Introduction and aims. We aimed to explore the impact of infection diagnosed upon admission and of other clinical baseline parameters on mortality of cirrhotic patients with emergency admissions.
Material and methods. We performed a prospective observational monocentric study in a tertiary care center. The association of clinical parameters and established scoring systems with short-term mortality up to 90 days was assessed by univariate and multivariable Cox regression analysis. Akaike’s Information Criterion (AIC) was used for automated variable selection. Statistical interaction effects with infection were also taken into account.
Results. 218 patients were included. 71.2% were male, mean age was 61.1 ± 10.5 years. Mean MELD score was 16.2 ± 6.5, CLIF-consortium Acute on Chronic Liver Failure-score was 34 ± 11. At 28, 90 and 365 days, 9.6%, 26.0% and 40.6% of patients had died, respectively. In multivariable analysis, respiratory organ failure [Hazard Ratio (HR) = 0.15], albumin substitution (HR = 2.48), non-HCC-malignancy (HR = 4.93), CLIF-C-ACLF (HR = 1.10), HCC (HR = 3.70) and first episode of ascites (HR = 0.11) were significantly associated with 90-day mortality. Patients with infection had a significantly higher 90-day mortality (36.3 vs. 20.1%, p = 0.007). Cultures were positive in 32 patients with resistance to cephalosporins or quinolones in 10, to ampicillin/sulbactam in 14 and carbapenems in 6 patients.
Conclusion. Infection is common in cirrhotic ED admissions and increases mortality. The proportion of resistant microorganisms is high. The predictive capacity of established scoring systems in this setting was low to moderate.
Patients with advanced liver cirrhosis live in a state of constant circulatory dysregulation, instability of the coagulation system and immune-deficiency.1 Bacterial translocation and infection are common triggers of clinical deterioration and are important causes of morbidity and mortality.2–4 Due to the instability of homeostasis and the exhaustion of compensatory mechanisms already minor disturbances can lead to dramatic consequences. Hence, medical interventions must be based on rapid assessment of the medical problem and early treatment in the appropriate medical setting. Traditional prognostic tools for stable cirrhosis such as the Child-Pugh-Turcotte (CPT) score have little value in cirrhotic patients with an acute clinical deterioration.5 While acute decompensation (AD) of cirrhosis may take a benign course, organ dysfunction and in particular renal failure are associated with worse outcome. Consequently the sequential organ failure assessment score (SOFA-score) has been shown to be of prognostic value in critically ill cirrhotic patients.6,7 It has lately been modified for patients with acute on chronic liver failure on the basis of data derived from a large multicenter trial by the CLIF consortium. A derived new score, the CLIF-C-ACLF, displayed good predictive value for hospitalized patients with cirrhosis and AD.8
The role of inflammation in the development of organ failure in the setting of ACLF has drawn increasing attention.9–11 With increasing portal hypertension, bacterial translocation becomes more pronounced and contributes to an activation of the immune system. Overt infection may have an even higher impact on the development of organ failures and thus may be an important prognostic factor.12
Cirrhotic patients with an acute deterioration of their condition are likely to be admitted in medical emergency care departments. In the ED setting, the need for rapid recognition of the nature of the medical problem and rapid triaging to provide focused medical care are obvious.
We performed a prospective observational study of an unselected cohort of consecutive cirrhotic patients admitted to a tertiary care emergency department to evaluate the importance and clinical pattern of infections in this setting and to assess the prognostic capacity of clinical parameters and some derived scores available upon admission.
Material and MethodsPatientsThis study enrolled all consecutive patients who presented to the non-operative Emergency Department (ED) of our tertiary care center and fulfilled the following inclusion criteria: Age above 18 years, history of liver cirrhosis or a first diagnosis of liver cirrhosis according to clinical and ultrasound criteria.
Patients were excluded, if referred by another medical institution after more than 6 h of treatment. Only the first admission of each patient during the observation period was included into the analysis.
Clinical data during the first hours of admission were collected using pre-specified forms. Microbiological results of specimens drawn upon admission were added upon availability.
Follow up at 90 days and one year was done by telephone interview either with the patients or their primary care physician to record survival and hospital readmissions, or by assessing insurance data. The main focus was on 90-day mortality with mortality at 28 and 365 days as secondary end-points.
Patients were considered to have an infection when the treating ED physician issued a presumptive clinical diagnosis of infection and/or culture material obtained from sterile places showed growth of bacteria. To be diagnosed with pneumonia, chest X-rays had to show infiltrates and either fever or elevated inflammatory parameters had to be present. Urinalysis was routinely performed and urinary tract infection was diagnosed if patients had leukocyturia (more than 10 leukocytes per high power field) in the setting of suspected infection and either positive urinary nitrates or bacteriuria. For a diagnosis of spontaneous bacterial peritonitis an ascitic neutrophil count of more than 250 G/L was sufficient in a patient without proof of a secondary cause. Diagnoses of infection are detailed in the results section.
Admission due to acute decompensation was defined as the acute development or deterioration of the following complications of liver cirrhosis as main complaint at presentation to the ED: ascites, gastrointestinal hemorrhage (hematemesis, melena, hematochezia or symptomatic anemia), hepatic encephalopathy (drowsiness, impaired mentation) or jaundice.
SOFA-score13 and CLIF-SOFA14 scores were defined or calculated as published, as were acute-on-chronic liver failure classes (ACLF 1 through 3)14 and CLIF-consortium ACLF-score (CLIF-C-ACLF).8 Calculation was performed during data analysis based on parameters obtained upon admission.
The study was approved by the institutional ethics committee and informed consent was obtained from all patients.
Statistical analysisData were analyzed using R 3.2.0 (R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS 23 for Mac (IBM Corporation, Armonk, NY, USA). The distribution of continuous data is presented by mean ± standard deviation or median (25th to 75th percentiles) depending on whether visual inspection of histograms revealed major deviations from the normal distribution. Respective group comparisons were performed by t-tests and Mann-Whitney-U tests, respectively. Categorical data are presented by absolute and relative frequencies and were compared between groups using the chi-square test.
Group comparisons of mortality rates for 28, 90 and 365 days were calculated using truncated uncensored KaplanMeier curves with a log-rank test.
The association between various parameters and mortality up to 90 days was assessed by univariate Cox regression models, which enables cause specific analysis. In the multivariable analysis, missing values that were present in the data were imputed by the missForest15 method to construct 20 complete datasets. A stepwise forward variable selection based on the Akaike Information Criterion (AIC) was restricted to the inclusion of not more than six parameters (for 57 events) and performed on each of the data sets to yield consistent estimation of the multivariable Cox regression models.16 Following a dominance statistics approach, the six variables most often selected across all data sets were included in the final model. The latter was fit to the original data as almost no missing values were involved. The same approach was repeated separately for the subsets of patients that were diagnosed with infection during the first 48 h of their admission and patients without infection, while the inclusion of only three variables into the models was allowed due to the decreased number of events. All statistical testing was performed on two-sided, exploratory 5% significance levels.
Censoring of transplanted patientsA small number14 of patients were transplanted during follow-up. As after transplantation, a different set of variables would be associated with mortality, time of survival after transplantation could not be used for analysis of baseline-factors associated with mortality. Also, laboratory MELD-scores at transplantation for these patients were fairly low (median 18, range 10 to 33) with an expected 90-day mortality without transplant of under 10%. Consequently, these patients were not transplanted in a critical condition, and transplantation was assumed to be independent from mortality-risk. Therefore, a competing risk model was not necessary but patients were censored at the time of transplant.
ResultsAll patientsFrom January 2010 through September 2012, 219 Patients [71.5% male, age 62.0 ± 10.4 years, MELD score 16.2 ± 6.6; CPT classes: A 30 (13.9%); B 116 (53.0%); C 73 (33.3%)] fulfilled the inclusion criteria. In 199 patients, the diagnosis of cirrhosis had been made previously, whereas cirrhosis was diagnosed upon the current admission in 22 according to clinical and ultrasound-criteria. 198 patients (90%) presented with acute decompensation of cirrhosis.
124 patients had a history of at least one episode of ascites, 52 had been treated for an episode of hepatic encephalopathy and 45 had previously suffered variceal hemorrhage.
71 patients were treated with non-selective beta-blockers (NSBB) as primary (n = 45) or secondary (n = 26) prophylaxis for variceal hemorrhage. Of these 63 were taking propranolol in doses that ranged from 10 mg/d to 120 mg/d (median 30 mg/d) and 8 carvedilol in doses from 6.25 mg/d to 37.5 mg/d (median 12.5 mg/d). 109 patients were currently on spironolactone in doses ranging from 50 to 400 mg/d (median 100 mg/d). Of these 95 had also furosemide (n = 69; range 20 to 160 mg/d; median 50 mg/d) or torasemide (n = 26; range 5 to 100 mg/d, median 20 mg/d), 28 patients only took loop diuretics. 72 patients were on a current medication of lactulose after a previous episode of HE and 2 had additional rifaximin. 56 patients had received antibiotics during the 3 months preceding the current admission for the treatment of, among others, UTI (n = 14), SBP (n = 9) and pneumonia (n = 8).
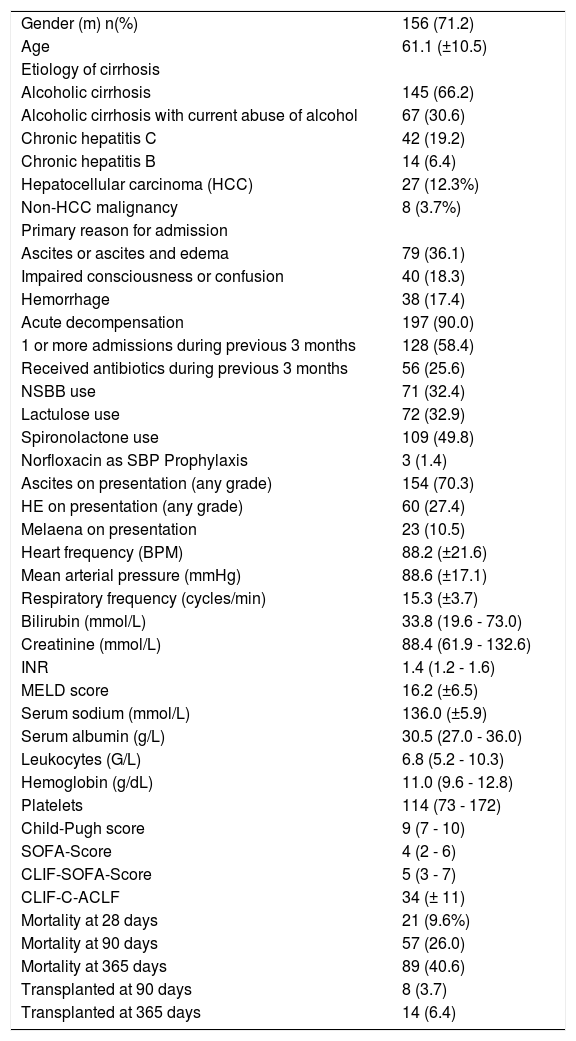
Parameters of all patients are presented in table 1.
Clinical data of all patients upon admission and outcome data.
| Gender (m) n(%) | 156 (71.2) |
| Age | 61.1 (±10.5) |
| Etiology of cirrhosis | |
| Alcoholic cirrhosis | 145 (66.2) |
| Alcoholic cirrhosis with current abuse of alcohol | 67 (30.6) |
| Chronic hepatitis C | 42 (19.2) |
| Chronic hepatitis B | 14 (6.4) |
| Hepatocellular carcinoma (HCC) | 27 (12.3%) |
| Non-HCC malignancy | 8 (3.7%) |
| Primary reason for admission | |
| Ascites or ascites and edema | 79 (36.1) |
| Impaired consciousness or confusion | 40 (18.3) |
| Hemorrhage | 38 (17.4) |
| Acute decompensation | 197 (90.0) |
| 1 or more admissions during previous 3 months | 128 (58.4) |
| Received antibiotics during previous 3 months | 56 (25.6) |
| NSBB use | 71 (32.4) |
| Lactulose use | 72 (32.9) |
| Spironolactone use | 109 (49.8) |
| Norfloxacin as SBP Prophylaxis | 3 (1.4) |
| Ascites on presentation (any grade) | 154 (70.3) |
| HE on presentation (any grade) | 60 (27.4) |
| Melaena on presentation | 23 (10.5) |
| Heart frequency (BPM) | 88.2 (±21.6) |
| Mean arterial pressure (mmHg) | 88.6 (±17.1) |
| Respiratory frequency (cycles/min) | 15.3 (±3.7) |
| Bilirubin (mmol/L) | 33.8 (19.6 - 73.0) |
| Creatinine (mmol/L) | 88.4 (61.9 - 132.6) |
| INR | 1.4 (1.2 - 1.6) |
| MELD score | 16.2 (±6.5) |
| Serum sodium (mmol/L) | 136.0 (±5.9) |
| Serum albumin (g/L) | 30.5 (27.0 - 36.0) |
| Leukocytes (G/L) | 6.8 (5.2 - 10.3) |
| Hemoglobin (g/dL) | 11.0 (9.6 - 12.8) |
| Platelets | 114 (73 - 172) |
| Child-Pugh score | 9 (7 - 10) |
| SOFA-Score | 4 (2 - 6) |
| CLIF-SOFA-Score | 5 (3 - 7) |
| CLIF-C-ACLF | 34 (± 11) |
| Mortality at 28 days | 21 (9.6%) |
| Mortality at 90 days | 57 (26.0) |
| Mortality at 365 days | 89 (40.6) |
| Transplanted at 90 days | 8 (3.7) |
| Transplanted at 365 days | 14 (6.4) |
NSBB: non-selective beta-blockers. INR: international normalized ratio. MELD: Model of End-Stage Liver Disease. SOFA: sequential organ failure assessment score.
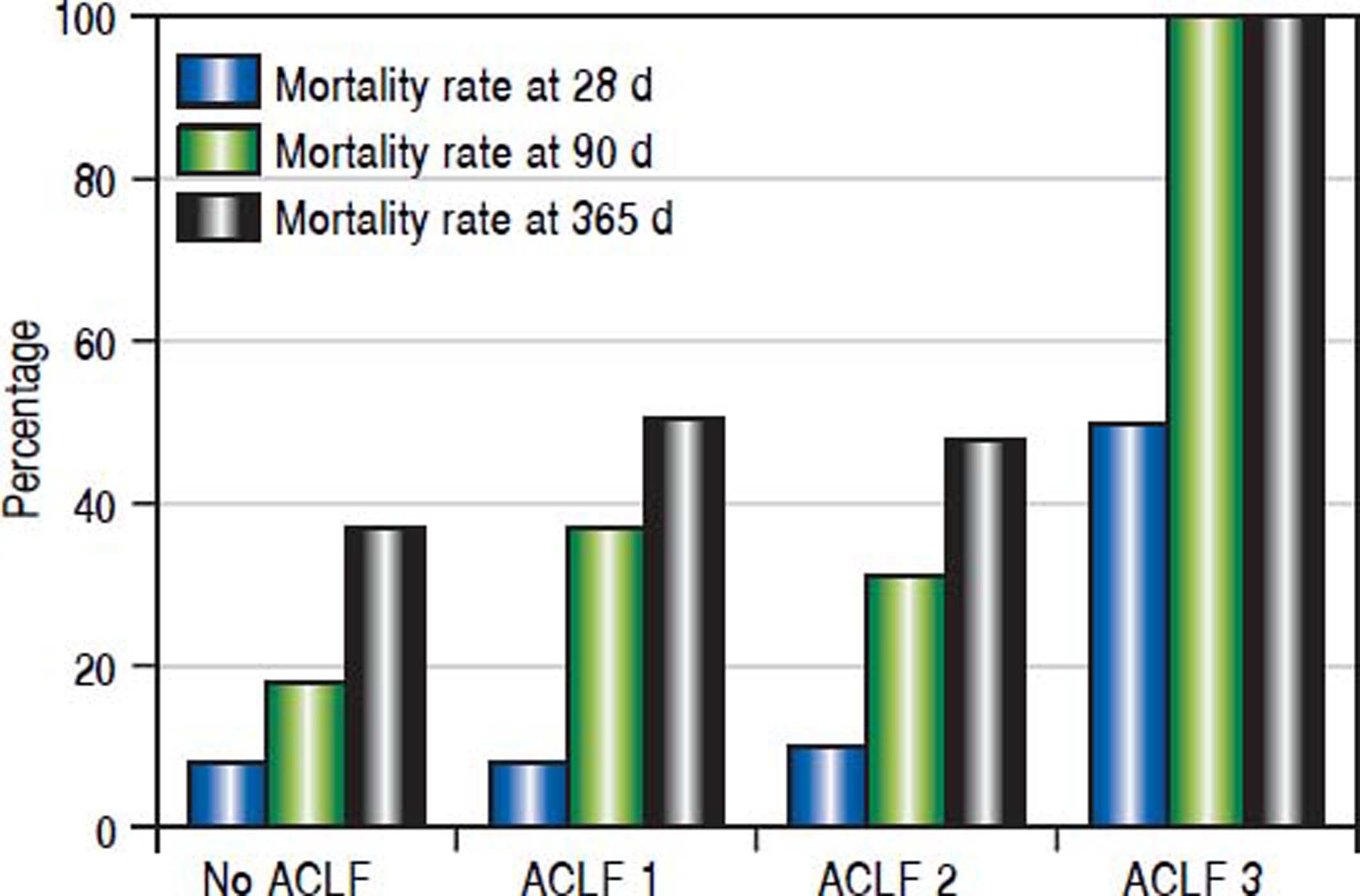
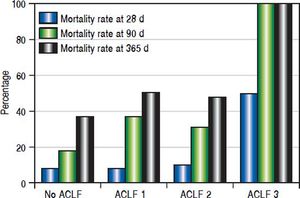
Twenty-one (9.6%), 57 (26.0%) and 89 (40.6%) patients had died at 28, 90 and 365 days of follow-up, respectively. Patients with no organ failure according to CLIF criteria (ACLF) (n = 145) had mortality rates at 28, 90 and 365 days of 8.3%, 19.3% and 33.8%, respectively. 43 patients had ACLF class 1, 29 had ACLF class 2 and only two patients had ACLF class 3. For patients with any grade of C-ACLF (n = 74) the corresponding mortality rates were 12.2%, 39.2% and 54.1%. The differences were significant for 90 days (p = 0.002) and 365 days (p = 0.002), but not for 28 days (p = 0.355) (Figure 1).
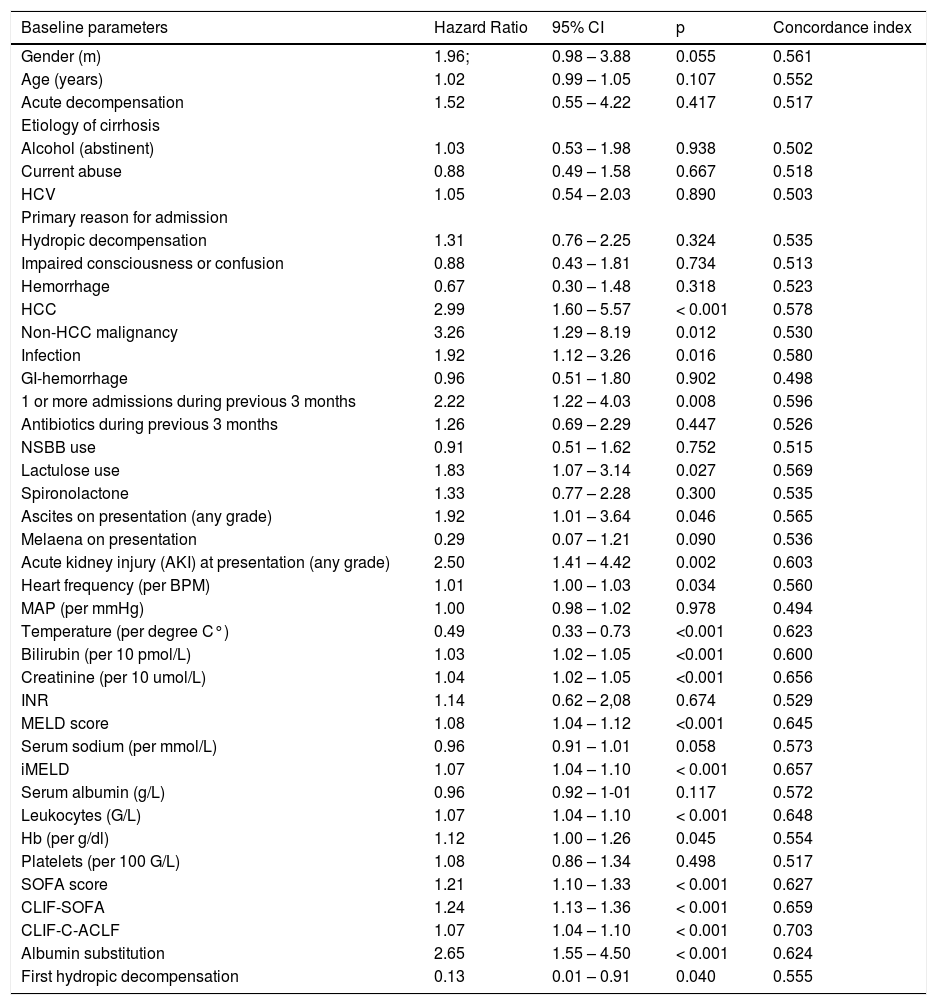
Univariable associations between clinical, laboratory and treatment-related parameters and mortality up to 90 days are presented in table 2.
Baseline parameters and their association with mortality up to 90 days.
| Baseline parameters | Hazard Ratio | 95% CI | p | Concordance index |
|---|---|---|---|---|
| Gender (m) | 1.96; | 0.98 – 3.88 | 0.055 | 0.561 |
| Age (years) | 1.02 | 0.99 – 1.05 | 0.107 | 0.552 |
| Acute decompensation | 1.52 | 0.55 – 4.22 | 0.417 | 0.517 |
| Etiology of cirrhosis | ||||
| Alcohol (abstinent) | 1.03 | 0.53 – 1.98 | 0.938 | 0.502 |
| Current abuse | 0.88 | 0.49 – 1.58 | 0.667 | 0.518 |
| HCV | 1.05 | 0.54 – 2.03 | 0.890 | 0.503 |
| Primary reason for admission | ||||
| Hydropic decompensation | 1.31 | 0.76 – 2.25 | 0.324 | 0.535 |
| Impaired consciousness or confusion | 0.88 | 0.43 – 1.81 | 0.734 | 0.513 |
| Hemorrhage | 0.67 | 0.30 – 1.48 | 0.318 | 0.523 |
| HCC | 2.99 | 1.60 – 5.57 | < 0.001 | 0.578 |
| Non-HCC malignancy | 3.26 | 1.29 – 8.19 | 0.012 | 0.530 |
| Infection | 1.92 | 1.12 – 3.26 | 0.016 | 0.580 |
| GI-hemorrhage | 0.96 | 0.51 – 1.80 | 0.902 | 0.498 |
| 1 or more admissions during previous 3 months | 2.22 | 1.22 – 4.03 | 0.008 | 0.596 |
| Antibiotics during previous 3 months | 1.26 | 0.69 – 2.29 | 0.447 | 0.526 |
| NSBB use | 0.91 | 0.51 – 1.62 | 0.752 | 0.515 |
| Lactulose use | 1.83 | 1.07 – 3.14 | 0.027 | 0.569 |
| Spironolactone | 1.33 | 0.77 – 2.28 | 0.300 | 0.535 |
| Ascites on presentation (any grade) | 1.92 | 1.01 – 3.64 | 0.046 | 0.565 |
| Melaena on presentation | 0.29 | 0.07 – 1.21 | 0.090 | 0.536 |
| Acute kidney injury (AKI) at presentation (any grade) | 2.50 | 1.41 – 4.42 | 0.002 | 0.603 |
| Heart frequency (per BPM) | 1.01 | 1.00 – 1.03 | 0.034 | 0.560 |
| MAP (per mmHg) | 1.00 | 0.98 – 1.02 | 0.978 | 0.494 |
| Temperature (per degree C°) | 0.49 | 0.33 – 0.73 | <0.001 | 0.623 |
| Bilirubin (per 10 pmol/L) | 1.03 | 1.02 – 1.05 | <0.001 | 0.600 |
| Creatinine (per 10 umol/L) | 1.04 | 1.02 – 1.05 | <0.001 | 0.656 |
| INR | 1.14 | 0.62 – 2,08 | 0.674 | 0.529 |
| MELD score | 1.08 | 1.04 – 1.12 | <0.001 | 0.645 |
| Serum sodium (per mmol/L) | 0.96 | 0.91 – 1.01 | 0.058 | 0.573 |
| iMELD | 1.07 | 1.04 – 1.10 | < 0.001 | 0.657 |
| Serum albumin (g/L) | 0.96 | 0.92 – 1-01 | 0.117 | 0.572 |
| Leukocytes (G/L) | 1.07 | 1.04 – 1.10 | < 0.001 | 0.648 |
| Hb (per g/dl) | 1.12 | 1.00 – 1.26 | 0.045 | 0.554 |
| Platelets (per 100 G/L) | 1.08 | 0.86 – 1.34 | 0.498 | 0.517 |
| SOFA score | 1.21 | 1.10 – 1.33 | < 0.001 | 0.627 |
| CLIF-SOFA | 1.24 | 1.13 – 1.36 | < 0.001 | 0.659 |
| CLIF-C-ACLF | 1.07 | 1.04 – 1.10 | < 0.001 | 0.703 |
| Albumin substitution | 2.65 | 1.55 – 4.50 | < 0.001 | 0.624 |
| First hydropic decompensation | 0.13 | 0.01 – 0.91 | 0.040 | 0.555 |
NSBB: non-selective beta-blockers. INR: international normalized ratio. MELD: Model of End-Stage Liver Disease. iMELD: integrated MELD (MELD + [age (years) × 0.3] × [0.7 × Na (mmol/L)] + 100). SOFA: sequential organ failure assessment score.
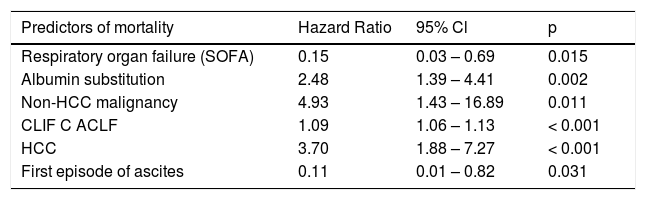
The six parameters selected by the AIC in multivariable analysis for all patients are presented in table 3.
Multivariable model of predictors of mortality up to 90 days (all patients).
| Predictors of mortality | Hazard Ratio | 95% Cl | p |
|---|---|---|---|
| Respiratory organ failure (SOFA) | 0.15 | 0.03 – 0.69 | 0.015 |
| Albumin substitution | 2.48 | 1.39 – 4.41 | 0.002 |
| Non-HCC malignancy | 4.93 | 1.43 – 16.89 | 0.011 |
| CLIF C ACLF | 1.09 | 1.06 – 1.13 | < 0.001 |
| HCC | 3.70 | 1.88 – 7.27 | < 0.001 |
| First episode of ascites | 0.11 | 0.01 – 0.82 | 0.031 |
In 80 (36.5%) patients [54 (68%) male] a clinical diagnosis of infection was established within the first 48 h. All of these patients fulfilled the criteria of acute decompensation. 29 (36.3%) fulfilled the criteria of sepsis,17 14 (17.4%) had severe sepsis and 5 (6%) were in septic shock. Spontaneous bacterial peritonitis (SBP) was diagnosed in 13 patients who had ascitic granulocyte counts of > 250/ mL. In all cases ascites was cultured but positive results were obtained only in 4. In 38 patients urinary tract infections were diagnosed based on the results of urinalysis. In all patients urine was cultured with positive results in 13 cases. In 28 patients pneumonia or broncho-pneumonia were diagnosed. Chest X-rays revealed lobar infiltrates in 8 and atypical infiltrates in the remainder. Sputum was cultured in 8 patients with positive results in 3 patients. In 4 patients blood cultures were positive without a focus of infection being determined. In 3 patients a diagnosis of infection was made based on fever, leukocytosis and elevated CRP levels without a focus of infection being determined. Three more patients were diagnosed with infectious gastroenteritis, two with soft tissue infection and one each with secondary peritonitis and pancreatic abscess, respectively.
• Clinical presentation and outcome. Patients with a diagnosis of infection upon admission significantly more often presented dyspnea (10.0 vs. 1.4%, p = 0.003) and fever (4.0 vs. 0.0%, p = 0.007) on admission, and less often GI-hemorrhage (7.5 vs. 23.6%; p = 0.003) compared to patients without infection. Impaired consciousness, hydropic decompensation, jaundice and abdominal pain were evenly distributed between both groups.
More patients with infection, compared to patients without infection had died at 90 days (36.3 vs. 20.1%, p = 0.007). The mortality rates of patients with infection were not significantly higher at 28 days (18.8 vs. 7.2; p = 0.117) and 365 days (46.3 vs. 37.4%, p = 0.096).
• Microbiology and resistance patterns. Overall, in 32 (40.0%) of these 80 cases at least one microbiological culture was positive. The following microorganisms were most frequently encountered: E. coli (n = 12, 15.0%) of which 2 showed resistance towards multiple antibiotics; K. pneumoniae (n = 4, 5.1%) of which 2 showed resistance towards multiple antibiotics; Enterococcus sp. (n = 5, 6.3%); Staph. aureus (n = 3, 3.8%), of which one was resistant to methicillin.
Resistance of the causative micro-organism to cephalosporins and quinolones was observed in cultures from 10 patients each, to ampicillin/sulbactam in 14, of which 10 had germs that were also resistant to piperacillin/ tazobactam, and to carbapenems in 6 patients. The initial calculated antibiotic regimen was found ineffective for cultured organisms obtained from 9 patients.
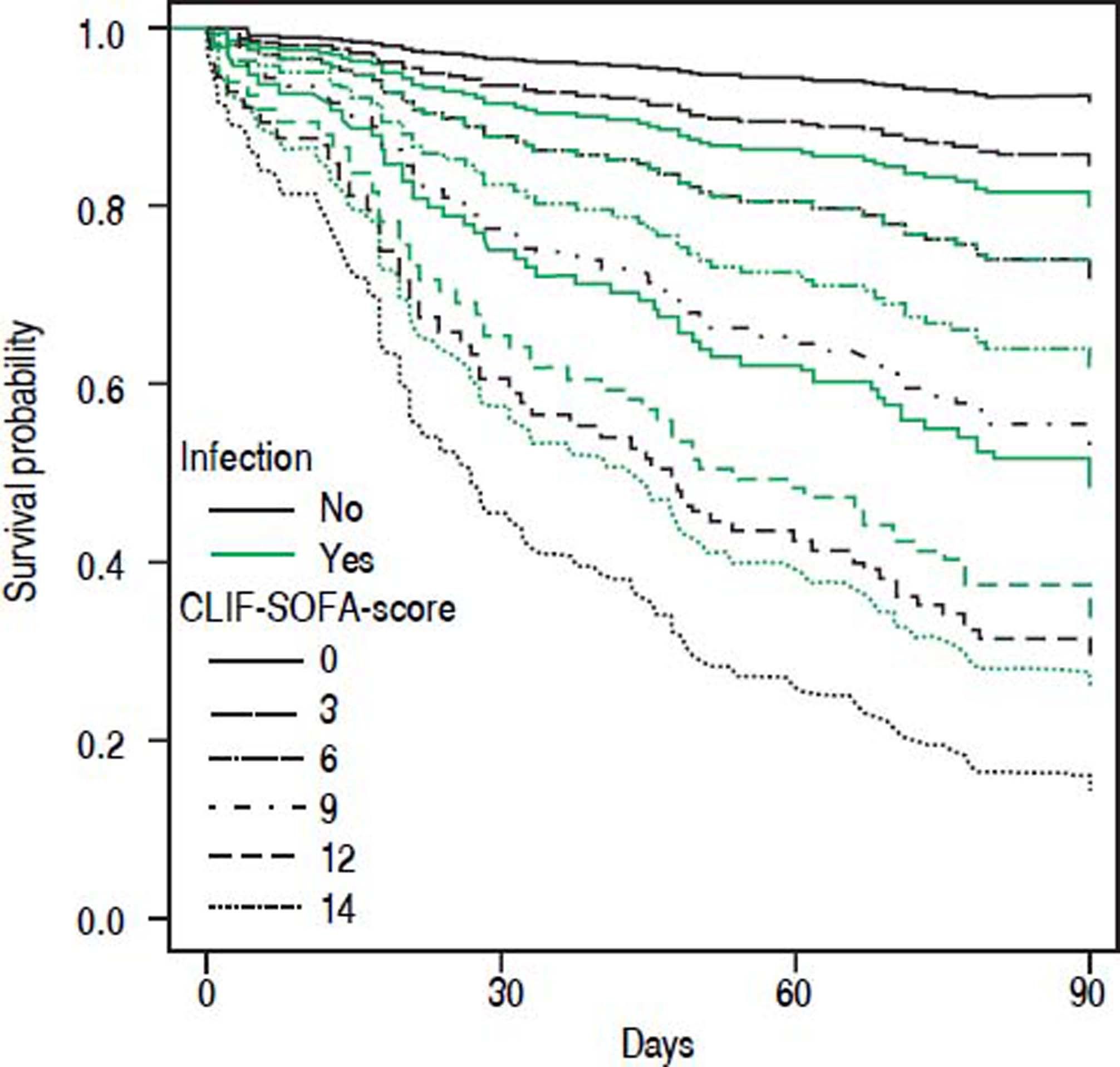
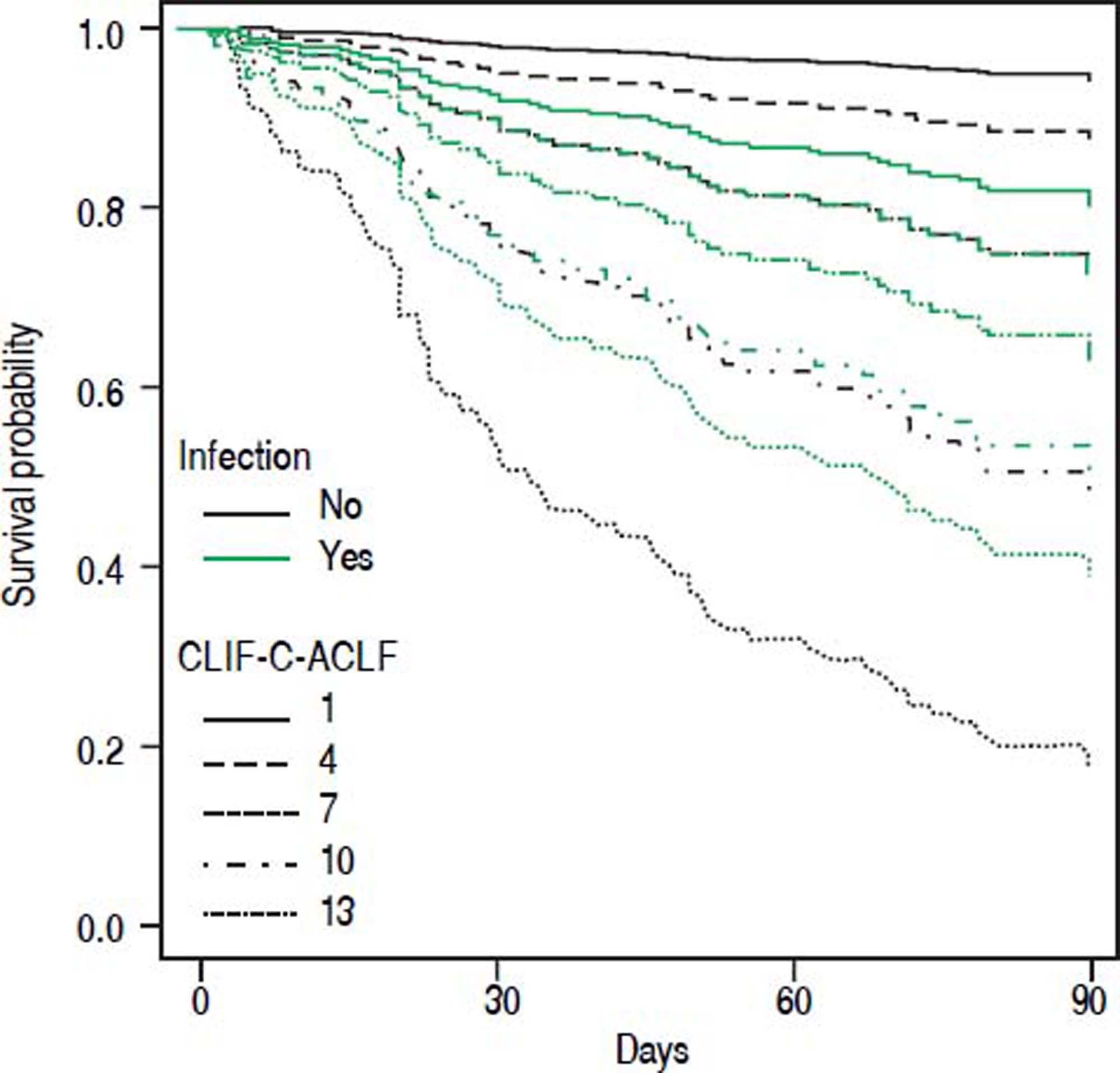
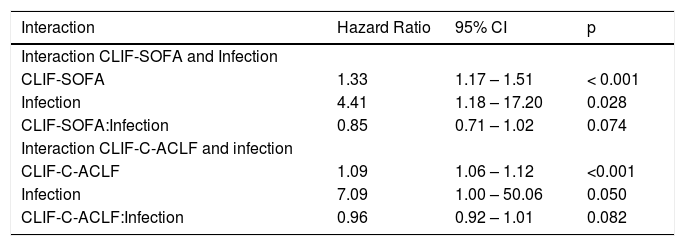
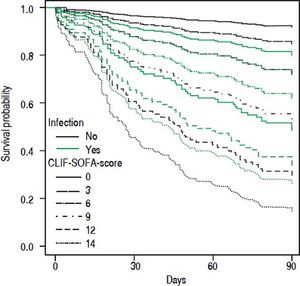
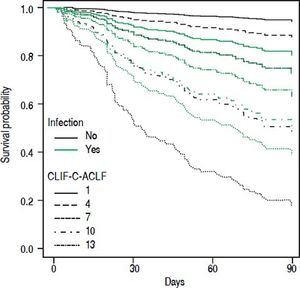
• Statistical interaction of the presence of infection on admission with prognostic parameters. Presence of infection modified the association between some scores and mortality. Infection reduced the positive correlation between CLIF-SOFA-score (HR = 0.85; p = 0.074), or CLIF-C-ACLF (HR = 0.96; p = 0.082), and mortality up to 90 days (Table 4). The influence of infection on mortality across different tiers of values of CLIF-SOFA and CLIF-C-ACLF, respectively, is illustrated in figures 2 and 3.
Interaction terms for infection and CLIF-SOFA and infection and CLIF-C-ACLF.
| Interaction | Hazard Ratio | 95% CI | p |
|---|---|---|---|
| Interaction CLIF-SOFA and Infection | |||
| CLIF-SOFA | 1.33 | 1.17 – 1.51 | < 0.001 |
| Infection | 4.41 | 1.18 – 17.20 | 0.028 |
| CLIF-SOFA:Infection | 0.85 | 0.71 – 1.02 | 0.074 |
| Interaction CLIF-C-ACLF and infection | |||
| CLIF-C-ACLF | 1.09 | 1.06 – 1.12 | <0.001 |
| Infection | 7.09 | 1.00 – 50.06 | 0.050 |
| CLIF-C-ACLF:Infection | 0.96 | 0.92 – 1.01 | 0.082 |
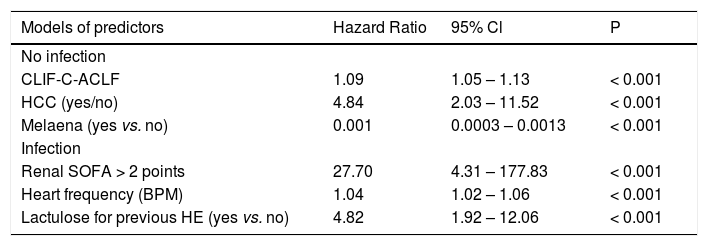
Cox regression analysis was run separately for patients with and without infection selecting three parameters each due to the reduced numbers of events in the subcohorts. Results are presented in table 5.
Multivariable models of predictors of mortality up to 90 days for patients with and without infection, respectively.
| Models of predictors | Hazard Ratio | 95% Cl | P |
|---|---|---|---|
| No infection | |||
| CLIF-C-ACLF | 1.09 | 1.05 – 1.13 | < 0.001 |
| HCC (yes/no) | 4.84 | 2.03 – 11.52 | < 0.001 |
| Melaena (yes vs. no) | 0.001 | 0.0003 – 0.0013 | < 0.001 |
| Infection | |||
| Renal SOFA > 2 points | 27.70 | 4.31 – 177.83 | < 0.001 |
| Heart frequency (BPM) | 1.04 | 1.02 – 1.06 | < 0.001 |
| Lactulose for previous HE (yes vs. no) | 4.82 | 1.92 – 12.06 | < 0.001 |
This study shows a high prevalence of organ failure and high mortality in a prospective cohort of cirrhotic patients who were admitted to a medical emergency department. Mortality at 90 days and one year was higher in patients who were diagnosed with infection during the first 48 h compared to patients without a diagnosis of infection. Infections have been reported to cause 25 to 35% of hospital admissions of cirrhotic patients4,18,19 and mortality has been found increased up to 4-fold in patients with cirrhosis and infection compared to cirrhotic patients without infection in a recent meta-analysis and 5-fold in a nationwide study of hospitalized cirrhotic patients in the US. Mortality rates at 1 year were around 50% and have not decreased over the past decades, despite improved survival rates in the intensive care setting.7,21 In addition, elevated inflammatory mediators have been associated with ACLF and mortality.10
In our study, mortality of patients with infection was similar to that found in the aforementioned studies, whereas mortality of patients without infection appeared higher. Hence patients with infection still had a higher 90-day mortality compared to patients without infection, but the difference of mortality rates was smaller than in previous reports. This higher observed mortality of patients without infection in our study may be the result of a different selection of patients in the emergency care setting.
Infection is not only associated with higher mortality in cirrhotic patients, it is also more common in cirrhotic vs. non-cirrhotic patients: A recent subgroup analysis of 410 (2,97%) cirrhotic patients among 13,796 patients from 1,265 Intensive Care Units showed a higher prevalence of infection in critically ill patients with cirrhosis than in patients without cirrhosis (59 vs. 51%). In that study, the most common infections were pulmonary infections. Mortality rates were again higher in cirrhotic patients with infection, with a hospital mortality rate of 51% compared to 29% for non-infected cirrhotic patients. It has been suggested that infection may be an important prognostic parameter in hospitalized cirrhotic patients.
Differences of clinical presentation between patients with and without infection were small in our study. Only dyspnea and fever were significantly more common in patients with infection. Overall, however, fever was rare even in infected patients with cirrhosis. It has been previously found that the presentation of infection in patients with cirrhosis may be subtle and that conventional criteria of SIRS (particularly body temperature) and of sepsis lack sensitivity and specificity in cirrhotic patients.23,24 Therefore clinical suspicion and an appropriate clinical workup are of importance.
Among variables associated with mortality up to 90 days, CLIF-C-ACLF-score displayed the highest concordance. This score comprises age, the number of organ failures according to the SOFA-score, as modified by the CLIF-consortium, and also the leukocyte-count as a parameter of inflammation. The inclusion of leukocytes renders this score sensitive to the presence of inflammation and infection and thus may explain its superiority in comparison to scores that just include parameters of organ dysfunction.8 In our multivariable analysis a model comprising CLIF-C-ACLF score, presence of HCC, non-HCC malignancy, first hydropic decompensation, albumin substitution and respiratory organ failure according to the original SOFA-score was selected. CLIF-C-ACLF, HCC and non-HCC-malignancy and albumin substitution within the first 72 h were positively associated with mortality. Patients with HCC (but-in contrast to our study only within Milan criteria) and non HCC-malignancy were also included in the CANONIC cohort but apparently HCC was not analyzed as a predictor of mortality.8,14
Albumin was used in our cohort to prevent post-paracentesis circulatory dysfunction in 55 patients presenting tense ascites and for volume expansion in 12 patients with acute kidney injury and in 3 patients with hepatic encephalopathy, respectively. We have no explanation for the overall statistical association of albumin substitution with an increased mortality seen in our study. We do not believe that this is a causal effect, as albumin substitution has been found to reduce mortality in SBP,25 and was independently associated with improved survival in non-SBP-infections.26 A recent retrospective analysis of patients with recent admission for acute kidney injury or SBP also found a dose-dependent association of albumin substitution during the first 48 h with a reduced mortality.27 Furthermore, albumin substitution has been shown to contribute to the resolution of hepatorenal syndrome28 and to reduce morbidity and mortality after large volume paracentesis.29 We suppose that in our study decision for albumin substitution by the treating physicians may have captured negative confounding factors, such as more pronounced portal hypertension, carrying a worse prognosis that were not otherwise accounted for by the parameters we analyzed. In our data we did not detect any significant correlation between albumin infusion and other predictors of worse outcome such as CLIF-C-ACLF or serum creatinine.
A first episode of ascites as reason for admission was associated with an improved survival in our cohort. It has previously been found that patients with repeated episodes of ascites despite treatment fare worse than cirrhotic patients with a first episode.14 Those patients without a previous episode of ascitic decompensation thus may constitute a cohort with a relatively benign prognosis. By the automated model selection using the AIC, a negative association of mortality with respiratory organ failure (which is already included in the CLIF-C-ACLF) became apparent in our ED cohort. A possible explanation could be that respiratory failure may carry too much weight within CLIF-C-ACLF for an emergency care setting like ours, where nosocomial pneumonia with its worse prognosis is excluded.
Currently most experts recommend third generation cephalosporins or amoxicillin/clavulanic acid (with macrolides in the case of pneumonia), or quinolones in the first-line treatment for community acquired infections in cirrhotic patients.1 Historically, gram-negative bacteria have been found in the vast majority of infections of cirrhotic patients. More recently a trend towards gram-positive and resistant bacteria for both, spontaneous bacterial peritonitis30–35 and infections in general,18,36 has been observed and attributed to increasing rates of invasive procedures, antibiotic prophylaxis and increasing time spent in health-care environments. In our study, by definition of the study group, no patient had nosocomial infection, but a large proportion of patients had recently been exposed to antibiotic treatment and hospital care. It has been previously found that prior hospital admissions and invasive procedures were associated with a trend towards more multi-resistant infections.19 In our study more than half of the patients had received hospital care during the 3 months preceding admission. A quarter had received antibiotics during the same period. We could not detect an influence of these factors on mortality. This may reflect knowledge of the changing microbiological epidemiology and consecutive adaptation of empirical antibiotic schedules. The number of positive results of microbiological cultures was too small to permit an analysis of risk-factors for microbiological resistance.
In our study the urinary tract was the most common site of infection followed by lungs and ascites (SBP). In cirrhotic patients, higher rates of asymptomatic bacteriuria and symptomatic UTI than in the general population have been reported and have ben associated with an increased mortality.37,38 Similarly, others have found a high prevalence of leukocyturia associated with an inferior outcome in a large cohort of cirrhotic patients upon hospital admission.39 The high incidence of UTI mirrors the distribution found previously in community acquired infections of cirrhotic patients40 and, recently, in a large analysis using a national database of new hospitalizations of cirrhotic patients.4 In this latter study the incidence of SBP was relatively low. This was also the case in our cohort, despite an astonishing underuse of prophylactic treatment with norfloxacin as is recommended by current guidelines41 which was used in only one of the 9 patients who had received antibiotics for a recent episode of SBP.
Overall, the yield of microbiological cultures drawn was relatively low. This is in line with previous studies that found low rates of bacterial isolation in patients with cirrhosis and infection.12,18 In positive cultures, a third of cultured microorganisms were resistant to the initial antibiotic regimen. Approximately a third of our patients received non-selective beta-blockers, mostly propranolol. The beneficial effect of non-selective beta-blockers (NSBB) in patients with cirrhosis has recently been questioned and NSBB-treatment has been associated with renal failure and increased mortality in patients with refractory ascites42 and in patients after an episode of SBP.43 In contrast, more recently, a retrospective analysis of a large database reported a protective effect of propranolol in doses up to 160 mg/d, such as used in our patients.44 In our study we could not detect a decisive association between the use of NSBB and mortality or the occurrence of renal failure.
Overall, our study suggests high mortality rates for cirrhotic patients admitted to an emergency department. The setting of our study was different from that of the CANONIC study in that in our study only new admissions to an emergency department and only data upon admission were analyzed. Despite that, mortality was found to be in the same range as reported previously and the examined prognostic parameters showed similar moderate predictive capacity in our cohort.
Since its development CLIF-SOFA score has been validated in unrelated cohorts of ICU patients, displaying good predictive power concerning hospital mortality45 and mortality at 6 months.46 A study from Brazil47 found excellent predictive power of CLIF-SOFA in a cohort of emergency admissions of cirrhotic patients that were remarkably similar to our cohort as far as clinical baseline parameters are concerned. However, overall mortality was lower, perhaps reflecting the lower proportion of patients with infection in that study. In our cohort CLIF-C-ACLF showed the highest concordance index among all tested scores. This score was derived from CLIF-SOFA and includes white blood cell count as a parameter of inflammation. Despite that, we detected statistical interaction between presence of infection and the association between either CLIF-SOFA or CLIF-C-ACLF and mortality (Figures 2 and 3). Upon separate analysis of parameters predicting mortality, two different models were found for patients with and without infection, respectively.
Our analysis therefore suggests that different sets of variables may be of varying prognostic importance in patients with and without infection. This issue should be explored and validated in larger cohorts.
Limitations of our study are the monocentric design and the limited number of patients. Obviously the setting of this study is a general medical emergency department, and the results may not be generalized to specialized care. On the other hand, we present a well-described real-life cohort of unselected cirrhotic emergency care admissions, a setting for which as yet little data has been published.
In conclusion our study shows a high incidence of infection among cirrhotic patients who were admitted from the community to an emergency care department. Mortality was increased in patients with infection and with severity of impairment and number of affected organ systems. The proportion of bacterial resistance to first-line antibiotics commonly used in emergency care was high. The use of broader initial antibiotic regimens should therefore be considered and efforts should be made to culture the offending micro-organisms. Established scoring systems for prognostication in cirrhotic patientswith acute decompensation showed moderate to good concordance with mortality in these emergency care admissions. The interaction of infection with the association of established scoring systems and mortality should be further investigated.
Abbreviations- •
ACLF: acute on chronic liver failure.
- •
AD: acute decompensation.
- •
ADQI: acute Dialysis Quality Initiative.
- •
AIC: akaike information criterion.
- •
AKI: acute kidney injury.
- •
AKIN: acute kidney injury network class.
- •
CLIF-SOFA: modified SOFA-score according to the Chronic Liver Failure Consortium.
- •
CLIF-C-ACLF: CLIF-Consortium Acute-on Chronic Liver Failure Score.
- •
CPT: Child-Pugh-Turcotte class.
- •
ED: Emergency Department.
- •
HE: hepatic encephalopathy.
- •
HCC: hepatocellular carcinoma.
- •
MELD: Model of End-Stage Liver Disease.
- •
SOFA: sequential organ failure assessment.
The authors declares that there is no conflict of interest regarding the publication of this article.