Microvascular invasion (MVI) of is generally considered to be an important prognostic factor for hepatocellular carcinoma (HCC) after operation, An accurate prediction of MVI before operation is helpful for clinical decision-making before operation.
Material and methodsA retrospective analysis of 227 cases of hepatocellular carcinoma patients after hepatectomy has been confirmed the pathological result whether there was MVI, and has been determined the independent risk factors of MVI. Based on these independent risk factors, we constructed a clinical scoring risk model for predicting MVI.
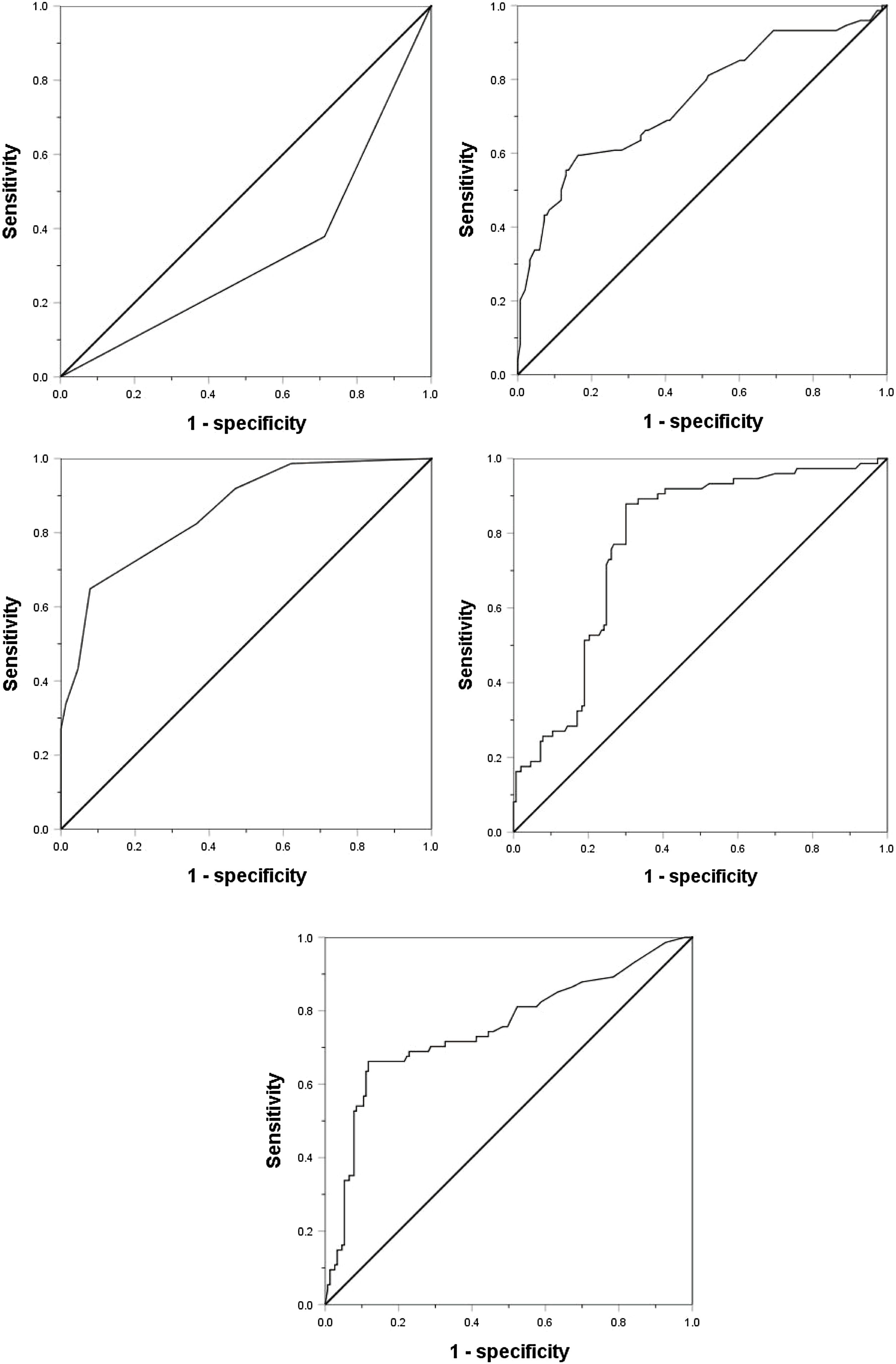
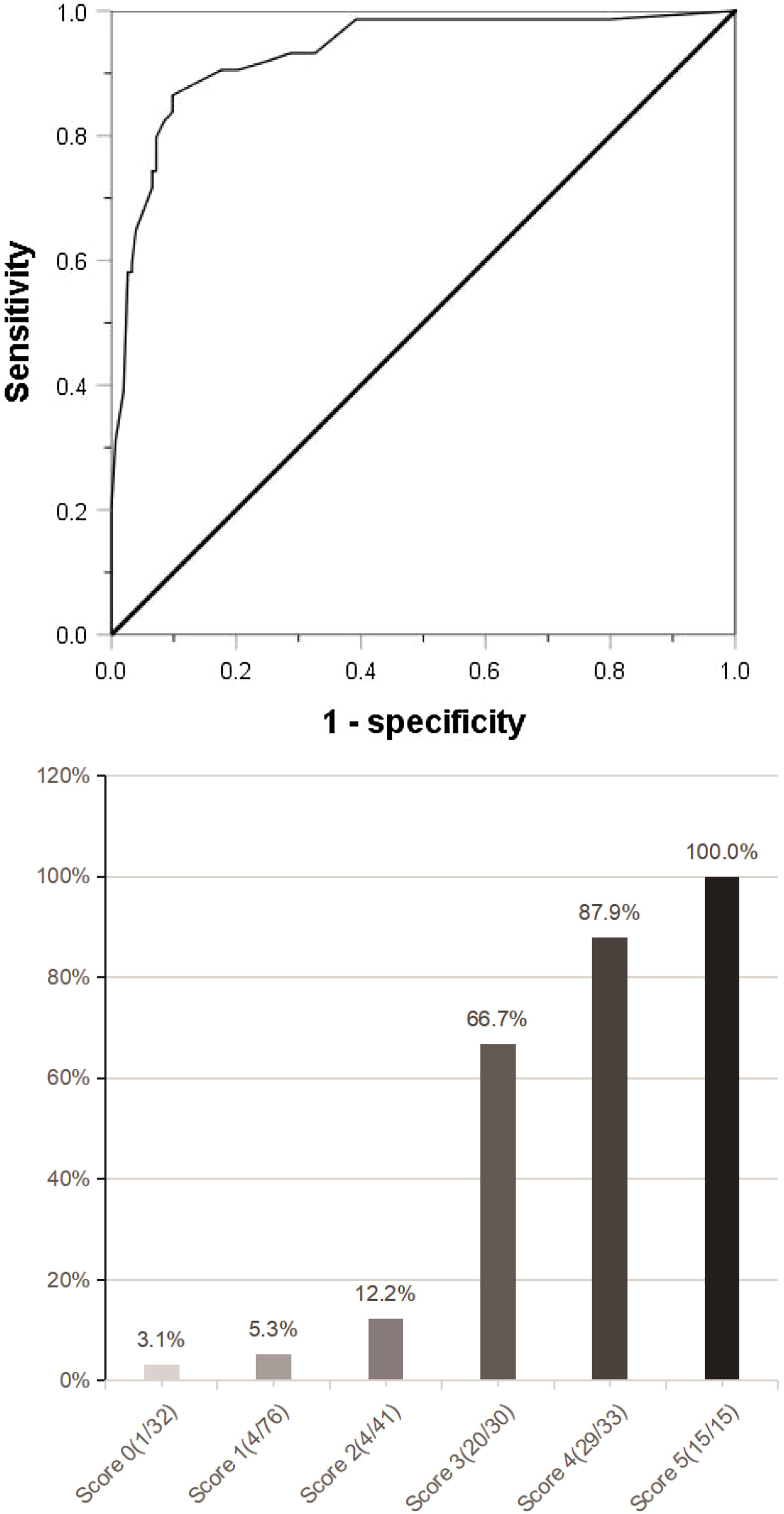
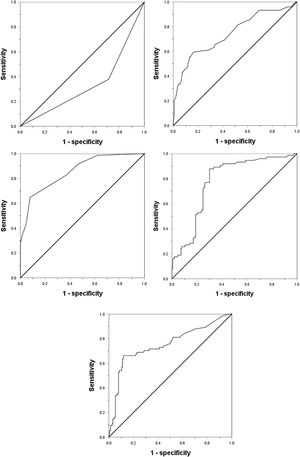
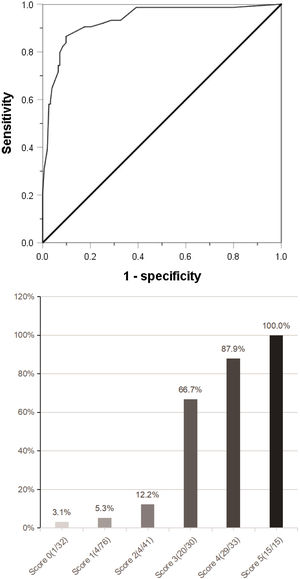
ResultsAmong the 227 patients with HCC, 74 (34.6%) were MVI positive. Using receiver operating characteristic (ROC) curve and logistic regression model, we found that alpha-fetoprotein(AFP)≥158 ng/mL(odds ratio[OR] = 4.152,95% confidence interval [95%CI]:1.602∼10.760,p = 0.003), Des-γ-carboxy prothrombin (DCP)≥178mAU/mL(OR = 9.730,95%CI:3.392∼27.910,p < 0.001), circulating tumor cells (CTCs)≥3/3.2 ml(OR = 7.747,95%CI:3.019∼19.881,P < 0.001), maximum tumor diameter≥59 mm(OR = 3.467,95%CI:1.368∼8.669,p = 0.008) and tumor margin unsmoothness(OR = 0.235,95%CI:0.096∼0.573,p = 0.001) were independent risk factors for MVI, they predicted that the area under the curve of MVI was 0.752, 0.777, 0.857, 0.743 and 0.333, respectively. Based on these five independent risk factors, we constructed a clinical scoring risk model for predicting MVI. The model predicts that the area under the curve of MVI is 0.922, and its prevalence rate from 0 to 5 are 3.1%(1/32), 5.3%(4/76), 12.2%(5/41), 66.7%(20/30), 87.9%(29/33), 100%(15/15), respectively (P < 0.001).
ConclusionBased on AFP, DCP, CTC, maximum tumor diameter and tumor margin unsmoothness, we constructed a model to predict the risk of MVI clinical score, so as to make a more accurate individualized treatment plan before operation, which has important clinical significance and application prospect to improve the curative effect of HCC.
Hepatocellular carcinoma (HCC) is the highest incidence of malignant tumor in the liver, ranking second in the world and the third leading cause of death from malignant tumors [1,2]. At present, hepatectomy is still the first choice for the treatment of hepatocellular carcinoma, but its 5-year recurrence rate is still as high as 40 %–70 % [3]. There are many high risk factors for postoperative recurrence of liver cancer, including residual focus, tumor diameter, number of tumors, degree of liver cirrhosis, mode of operation and so on [4–6]. Among them, the microvascular invasion (MVI) of hepatocellular carcinoma is an invasive manifestation of tumor behavior, and it is an independent factor leading to poor postoperative prognosis, which has been recognized and confirmed by many scholars [7,8]. MVI is defined as the nesting mass of cancer cells in the vascular lumen lined with endothelial cells under the microscope, which is dominated by branches of the portal vein adjacent to the cancer and contains intracapsular vessels [9]. At present, in clinical work, the diagnosis of MVI still depends on the pathological diagnosis after surgical resection of liver cancer, but it is difficult to judge the existence of MVI before treatment, which affects the next treatment strategy. Therefore, it is very important to accurately predict the choice of the best individualized treatment for hepatocellular carcinoma before operation.
In recent years, circulating tumor cell (CTC) is considered to be a major source of postoperative recurrence and metastasis of liver cancer, and has become an important starting point to study the process and mechanism of tumor metastasis and recurrence [10]. CTC is a tumor cell that metastases from the primary tumor to the blood or lymphatic system, and then locates in the blood, bone marrow, lymph nodes and other healthy organs. The existence of it is the process of tumor growth and distant formation of metastatic foci [11,12]. Although a large number of previous studies have found that CTC is related to the formation of portal vein tumor thrombus in hepatocellular carcinoma and affect the postoperative prognosis, there is no report on whether there is a correlation between CTC and MVI [13,14]. Tumor cells break away from the primary focus and enter the blood circulation to form CTC, and MVI and CTC are important indicators to reflect tumor invasiveness [7,15]. It can be seen that CTC may be directly involved in the occurrence and development of MVI and has important diagnostic value in predicting MVI in patients with liver cancer before operation. On the other hand, in order to improve the accuracy of MVI prediction before surgery, many studies have combined with various factors related to MVI to construct a model for predicting MVI, which has made significant progress [16,17]. In this study, we retrospectively analyzed the imaging and serological features of patients with HCC, and constructed a clinical scoring risk model based on circulating tumor cells and valuable predictors of MVI, which can be used to predict preoperative MVI in hepatocellular carcinoma.
2Materials and methods2.1Study subjectsFrom January 2018 to March 2020, 227 patients were diagnosed as primary liver cancer and underwent radical hepatectomy in the Department of Hepatobiliary surgery, Zhongshan Hospital Affiliated to Sun Yat-sen University. The preoperative diagnosis of hepatocellular carcinoma mainly depends on the typical imaging performance of dynamic enhanced computed tomography (CT) combined with contrast enhanced magnetic resonance imaging (MRI). At the same time, the level of tumor markers and the history of hepatitis virus infection are also complementary factors in the diagnosis of liver cancer. The inclusion criteria of this study are as follows: (1) all patients were diagnosed as hepatocellular carcinoma by pathological examination after radical tumor resection; (2) there was no evidence of portal vein invasion or extrahepatic metastasis by Computed Tomography (CT); (3) there was no history of other malignant tumors; (4) they had not received any preoperative radiotherapy and chemotherapy; and (5) all patients provided written informed consent. This retrospective study was conducted with the approval of the Hospital Clinical Research Ethics Committee in accordance with the principles of the Helsinki Declaration.
2.2Preoperative clinical characteristicsWe reviewed the clinical features of all patients, including sex, age, liver cirrhosis, hepatitis B virus infection, Circulating tumor cells (CTCs), Alpha-fetoprotein (AFP), Des-γ-carboxy prothrombin (DCP), Total bilirubin (TB), Alanine aminotransferase (ALT), Aspartate aminotransferase (AST), Albumin (Alb), Glutamyltransferase (GGT), Alkaline phosphatase (ALP), Child-Pugh grade, maximum tumor diameter, number of tumor nodules and smoothness of tumor margin.
2.3Preoperative image analysisThe liver images of all patients with hepatocellular carcinoma were obtained by enhanced computed tomography (Siemens Somatom Sensation 16- Dedector CT, Germany) and enhanced magnetic resonance imaging (MRI,1.5 T, Siemens, Magnetom Symphony, Erlangen, Germany) one week before operation. Finally, the diagnosis was confirmed by the pathological examination of postoperative specimens as the gold standard. When contrast-enhanced CT was performed, the patients were injected with Nonionic contrast medium (iopramine,300mgI/mL) at the dose of 1.5 mL/kg, and then plain scan and post-enhanced three-phase dynamic imaging were performed. When contrast-enhanced MRI was performed, gadolinium ethoxybenzyldiethy-lenetriamine pentaacetic acid (Gd-EOB-DTPA,Primovist; Bayer Schering Pharma, Berlin,Germany) was injected into the patient at the dose of 0.1 mL/kg, and then the hepatobiliary phase-enhanced images were taken 15 min later. The margin of the tumor was evaluated in the venous phase of enhanced CT and hepatobiliary phase of enhanced MRI, and its type was finally determined by enhanced MRI. The edge of the tumor can be divided into two types: smooth and non-smooth. If the tumor capsule is intact and there is no extra-nodular sprouting part, the tumor edge is considered smooth; if the tumor capsule edge is blurred, there are local protruding areas, multi-nodular confluence or lobulation, the tumor edge is not smooth. All imaging features were independently evaluated by two radiologists with more than 5 years experience, who has turned a blind eye to the clinical features of the patients. The third radiologist with 20 years of experience joined the consensus meeting when there was a need to resolve differences in the evaluation. Finally, according to the imaging characteristics, they summed up the maximum diameter of the tumor, the number of tumor nodules and the smooth edge of the tumor capsule.
2.4Detection of CTCsThe blood samples of each patient with hepatocellular carcinoma were collected within one week before operation, and the CTC in the peripheral blood of each patient was detected by Cyttel (Jiangsu, China). First of all, when taking blood samples, the first step is to discard 2 mL blood after stirctly disinfecting the puncture site, and then use BD vacuum container tube to collect 3.2 mL of empty elbow vein blood to avoid contamination by skin and vascular endothelial epithelial cells. Then, the negative immunomagnetic particle method was sent for examination within 24 h, It is mainly used the human peripheral blood leukocyte removal kit with immunomagnetic particles as the carrier, using the centrifugation technology based on the antigen-antibody reaction principle in order to remove white blood cells in vitro. Afterwards, rare cells in blood can be seperated, so that CTC in blood can be obtained and enriched. Finally, immunofluorescence in situ hybridization (im-FISH) was performed within 24 h, and the glass slides were fixed with fixed solution, dehydrated with ethanol and dried, and hybridized with chromosome centromere probe 1 and chromosome centromere probe 8. After cleaning the cleaning solution at the end of hybridization, the plates were sealed with 4-diamidinyl-2-phenylindole (DAPI) staining solution and were observed and counted under photoluminescence microscope [18,19].
2.5Histopathological assessmentDuring the operation, the pathological specimens were collected using the "7 o'clock" baseline sampling method: (1) At the junction of the cancer at 12 o'clock, 3 o'clock, 6 o'clock and 9 o'clock in the direction of the cancer and adjacent tissues, the materials were collected at a ratio of 1:1; (2) At least within the tumor 1 piece of material; (3) 1 piece of material in each area ≤1 cm and >1 cm from the edge of the tumor. When two senior pathologists turned a blind eye to the imaging and clinical features, hematoxylin-eosin staining and immunohistochemistry were used to explain and confirm the pathological diagnosis, the result judged whether there was microvascular invasion and were divided into MVI positive group and MVI negative group. MVI refers to the presence of cancer cell nests under the microscope in the vascular cavity lined by endothelial cells, which are more common in the small portal vein branch (including the tumor capsule blood vessel) in the liver tissue adjacent to the cancer, followed by the hepatic vein branch and hepatic artery, and bile duct and small branches such as lymphatic vessels which are rarer.
2.6Statistical analysisSPSS 25.0 statistical software (IBM Corp,Armonk,NY,USA) was used to analyze the data. The following statistical methods were used to analyze the relationship between CTC, MVI and clinical parameters: independent sample t-test was used to express the mean ± standard deviation of continuous data in accordance with normal distribution; Wilcoxon rank sum test was used to express median (quartile) in continuous data of non-normal distribution; chi-square test or Fisher exact-test was used to compare classified data. The single factor with significance to MVI was further analyzed by Logistic regression model, and the odds ratio (OR) and 95% confidence interval (95%CI) were calculated. Afterwards, the ROC curve is used to calculate the cut-off value of the prediction index and the prediction score of MVI. All P values were obtained by double-tail test, and P < 0.05 was considered to be statistically significant.
3Results3.1Patient characteristicsThe 227 patients included 198 males and 29 females, with an average age of 55.39 ± 10.75 years (median age 56 years, range 27 years 86 years). According to the imaging features, the average maximum tumor diameter was 50.4 ± 31.2 mm (median 43.0 mm, range 2-200 mm), of which 171cases were single lesions and 56 cases were multiple lesions (43 cases were 2 lesions and 13 cases were 3 lesions). As far as the smooth type of tumor capsule was concerned, 137 patients' tumor capsule was not smooth (60.4%), and only 90 patients were smooth tumor capsule (39.6%).
3.2Relationship between CTCs and clinical characteristicsThe CTC counts detected this time ranged from 0 to 20 cells / 3.2 mL of peripheral blood, and 165 of 227 patients (72.7%) had detected one or more CTCs. According to the ROC curve, it is determined that CTCs = 3/3.2 mL is the cutoff value for the diagnosis of MVI, so we define the CTC value of peripheral blood ≥ 3/3.2 mL as positive and the CTC value of < 3/3.2 mL as negative. Among the 227patients, 117cases were CTC positive and 110cases were CTC negative. Table 1 showed that the preoperative AFP (P < 0.001) and the maximum tumor diameter (P = 0.023) were significantly correlated with the positive / negative rate of CTC, respectively.
Relationship between CTCs and clinical characteristics.
| Clinical characteristics | CTC≥3/3.2 mL | CTC<3/3.2 mL | P value |
|---|---|---|---|
| Positive(n = 117) | Negative(n = 110) | ||
| Age, years | 57(46.0−63.5) | 55(50.0−62.3) | 0.488 |
| Sex, n (%) | 0.414 | ||
| male | 100(85.5%) | 98(89.1%) | |
| female | 17(14.5%) | 12(10.9%) | |
| Hepatitis B virus infection, n (%) | 0.504 | ||
| Yes | 95(81.2%) | 93(84.5%) | |
| No | 22(18.8%) | 17(15.5%) | |
| Liver cirrhosis, n (%) | |||
| Yes | 75(64.1%) | 66(60.0%) | |
| No | 42(35.9%) | 44(40.0%) | |
| Child–Pugh, n (%) | 0.290 | ||
| A | 105(89.7%) | 103(93.6%) | |
| B | 12(10.3%) | 7(6.4%) | |
| AFP, ng/mL | 123.0(11.5−645.0) | 9.5(4.0−70.8) | <0.001 |
| DCP, mAU/mL | 206(60.0−400.5) | 127(38.0−465.5) | 0.115 |
| TB, μmol/L | 14.6(8.7−31.0) | 14.3(10.5−20.1) | 0.797 |
| ALT, U/L | 41.97 ± 39.24 | 39.75 ± 35.95 | 0.659 |
| AST, U/L | 45.10 ± 35.23 | 40.09 ± 42.56 | 0.334 |
| Alb, g/L | 39.68 ± 4.76 | 40.64 ± 5.32 | 0.153 |
| GGT, U/L | 91.44 ± 104.76 | 92.33 ± 99.00 | 0.948 |
| ALP, U/L | 92.59 ± 47.90 | 91.15 ± 49.01 | 0.824 |
| Maximum tumor diameter, mm | 50(35.0−80.0) | 36(25.0−55.0) | 0.023 |
| Number of tumors, n (%) | 0.726 | ||
| single | 87(74.4%) | 84(76.4%) | |
| multiple | 30(25.6%) | 26(23.6%) | |
| Tumor margin, n (%) | 0.210 | ||
| smooth | 66(56.4%) | 71(64.5%) | |
| non-smooth | 51(43.6%) | 39(35.5%) |
CTC, Circulating tumor cell; AFP, Alpha-fetoprotein; DCP, Des-γ-carboxy prothrombin; TB, Total bilirubin; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; Alb, Albumin; GGT, Gamma-glutamyltransferase; ALP, Alkaline phosphatase.
Considering the significant changes of AFP, DCP, CTC and the maximum tumor diameter, we drew ROC curves to determine their cutoff value. Except that the age is divided according to the median age of 55 years (actual 56 years), the other indicators are divided according to the normal value / abnormal value. The ROC curve shows that the area under the curve (AUC value) of AFP, DCP, CTC, maximum tumor diameter and tumor margin (smooth / uneven) are 0.752, 0.777, 0.857, 0.743, 0.333, respectively, and their best cut-off values are 158 ng/mL, 178mAU/mL, 3/3.2 ml, 59 mm, respectively(Fig. 1). As shown in Table 2, in order to evaluate the preoperative predictors of MVI, 227 patients were divided into two groups: MVI positive group (32.6%) and MVI negative group (67.4%), and the clinicopathological factors of the two groups were compared. Univariate analysis showed that AST > 40U/L(P = 0.029), AFP≥158 ng/mL(P < 0.001), DCP≥178mAU/mL(P < 0.001), CTC ≥ 3/3.2 ml(P < 0.001), maximum tumor diameter≥59 mm(P < 0.001) and unsmooth tumor margin(P < 0.001) were potential risk factors for MVI. Then, after all the potential risk factors were substituted into the multivariate analysis, it was found that only AFP≥158 ng/mL(OR = 4.152,95%CI:1.602∼10.760,p = 0.003), DCP≥178mAU/mL(OR = 9.730,95%CI:3.392∼27.910,p < 0.001), CTC ≥ 3/3.2 ml(OR = 7.747,95%CI:3.019∼19.881,p < 0.001), the maximum tumor diameter≥59 mm(OR = 3.467,95%CI:1.368∼8.669,p = 0.008) and unsmooth tumor margin(OR = 0.235,95%CI:0.096∼0.573,p = 0.001) were the independent risk factors for MVI (Table 3). Therefore, as shown in Fig. 2a, we combine AFP ≥ 158 ng/mL, DCP ≥ 178mAU/mL, CTC ≥ 3 / 3.2 ml, maximum tumor diameter ≥ 59 mm and tumor margin unsmoothness to draw the Roc curve for predicting MVI. The AUC value, sensitivity and specificity are 0.933, 86.49% and 90.20%, respectively.
Univariate analysis of potential risk factors for microvascular invasion.
| Clinical characteristics | Microvascular invasion, n (%) | P value | |
|---|---|---|---|
| Positive (n = 74, 32.6%) | Negative (n = 153, 64.7%) | ||
| Age, years | 0.244 | ||
| ≥55 | 36(48.6%) | 87(56.9%) | |
| <55 | 38(51.4%) | 66(43.1%) | |
| Sex | 0.537 | ||
| male | 66(89.2%) | 132(86.3%) | |
| female | 8(10.8%) | 21(13.7%) | |
| Hepatitis B virus infection | 0.914 | ||
| Yes | 61(82.4%) | 127(83.1%) | |
| No | 13(17.6%) | 26(17.0%) | |
| Liver cirrhosis | 0.763 | ||
| Yes | 47(63.5%) | 94(61.4%) | |
| No | 27(36.5%) | 59(38.6%) | |
| Child–Pugh | 0.151 | ||
| A | 65(87.8%) | 143(93.5%) | |
| B | 9(12.2%) | 10(6.5%) | |
| AFP, ng/mL | <0.001 | ||
| ≥158 | 49(66.2%) | 19(12.4%) | |
| <158 | 25(33.8%) | 134(87.6%) | |
| DCP, mAU/mL | <0.001 | ||
| ≥178 | 65(87.8%) | 47(30.7%) | |
| <178 | 9(12.2%) | 106(69.3%) | |
| CTC,n/3.2 ml | <0.001 | ||
| ≥3 | 61(82.4%) | 56(36.6%) | |
| <3 | 13(17.6%) | 97(63.7%) | |
| TB, μmol/L | 0.561 | ||
| >20.4 | 18 (24.3%) | 32(20.9%) | |
| ≤20.4 | 56 (75.7%) | 121(79.1%) | |
| ALT, U/L | 0.141 | ||
| >50 | 17(23.0%) | 23(15.0%) | |
| ≤50 | 57(77.0%) | 130(85.0%) | |
| AST, U/L | 0.029 | ||
| >40 | 31(41.9%) | 42(27.5%) | |
| ≤40 | 43(58.1%) | 111(72.5%) | |
| Alb, g/L | 0.553 | ||
| >35 | 58(78.4%) | 125(81.7%) | |
| ≤35 | 16(21.6%) | 28(18.3%) | |
| GGT, U/L | 0.895 | ||
| >60 | 36(48.6%) | 73(47.7%) | |
| ≤60 | 38(51.4%) | 80(52.3%) | |
| ALP, U/L | 0.588 | ||
| >125 | 9(12.2%) | 15(9.8%) | |
| ≤125 | 65(87.8%) | 138(90.2%) | |
| Maximum tumor diameter, mm | <0.001 | ||
| ≥59 | 44(59.5%) | 26(17.0%) | |
| <59 | 30(40.5%) | 127(83.0%) | |
| Number of tumors, n (%) | 0.059 | ||
| single | 50(67.66%) | 121(79.1%) | |
| multiple | 24(32.4%) | 32(20.9%) | |
| Tumor margin, n (%) | <0.001 | ||
| smooth | 28(37.8%) | 109(71.2%) | |
| non-smooth | 46(62.2%) | 44(28.8%) |
AFP, Alpha-fetoprotein; DCP, Des-γ-carboxy prothrombin; CTC, Circulating tumor cell; TB, Total bilirubin; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; Alb, Albumin; GGT, Gamma-glutamyltransferase; ALP, Alkaline phosphatase.
Multivariate analysis to predict independent risk factors for microvascular invasion.
| Clinical characteristics | OR | 95% CI | P value |
|---|---|---|---|
| AFP ≥158 ng/mL | 4.152 | 1.602−10.760 | 0.003 |
| DCP ≥178mAU/mL | 9.730 | 3.392−27.910 | <0.001 |
| CTC ≥3/3.2 ml | 7.747 | 3.019−19.881 | <0.001 |
| AST >40U/L | 0.716 | 0.270−1.901 | 0.503 |
| Maximum tumor diameter ≥59 mm | 3.467 | 1.368−8.669 | 0.008 |
| Unsmooth tumor margin | 0.235 | 0.096−0.573 | 0.001 |
AFP, Alpha-fetoprotein; DCP, Des-γ-carboxy prothrombin; CTC, Circulating tumor cell; AST, Aspartate aminotransferase.
(a) Combined with the five indexes of AFP ≥158 ng/mL, DCP ≥178 mAU/mL, CTC ≥3/3.2 ml, maximum tumor diameter ≥59 mm and tumor margin unsmoothness, the Roc curve predicting MVI was jointly drawn. (b) The clinical scoring risk model predicts the existence probability of microvascular invasion. AFP: Alpha-fetoprotein; DCP: Des-γ-carboxy prothrombin; CTC: Circulating tumor cell; Roc: Receiver operating characteristic.
The independent risk factors of MVI were used to establish a clinical scoring risk model of MVI. Fig. 2 shows that the prevalence of MVI was 3.1%(1/32) with a score of 0, 5.3%(4/76) with a score of 1, 12.2%(5/41) with a score of 2, 66.7%(20/30) with a score of 3, 87.9%(29/33) with a score of 4,and 100%(15/15) with a score of 5 (P < 0.001). According to the analysis of ROC curve, the AUC value of the system is 0.922 and the critical value is 2 points. As the percentages of 0–2, 4–5 were similar, the best odds ratio of 0–2, 3 and 4–5 was established, and their OR values were 1,27.8(95%CI:10.289∼75.115,p < 0.0001) and 152.9(95%CI:45.682∼511.763,p < 0.0001), respectively. Thus, the higher the score, the higher the prevalence of MVI (Table 4).
4DiscussionMVI is generally considered to be an important prognostic factor for hepatocellular carcinoma after treatment, and an accurate prediction of MVI before treatment is helpful for preoperative clinical decision-making [7,20]. In this analysis, we unearth that AFP ≥ 158 ng/mL, DCP ≥ 178mAU/mL, CTC ≥ 3 / 3.2 ml, maximum tumor diameter ≥ 59 mm and unsmooth tumor margin were independent risk factors for MVI. In order to predict the existence of MVI more accurately before operation, we established a clinical score model to predict the risk of MVI based on the independent risk factors of these five MVI.
It is worth paying attention to that CTC is one of the independent risk factors for MVI in this research. CTC has similar or same biological characteristics as the primary tumor, and histopathological examination can be obtained by detecting CTC in blood [21,22]. As the patient's peripheral blood is easy to collect, the detection of CTC in peripheral blood has become a convenient and repeatable non-invasive technique, and then the "liquid biopsy" of primary tumor and metastatic focus can be realized [23]. In addition, as a marker reflecting tumor invasion, CTC has long been used in prognosis monitoring, individualized treatment and curative effect evaluation of malignant tumors such as breast cancer, lung cancer and colorectal tumors, so its role in malignant tumors has attracted more and more attention [19]. Sun et al. and Wang et al. found the prognostic value of circulating tumor cells in the blood of patients with HCC, reflecting that the higher the level of peripheral CTC in patients with HCC, often leads to poor prognosis after HCC [24,25]. On the other hand, Jonathan et al. believe that inflammatory stimulating factors produced by hepatocellular carcinoma can up-regulate cell adhesion molecules, combine with platelets, tissue factors and various immune cells, and further promote the polymerization of hepatoma CTC in the vascular endothelium of portal vein and hepatic vein of the liver, which is beneficial to the formation of vascular tumor thrombus [26]. At the same time, Liu et al. found that a large number of CTCs were detected in patients with HCC accompanied by MVI. It can be seen that there is a close relationship between CTC and MVI, which is the key point for CTC to become an independent risk factor of MVI in this study [27].
AFP is a wide range of tumor marker closely related to liver cancer, not only related to the malignant potential and high incidence of liver cancer, but also found in patients with chronic hepatitis or liver cirrhosis. There is little correlation between itself and MVI. On the contrary, in the independent risk factor analysis of MVI in our study, AFP≥158 ng/mL(AUC = 0.752) is one of the indicators to predict the existence of MVI. It can be seen that high levels of AFP can also reflect the erosion of tumor cells [28,29]. Previous studies have revealed that DCP is the strongest predictor of MVI among tumor markers, which is due to the fact that high levels of DCP are associated with histological vascular invasion of cancer cells [28–30]. However, in our research, we found that CTC was the strongest predictor of MVI in this article, with an AUC value of 0.857, which is much higher than the independent risk factors of the other four MVI. Previously, we found that CTC was positively correlated with the maximum straight diameter of tumor and the level of AFP, and the maximum diameter of tumor and the level of AFP were two of the five independent risk factors of MVI in this analysis, which may be the key to make CTC the strongest predictor of MVI. The larger the diameter of the tumor and the higher the level of AFP are, the higher the level of CTC is. In fact, before this analysis, some scholars have confirmed the accuracy of this conclusion [31,32].
In the clinical scoring risk model, we conclude that the sensitivity and specificity of evaluating the risk of MVI are much higher than those of individual predictors, which means that the model can be used as a very helpful tool for accurate prediction of MVI before operation(the AUC value of the clinical scoring risk model is 0.922). For the clinical application of this model, we know that HCC patients with clinical scores of 3[66.7%(20/30)], 4[87.9%(29/33)] and 5[100%(15/15)] can be classified as high-risk groups of MVI. In contrast, patients with a score of 0 to 2[0 points 3.1% (1/32), 1 point 5.3% (4/76), 2 points 12.2% (5/41)] on the model had a similar risk of MVI and a significantly lower risk of MVI. At present, there are many liver cancer staging systems that predict the survival rate of liver cancer after resection, but the most commonly used are the four staging systems: the Barcelona Clinic Liver Cancer (BCLC) staging, the Okuda staging, the Cancer of the Liver Italian Program (CLIP) scoring system, and the tumor-node-metastasis (TNM) staging system. However, these staging systems do not include MVI as a risk factor. There was a large-scale multi-center study that used various risk factors related to MVI to jointly design the MVI scoring system. Compared with the other four liver cancer staging systems, it can more accurately predict the prognostic effect of liver cancer patients after surgery. Moreover, the scoring system of this multi-center study was merged into the other four liver cancer staging systems, and it was found that the prognosis of the groups with different scores also had significant differences [4]. In addition, some scholars have found that among MVI patients with BCLC stage, compared with MVI patients who received only hepatectomy, the tumor-free survival rate and overall survival rate of MVI patients who received postoperative TACE adjuvant therapy were significantly improved [33,34]. Therefore, for patients with hepatocellular carcinoma with high suspicion of MVI, the clinical scoring model we designed, combined with their liver cancer staging, has certain application value in making preoperative clinical decisions. As a retrospective analysis of this study, our MVI clinical scoring risk model is not only lack of external review, but also did not combine the score with different liver cancer staging systems to compare the prognosis of patients, which leads to certain limitations of our research. We hope that in the future, there will be a large number of clinical studies to verify our clinical scoring risk model, so that more patients can get timely, reasonable and effective treatment.
5ConclusionTo sum up, based on the five independent risk factors of AFP≥158 ng/mL, DCP≥178mAU/mL, CTC ≥ 3/3.2 ml, maximum tumor diameter≥59 mm and unsmooth tumor margin, we constructed a simple clinical score model to predict the risk of MVI, in order to make a more accurate individualized treatment plan before operation, which is of great clinical significance and application prospect to improve the curative effect of liver cancer.
Compliance with ethical standardsThe single-center study was approved by the Ethics Committee of Zhongshan Hospital Affiliated to Sun Yat-sen University and followed the guidelines of the Declaration of Helsinki.
Author contribution statementYong Zhu He: Concept, design, data collection, analysis, manuscript preparation, editing; Kun He: Concept, design, data collection, analysis, manuscript preparation, editing; Rui Qin Huang: Concept, design, data collection, analysis, manuscript preparation, editing; Ze Liang Wang: Data collection, analysis, manuscript preparation, editing; Shao Wei Ye: Data collection, analysis, manuscript preparation, editing; Li Wen Liu: Data collection, analysis, manuscript preparation, editing; Qi Jie Luo: Analysis, manuscript preparation, editing; Ze Min Hu: Guarantees the integrity of the entire study and manuscript review. All authors have read and approved the final version to be submitted.
Competing interestsThe authors declare that they have no competing interests