronic hepatitis B (HBV) or C infections (HCV) who are at high risk for HCC [3,4]. Diagnosis specificity is higher than 90% in these clinical conditions when presence of radiological hallmarks such as arterial phase hyper-enhancement (APHE) and wash-out during late or portal phases [5,6] are detected. However, some nodules may not present these typical signs, and biopsy may still be required in some cases [3,4].
In 2008, the American college of Radiology proposed the Liver Imaging Reporting and Data System (LI-RADS) to standardize interpretation and reporting of nodular liver lesions in patients at high risk for HCC [5,7]. The first version of LI-RADS appeared online in 2011 and it has been updated four times since then [7,8]. Five categories determine the probability or likelihood of malignancy. While LI-RADS 2 (LR-2) is more likely to be a benign nodule, LI-RADS 3–5 have increasing probabilities of HCC. LI-RADS M defines malignancy, and is not specific for HCC [7,8].
Since 2013, Argentina has required LR-5 category be present for MELD priority points to be authorized for HCC liver transplant (LT) candidates. This was based on previous local reports showing high false positive imaging rates compared to explant pathology results [9].
However, the clinical benefit of stratifying imaging observations into HCC probabilities has not been prospectively validated [10]. In addition, likelihood of HCC for each LI-RADS category may overlap to some degree [11]. We hypothesize that when radiological reports are compared to explant pathology findings there may be not sufficiently significant difference between categories LR-4 and LR-5. Our aim was to compare imaging reports based on LI-RADS categories to histological findings of explant pathology after LT.
2Materials and methodsThis prospective observational cohort study was conducted in a single LT center in Buenos Aires, Argentina, from January 1st 2012 to January 1st 2018. All procedures followed STROBE guidelines [12]. The Austral University Institutional Ethics Committee approved the study protocol (CIE 17-065), which complied with ethical standards, confidentiality and the Helsinki Declaration of 1975, as revised in 2008. Study protocol was registered as part of a multicenter study (NCT03775863).
2.1Cohort characteristics and study variablesAll eligible patients were included on a sequential basis to prospectively record data from medical charts. Inclusion criteria required patients be adults (>17 years of age) with a) HCC imaging diagnosis according to international guidelines [3,4]; b) cirrhosis or chronic HCV or HBV infection and c) be listed for LT either for HCC or decompensated cirrhosis. Exclusion criteria considered patients with a) LI-RADS non-applicability criteria (chronic portal vein thrombosis, cardiac congestion, diffuse nodular hyperplasia, hereditary hemorrhagic telangiectasia, Budd-Chiari syndrome) [7,8] or b) Incidental HCC diagnosed in explant pathology specimen.
Standard selection criteria used for LT at our center are the Milan criteria, in agreement with local allocation policies [13]. However, patients exceeding Milan criteria are also evaluated for LT if they meet the French AFP score criteria [14,15]. Systematic data recording was conducted in all included patients in the study protocol. Patient demographics, liver function test results, alpha-fetoprotein levels (AFP) and tumor characteristics at listing were recorded. Tumor burden based on radiological images was categorized according to Milan criteria and the AFP model.
All enhanced MRI or CT scans were evaluated by senior radiologists with more than 5 years’ experience in liver imaging diagnosis. In non-concordant cases, agreement needed to be reached prior to reporting. All visible nodules were prospectively categorized following LI-RADS recommendations (v.2011, v.2014, v.2017 and v.2018). The most recent LI-RADS v.2018 incorporates to the LR-5 category the criteria already validated by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver [3,4]. Nodules were assigned numbers according to size, such that NOD1 corresponded to the nodule with the largest diameter, followed by NOD2, NOD3, etc. Each nodule was measured during the arterial phase if margins were clearly visible, otherwise they were assessed during the vascular phase that showed its characteristics best [3,4].
Local/regional tumor treatment was recorded, namely: liver resection, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), trans-arterial chemoembolization (TACE) and trans-arterial radioembolization (TARE). Individual treatment was discussed on a case-by-case basis. Tumor imaging reassessment was conducted while patient were on the waiting list following international guidelines for western countries (AASLD and EASL) [3,4], with a minimum interval of once every 3 months. In patients receiving locoregional treatment, image re-evaluation was obtained 4–6 weeks after each procedure. Study protocol adopted RECIST 1.1 instead of modified RECIST criteria (M-RECIST) [16] to avoid misinterpretation of necrotic areas or heterogeneous evaluation of hyper vascular enhancement. We did not conduct post-treatment LI-RADS evaluation (v.2018) because at time of study analysis it had not yet been appropriately validated in randomized clinical trials, nor included in international guidelines.
The senior pathologist performing explant analysis was unaware of LI-RADS imaging categorization. Explant findings included macroscopic and microscopic evaluation reporting: total number of nodules, diameter of each nodule (cm), presence of microvascular invasion (MVI) and degree of tumor differentiation according to Edmonson-Steiner grading system (tumors were considered dedifferentiated when nuclear grade > II was observed). Site of each nodule in the explant was then specifically compared to its location on imaging reports. Nodules with 100% necrosis were excluded from HCC likelihood comparison between imaging and histology, but were still considered properly treated HCC nodes.
All patients were followed from time of listing to most recent control visit or death. Tumor progression was recorded following RECIST 1.1 criteria, as well as cause of dropout or death.
2.2Study end-pointsPrimary end-point was HCC probability and 95% confidence intervals (CI), assessed comparing LR-3 to LR-5 categories to histological findings on explant pathology. Specifically, we compared rates of definite HCC diagnosis between categories LR-4 and LR-5 in NOD1 nodules based on most recent image prior to LT, except patients with images from other institutions at last evaluation. In order to unify comparison criteria between different LI-RADS versions throughout the study period, each nodule was re-categorized according to the 2018 version (https://www.acr.org › media › ACR › Files › LI-RADS).
Ancillary features favoring HCC in particular, including restriction on diffusion, fat sparing, nodule-in-nodule, mosaic architecture, were reported by radiologists but not used to upgrade to LR-5 category, as recommended [17]. In cases of uncertainty between categories, the one with the lowest certainty of HCC was chosen, also as recommended. Discrepancies were resolved through agreement between radiologist.
2.3Statistical analysisStatistical significance is considered at p < 0.05. Categorical data were compared using Fisher’s exact test or Chi-Square test. Continuous variables are shown as mean (± standard deviation) or median (interquartile range 25–75%, IQR) and were compared with Student’s T test or Mann-Whitney U test, respectively, depending on distribution. Multiple comparisons for continuous data were carried out according to distribution using ANOVA or Kruskal Wallis tests, as appropriate. HCC probability for each LI-RADS category and the corresponding 95% confidence interval (CI) were calculated. Collected data was analyzed using STATA 13.0.
3ResultsA total of 130 patients with HCC listed for LT were included. Of these, 96.1% (n = 123) had cirrhosis and only 3.9% had HCC in a non-cirrhotic liver (Table 1). The most frequent chronic liver disease etiology was HCV in 35.6% (n = 46), followed by non-alcoholic fatty liver disease (NAFLD) in 20.1% (n = 26) and chronic alcohol consumption in 18.6% (n = 24).
Baseline patient characteristics (n = 130).
| Variable | Values |
|---|---|
| Age, years (± SD) | 57 ± 9 |
| Gender, Male, n (%) | 109 (84.5) |
| Median time on waiting list, (IQR), months | 10.5 (2.9−17.5) |
| Cirrhosis, n (%) | 123 (96.1) |
| Child Pugh A/B/C, n (%) | 57 (45)/42 (33)/28 (22) |
| Etiology of liver disease, n (%) | |
| Hepatitis C | 46 (35.6) |
| Hepatitis B | 7 (5.4) |
| Alcohol | 24 (18.6) |
| Cholestatic (PBC, SSC, PSC) | 6 (4.6) |
| NASH | 26 (20.1) |
| Cryptogenic | 14 (10.8) |
| Autoimmune | 1 (0.8) |
| Iron metabolism | 5 (3.9) |
| Locoregional treatments, n (%) | 67 (51.9) |
Abbreviations: PBC: primary biliary cholangitis; SSC: secondary sclerosing cholangitis; PSC: primary sclerosing cholangitis.
During a median time on the waiting list of 10.5 months (IQR 2.9−17.5), locoregional tumor bridging therapies were performed in 51.9% of patients (n = 67); the most of which was TACE. According to RECIST 1.1 criteria, 22.6% (CI 15.5–30.4) of listed patients presented tumor progression (n = 30) while on the waiting list. Only 5 patients progressed to macrovascular invasion (n = 1) or extrahepatic involvement (n = 4). Dropout rate or delisting was observed in 22.3% (CI 15.5–30.4), due either to tumor progression (n = 18), death (n = 8) or other causes (n = 3). At time of study analysis, 55.4% of patients had undergone LT (n = 72).
Explant pathology analysis showed median number of HCC nodules was 1 (IQR 1−2) with one median major nodule 22 mm in diameter (IQR 15−34). Presence of MVI and dedifferentiated tumors was observed in 30.3% (n = 14) and 8.0% (n = 6) of the cohort, respectively (Table 2).
Events during time on the waiting list and explant pathology findings.
| Variable | Values |
|---|---|
| Treatment during WL, n (%) | 67 (51.9) |
| PD (RECIST 1.1), n (%) | 30 (22.6) |
| Type of Progressive Disease (PD), n (%) | |
| Uninodular intrahepatic | 1 (3.4) |
| Multinodular intrahepatic | 19 (65.5) |
| Diffuse intrahepatic pattern | 4 (13.8) |
| Vascular invasion | 1 (3.4) |
| Extrahepatic disease | 4 (13.8) |
| Drop out, n (%) | 29 (22.3) |
| Tumor Progression | 18 |
| Death | 8 |
| Other reasons | 3 |
| Liver transplantation, n (%) | 72 (55.4) |
| -Explanted Liver Features | |
| Median number of HCC nodules (IQR) | 1 (1−2) |
| Median major nodule diameter, mm (IQR) | 22 (15−34) |
| Microvascular invasion, n (%) | 14 (20.3) |
| Nuclear grade > II, n (%) | 6 (8.0) |
Overall, of 130 patients included, 65% had imaging HCC diagnosis based on MRI scan (n = 84), 26% on CT scan (n = 34) and 9% (n = 12) based on both imaging methods. Median number of liver nodules visible on imaging studies was 2 (IQR 1−2), while median number of HCC nodules was 1 (IQR 1−2); and median NOD1 diameter was 32 mm (IQR 23–46). Median AFP serum level was 7.7 ng/mL (IQR 3.7–52).
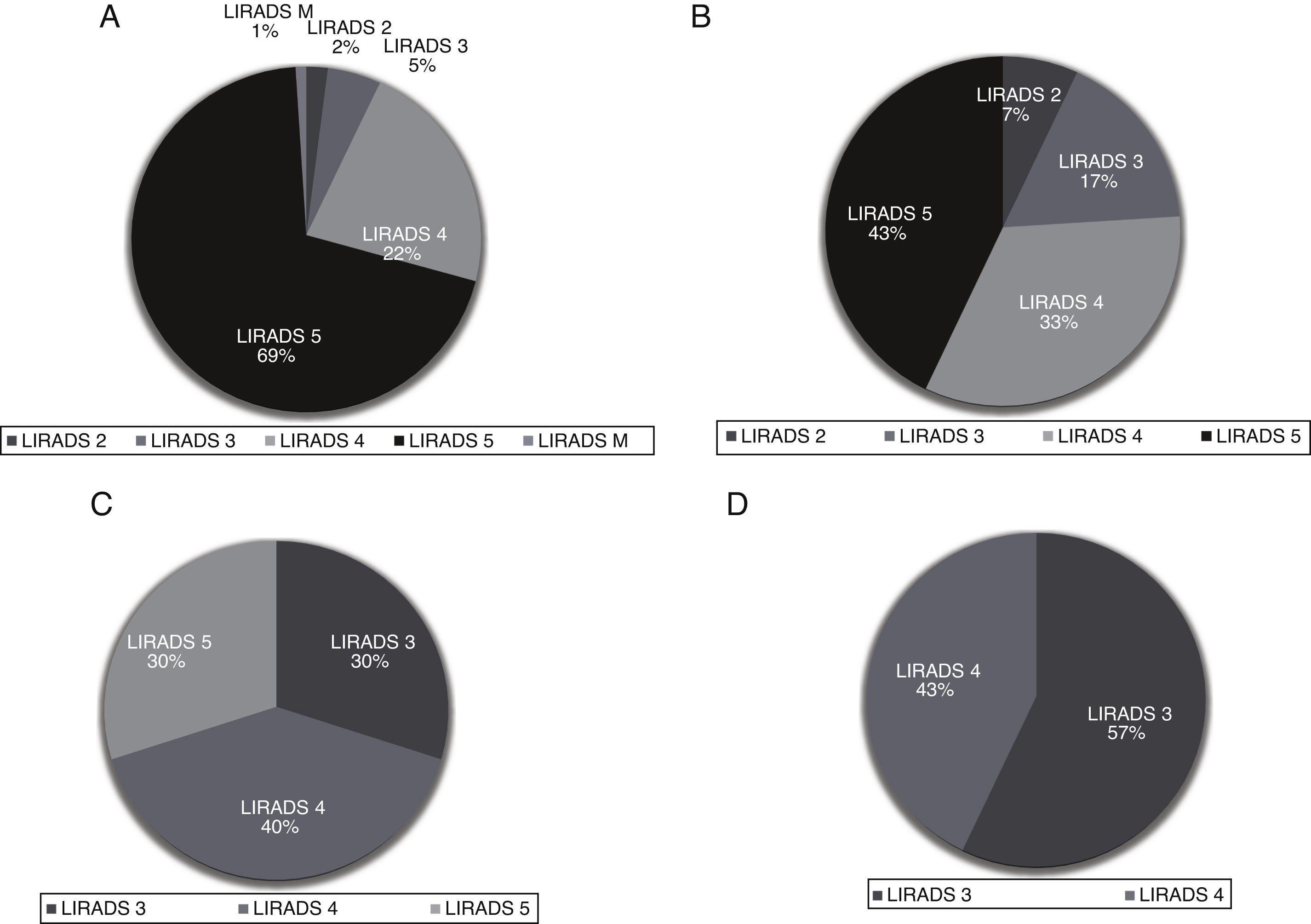
Most of the study population had a total number of HCC nodules ≤ 3 (n = 124); 60.8% (n = 79) had only 1 nodule, of which 87.2% presented with APHE and 73.1% delayed wash-out. In this group of patients, nodules were categorized as LR-5 in 69%, LR-4 in 22%, LR-3 in 5%, LR-2 in 2%, LR-M in 2% (infiltrative) (Fig. 1A). Thirty-two patients had a second liver nodule, with a median diameter of 14.5 mm (IQR 11.5–23), showing APHE in 74.3% and delayed wash-out in 61.3%. Of these, 43% were LR-5, 33% LR-4, 17% LR-3 and 7% LR-2(Fig. 1B). Twelve patients had a third lesion with a median diameter of 16 mm (IQR 11−25.5); 64% with APHE and 55% with delayed wash-out. These lesions were LR-4 in 40%, followed by LR-3 30% and LR-5 in 30% (Fig. 1C). Only 7 patients had 4 liver lesions, with a median diameter of 17 mm (13–25), 28.6% APHE and 28.9% wash-out. No LR-5 lesions were found; 57% were LR-3 and 43% LR-4 (Fig. 1D).
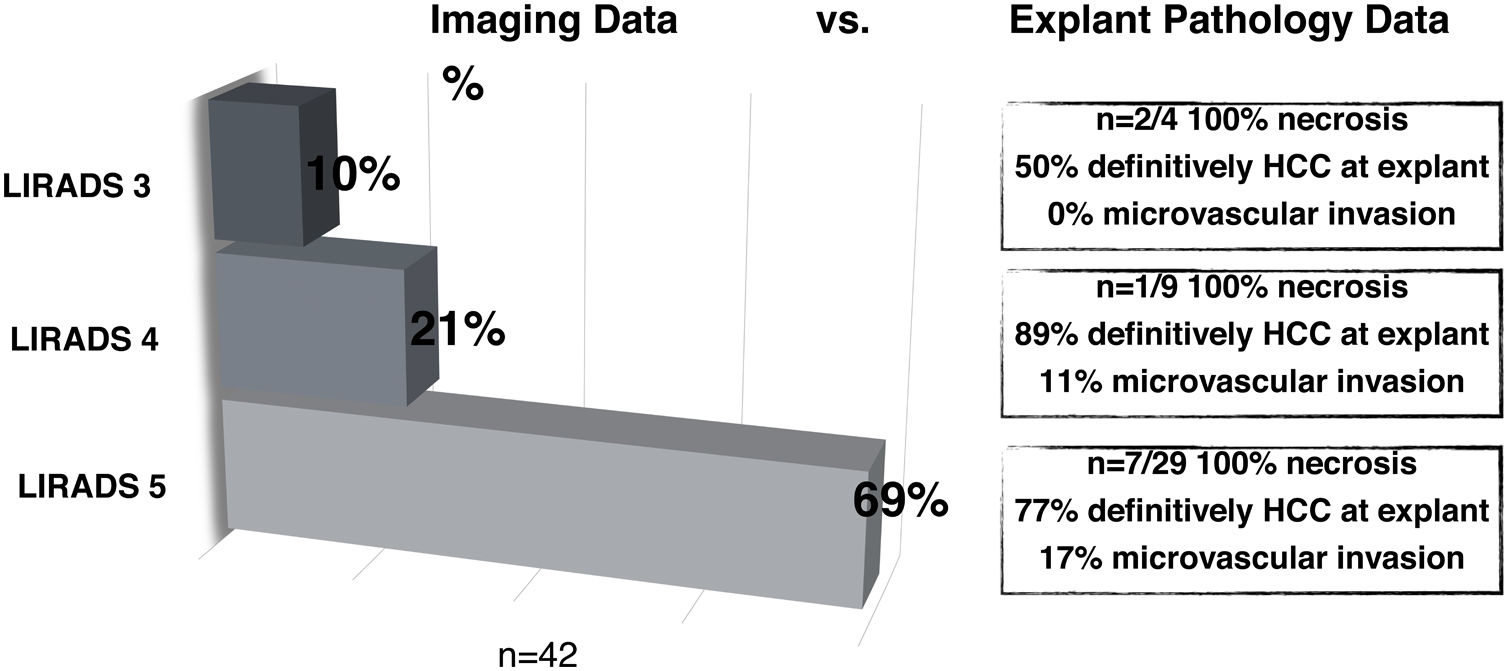
3.2HCC probabilities based on LI-RADS categorizationAll 72 patients who underwent LT had confirmation of HCC diagnosis explant pathology. Thus, there were no false positive diagnoses. When comparing imaging report of NOD1 to explant pathology findings in LT patients during in-house imaging reassessment (n = 42/72), 69% were LR-5, 21% LR-4 and 10% LR-3 (Fig. 2). Explant pathology showed 12 nodules with 100% necrosis and no residual tumor following bridging therapy. HCC diagnosis was confirmed in 50% of LR-3 nodules, none of which presented mvi. In LR-4 nodules, HCC diagnosis was confirmed in 89% of cases and 11% showed MVI; whereas in LR-5 nodules, 77% had confirmed HCC and 17% presented MVI. Consequently, probability of HCC for each LI-RADS category was: 50% (CI 18–90) for LR-3, 89% (CI 59–98) for LR-4 and 77% (CI 64–87) for LR-5. No statistically significant difference between LR-4 and LR-5 categories was found (Fig. 2).
4DiscussionOur study describes the real-world applicability of the LI-RADS system and compares findings to histological analysis. Imaging observations were compared based on extracellular-contrast MRI or CT scans to explant pathology following LT. Overall, almost seventy percent of major nodules were LR-5. Although HCC probability increased from LR-3 to LR-5 categories, fifty percent of LR-3 NOD 1 cases in this series were confirmed as HCC. When comparing LR-4 category to LR-5, although probability of HCC was over eighty percent, likelihood between categories did not differ, clinically or statistically. Previous papers have evaluated the use of LI-RADS, however, to our knowledge, this is the first study describing LI-RADS application in Latin America.
Strengths of the LI-RADS include reproducibility and ease of learning. It has a systematic, structured and standardized system for interpreting and reporting liver findings in patients with increased risk of HCC and can be used by experts and non-experts at academic or non-academic centers. It provides clearer language, enhancing communication between radiologists, hepatologists and liver surgeons [7,8]. However, some pitfalls in the diagnostic work-up algorithm exist in relation to probability of HCC [6,10] and inter-observer agreement needs be further evaluation in non-expert readers [18].
Although LIRADS reports the likelihood of HCC, the probability has not been validated. Indeed, LIRADS defines three groups of likelihood of HCC: definitive benign nodule, intermediate HCC probability (LR 2- to LR-4) and definite HCC diagnosis. Grey zone ranges from 20% to 80% [8,19]. In a recently published systematic review and meta-analysis, Van der Pol et al. showed that the likelihood of HCC for LR-2, LR-3, LR-4, LR-5 and LR-M categories was: 13% (CI 18–22), 38% (CI 31–45), 74% (CI 67–80), 94% (CI 92–96) and 36% (CI 26–48), respectively [19]. Confidence intervals showed clearly that intermediate risk populations have a wide range of HCC probability. This data came from retrospective cohort studies showing different levels of bias. Other studies not included in this meta-analysis, reported higher likelihood of HCC for LR-4 category in the HBV population [20]. In another Italian study of a retrospective cohort evaluating applicability of contrast-enhanced ultrasound, HCC probability for LR-3 to LR-5 categories was 47%, 85% and 98%, respectively [21].
From a clinical point of view, how reliable is it to stratify intermediate HCC probability if the likelihood is greater than eighty percent? Our results are similar to those reported in another prospective cohort study from Spain [19]. We found that almost fifty percent of LR-3 nodules were ultimately HCC with greater likelihood for LR-4 nodules, a figure higher than expected. We observed no significant differences in HCC probability between LR-4 and LR-5 categories. In contrast, authors of another prospective cohort study, reported HCC probability for LR-3 to LR-5 categories as 33%/41%, 53%/54% and 94%/91% in MRI or CT scans, respectively [22]. Previously published studies assessed LI-RADS using version 2014, whereas in this study we the latest version was used. One important factor that may explain our findings is that we compared imaging data at most recent pre-LT evaluation to findings in explant pathology only in patients with local images. In this regard, Burke L et al. have shown that thirty percent of LR-4 nodules may progress to LR-5 in a median time of 6 months, mostly due to threshold growth or new capsule development [23]. Cautious interpretation of HCC probability is key intermediate categories LR-3 and LR- 4.
LI-RADS inter-observer agreement is another important point to address. Razek et al., found excellent inter-observer agreement between senior radiologists, particularly for LR-1, LR-2, LR-5, LR-TIV and LR-M categories [24]. However, agreement was poor in LR-3 and LR-4 [24]. Moreover, agreement between non-expert radiologist has not been assessed. In our study, although the agreement kappa test was not done, discrepancies were resolved on a case-by-case basis.
Finally, recent studies have also used the LR-TR algorithm for post-treatment evaluation [25]. We decided not to do so for this study because appropriately validation in a randomized clinical trial has yet to be conducted. Moreover, in grey zones (LR-TR equivocally viable), inter-observer agreement was modest [25].
Our study has some limitations shared by all observational studies. First, not all patients who underwent a CT or MRI scan at our institution were included. Because patients who underwent a LT had final HCC diagnosis at explant pathology, we focused on NOD1, corresponding to the largest nodule. True overall rate of HCC probability may therefore not have been reported. However, we included all patients with institutional imaging reassessment and compared findings to explant pathology results. We did not report ancillary findings, which are not recommended to upgrade from category LR-4 to LR-5 [17] and may show less inter-observer agreement than major HCC features [26].
In conclusion, LI-RADS was useful to standardize liver imaging observations and to enhance communication between radiologists and clinicians [27]. However, clinicians should be cautious when interpreting HCC probability for decision-making purposes. LIRADS category 4 may confer enough HCC probability to decide therapy. If other diagnostic procedures are decided as a consequence of misinterpreting these results however, a delay in HCC diagnosis or deleterious decisions may ensue. Consequently, clinicians should interpret LIRADS categories based on individual pre-test HCC probability as a Bayesian algorithm to correctly decide whether diagnostic evaluation is sufficient or further testing is needed. With regard to LT policies, regional and national agencies for organ procurement should consider this issue, not only for transplant eligible patients but also for those cases in which additional nodules are observed. Should agencies preclude patients with LR-4 nodules from LT, this policy should also apply to patients who fall within transplant criteria based on LR-5 categorization, but present additional LR-4 nodules. The likelihood of exceeding transplant criteria in these patients may be much greater than currently appreciated. Further pitfalls and clinical decision-making processes will also likely improve future LI-RADS versions.
Supportive foundationThis research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Conflict-of-interestM Thompson has no conflict of interest. F Piñero has received Advisory Board and speaker honoraria and he is consultant of BAYER Cono-Sur; research grants from the Argentinean National Institute of Cancer (INC ID-190), Argentinean National Ministry of Science and Technology Development (PICT 2017, FONCYT) and from the Latin American Liver Research Educational and Awareness Network (LALREAN). M Silva has received has received speaker honoraria and is a consultant for Abbvie, Gador, Bristol-Myers Squibb, Merck, BAYER and research grants from the Argentinean National Institute of Cancer (INC ID-190), Argentinean National Ministry of Science and Technology Development (PICT 2017, FONCYT).AbbreviationsAFP
Alpha-fetoprotein
APHEarterial-phase hyper-enhancement
CIConfidence Interval
CTComputerized Tomography
HCCHepatocellular carcinoma
HBVHepatitis B virus
HCVHepatitis C virus
HRHazard ratio
IQRInterquartile range
MELDModel for End-stage Liver Disease
MRIMagnetic resonance image
MVIMicrovascular invasion
NAFLDNon-alcoholic fatty liver disease
NASHNon-alcoholic steatohepatitis
NODNodule
LRLIRADS
LTLiver transplantation
LI-RADSLiver imaging reporting and data system
TACETrans-arterial chemoembolization
TARETrans-arterial radioembolization
RFARadiofrequency ablation (RFA)
SSCSecondary sclerosing cholangitis
PBCPrimary biliary cholangitis
PEIPercutaneous ethanol injection (PEI)
PSCPrimary sclerosing cholangitis
We thank the Latin American Liver Research, Education and Awareness Network (LALREAN) for their support.