HCV is primarily hepatotropic, but there is mounting evidence pointing to infection and replication of extrahepatic sites. Here we evaluated the occurrence of HCV infection of peripheral blood mononuclear cells (PBMC) and explored the possible association between viral extrahepatic infection and the natural history of the disease. Forty seven Chilean, HCV infected, treatment naïve patients were included in the study. HCV RNA was isolated from plasma and PBMC and subsequently reverse transcribed, amplified and sequenced. Most patients harbored HCV 1b genotype and the most common route of infection showed to be blood transfusion. HCV RNA was readily detected in PBMCs of 34 out of the 47 patients (72%). We report that HCV sequences found in PBMC differ from those in plasma of the same subjects strongly suggesting HCV compartmentalization. In addition, we found that patients with detectable HCV RNA in PBMC had a tendency for being more likely cirrhotic [OR 3.8 (95% CI: 0.98 to 14)]. In conclusion, this study provides further arguments for the existence of HCV infection of extrahepatic sites and suggests that extrahepatic infection could be a factor influencing the natural history of the disease.
The present study was supported by FONDECYT No 1050782 and the JEAI-IRD Initiative. MIB is student of the Programa de Doctorado en Ciencias Biológicas, Facultad de Ciencias, Universidad de Chile and was initially funded by a CONICYT doctoral fellowship and currently hold an IRD doctoral fellowship. JV-O is student of the Programa de Doctorado en Microbiología, Universidad de Santiago de Chile and was supported by a MECESUP USACH doctoral fellowship. DM was partially supported through a Millennium Nucleus on Immunology and Immunotherapy (NMII) post-doctoral fellowship. MLL is member of the NMII.
Hepatitis C virus (HCV) infection is a worldwide distributed disease leading to chronic hepatitis, cirrhosis and hepatocellular carcinoma, causing significant disability and mortality.1 Aside from the well known hepatic complications of this infection, there are a number of less well understood extrahepatic manifestations of the disease, ranging from cryoglobulinemia to lymphoproliferative disorders, including neuropathy, dermatologic manifestations (e.g.: lichen planus and porphyria cutanea tarda), glomerulonephritis and several autoimmune disorders among others.2-4 Some of these extrahepatic manifestations could be attributed to an altered immune response to HCV infection, leading to autoimmunity.
One of the most striking aspects of HCV infection is the high likelihood of becoming a chronic disease, with approximately 75 to 85% of subjects with acute hepatitis C not being able to clear acute infection, despite an apparent immune response.5 There is evidence that the immune response may be insufficient or the virus may develop escape mutations in critical viral epitopes recognized by cytotoxic T cells (CTL),6 but mechanisms of immune escape are incompletely understood. Similarly, even though treatment of HCV infection has experienced great advances in the probability of achieving a sustained response,7 therapy of this infection has been invariably tempered by a considerable relapse rate. It has been shown that HCV can be detected long after spontaneous or treatment-induced viral clearance.8
HCV replication is thought to occur predominantly in hepatocytes, but several observations have shown that the virus can be detected in extrahepatic sites, including peripheral blood mononuclear cells (PBMC),9-18 dendritic cells,19 central nervous system,20-22 among several other different biological compartments.23-30 A number of specific approaches have aroused to clarify whether the presence of HCV RNA in extrahepatic tissues represents a contamination artifact, such as adsorption of virions onto cells, or real ongoing replication. Detection of non-structural proteins, negative-strand RNA (intermediary of replication) and demonstration of quasispecies compartmentalization have all been advocated as strong arguments for supporting HCV active extrahepatic replication. The significance of PBMC or other non-hepatic tissue infection by HCV as well as the possible implication of extrahepatic replication in viral pathogenesis is currently a matter of debate.
Most of the observations regarding replication of HCV in extrahepatic tissues have been performed in immunosuppressed15,20,30 or intravenous drug users (IDU),31 who are probably more permissive to infection due to altered immunity or multiple exposures. There is therefore, scanty data about detection of HCV in PBMC in immunocompetent subjects. In this study we explored the possible influence of viral extrahepatic infection in the natural history of hepatitis C. We report that HCV RNA is detectable in PBMC from treatment naïve Chilean patients, mainly infected by blood transfusion, suggesting that PBMC infection by HCV is not restricted, as previously suggested, to IDU, liver transplant recipients, nor HCV-HIV co-infected patients. Moreover, we show that viral sequences isolated from PBMC differ from those found in plasma, strongly suggesting virus compartmentalization. In addition, by comparison of the clinical features of HCV infected patients that harbor viral RNA in PBMCs with those of patients that show virus only in plasma we establish a direct correlation between PBMC infection and hepatitis C disease progression.
Patients and methodsStudy populationPatients were recruited prospectively from the outpatient clinic of the Liver Unit of the Clinical Hospital, Pontificia Universidad Católica de Chile from 2005 to 2007. We enrolled consecutive adult patients (older than 18 years old) with chronic hepatitis C defined as a positive anti-HCV antibody for more than 6 months with detectable viremia by Cobas Amplicor HCV Monitor Test, version 2.0 (Roche Diagnostic Systems). Patients were treatment naïve. Patients with anemia (hematocrit less than 20%), pregnancy, HIV or HBV co-infection or accumulated blood extraction greater than 6 mL/kg in the 6 previous weeks were excluded from the study. Other causes of liver disease were excluded. Mixed cryoglobulinemia was diagnosed on the basis of the manifestations of Meltzer and Franklin’s triad (purpura, asthenia, and arthralgia),32 the demonstration of a cryocrit level greater or equal to 2% and a positive determination of rheumatoid factor. Demographic, clinical and laboratory characteristics of the subjects were recorded prospectively. Duration of infection was defined as the interval between the time of the first unsafe blood transfusion or the time of the first intravenous drug use and the date of the blood sample. The protocol of the study was approved by the Ethics Committee of the Faculty of Medicine of the Pontificia Universidad Católica de Chile. All patients signed a written informed consent form approved by the same committee.
Blood processing, RNA isolation and RT-PCRPeripheral blood (50 mL) was collected into EDTA-containing Vacutainer tubes (Becton Dickinson®). Blood samples were diluted with an equal volume of PBS and a density gradient (Lymphocyte separation medium, Cellgro®) was used to isolate mononuclear cells by centrifugation. Total RNA was extracted using the protocol described by Chomczynski and Sacchi.33 RNA concentrations were determined by spectrophotometry (GeneQuant, Pharmacia). Total PBMCs were cultured in 24-well plates at a density of 1x106 cells per ml in R-10 media (RPMI 1640, GIBCO Life Technologies), 10% heat-inactivated fetal calf serum, 2 mM L-glutamine, antibiotics and 50 • • M 2-mercaptoethanol (Sigma). HCV-RNA was detected by a one step reverse transcription (RT)-polymerase-chain reaction (PCR) using the SuperScript™ III one step RT-PCR system with Platinum® Taq DNA Polymerase (Invitrogen) kit was carried out against the highly conserved 5’ UTR using the sense primer: 5’-TTG GGG GCG ACA CTC CAC CAT GAT C-3’ and the anti-sense primer: 5’-GTT ACG TTT GGT TTT TCT TTG AGG T-3’ generating a 370 bp amplicon. RT-PCR was also conducted against the NS5B coding region using the sense primer: 5’-TTC TCG TAT GAT ACC CGC TGT TTT GA-3’ and the anti-sense primer: 5’-TAC CTG GTC ATA GCC TCC GTG AA-3’ generating a 388 bp amplicon. The RT-reaction was conducted at 50°C for 45 min. The RT-enzyme was then inactivated and the Platinum® Taq DNA polymerase activated, by heating the mix at 95°C for 5 min. PCR amplifications were carried out for 40 cycles with each cycle at 95°C for 45 seg, 58°C (for the 5’UTR) or 56°C (for the NS5B coding region) for 45 seg, and 68°C for 45 seg. In vitro transcribed RNA (using T7-RNA polymerase, Fermentas) generated from plasmid pFK-I377neo/NS3-3’/wt34 (AJ242654; kindly provided by Dr. Ralf Bartenschlager, University of Heidelberg, Germany) or total RNA extracted from a human hepatoma cell line, Huh-7, constitutively expressing plasmid pFK-I377neo/NS3-3’/wt34 (kindly provided by Dr. R. Bartenschlager) were used as RT-PCR positive control while water, an unrelated T7-RNA polymerase in vitro synthesized RNA, or total RNA extracted from Huh-7 cell lines were used as negative control.
HCV genotyping and sequencingRNA was isolated from plasma and PBMC as described above. 5’-UTR genotyping was performed using the reverse-hybridization line probe assay, INNO-LiPA HCV II kit (Innogenetics, Ghent, Belgium) according to the manufacturer’s instructions. Briefly, the 5’ UTR is amplified with biotinylated primers. Biotin-labeled PCR products are reverse hybridized to specific probes attached to nitrocellulose strips. Development results in a purple precipitate that forms a positive line on the strip. The HCV type is deduced on the basis of the patterns of hybridizing bands by using the line probe assay (LiPA) interpretation chart. Amplification of the 5’UTR and the nonstructural region 5B (NS5B) followed by direct sequencing (Macrogen Corp, USA) were performed as previously described to confirm the 5’-UTR genotyping. 5’UTR and NS5B sequences were genotyped by using referenced and annotated HCV sequences.
Statistical analysisDescriptive statistics (mean, median, interquartile range) were used as appropriate for the main variables. Patients with detectable HCV RNA in PBMC (cases) were compared with those who did not have detectable HCV RNA (controls). Unpaired, two-tailed T test (with Welch correction for nonparametric variables) was used to compare both groups. Odds ratios with the corresponding 95% confidence interval (CI) were calculated for variables of interest. Significance was set at a p value lower or equal to 0.05.
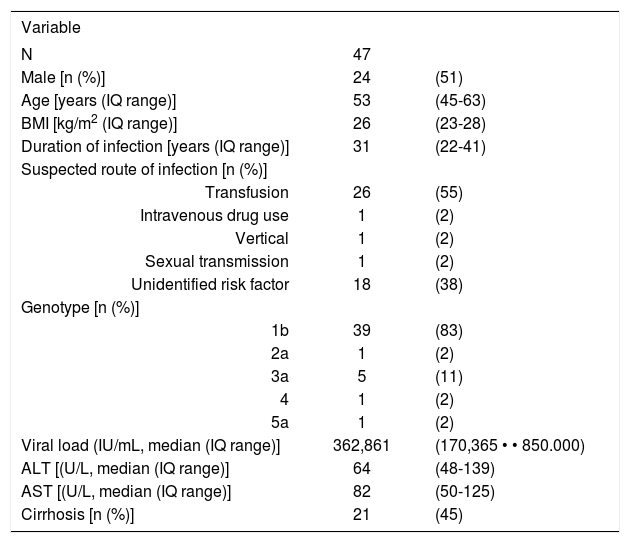
ResultsForty seven patients were eligible for the study. The basal characteristics of the patients are shown in Table I The predominant route of infection was blood transfusion (55%), with only 1 patient having a history of intravenous drug use. A considerable proportion of patients were cirrhotic (45%) and three patients had mixed cryoglobulinemia at the time of recruitment.
Clinical features of the 47 Chilean patients enroled.
| Variable | ||
|---|---|---|
| N | 47 | |
| Male [n (%)] | 24 | (51) |
| Age [years (IQ range)] | 53 | (45-63) |
| BMI [kg/m2 (IQ range)] | 26 | (23-28) |
| Duration of infection [years (IQ range)] | 31 | (22-41) |
| Suspected route of infection [n (%)] | ||
| Transfusion | 26 | (55) |
| Intravenous drug use | 1 | (2) |
| Vertical | 1 | (2) |
| Sexual transmission | 1 | (2) |
| Unidentified risk factor | 18 | (38) |
| Genotype [n (%)] | ||
| 1b | 39 | (83) |
| 2a | 1 | (2) |
| 3a | 5 | (11) |
| 4 | 1 | (2) |
| 5a | 1 | (2) |
| Viral load (IU/mL, median (IQ range)] | 362,861 | (170,365 • • 850.000) |
| ALT [(U/L, median (IQ range)] | 64 | (48-139) |
| AST [(U/L, median (IQ range)] | 82 | (50-125) |
| Cirrhosis [n (%)] | 21 | (45) |
IQ: Interquartile range ALT: Alanine-aminotransferase AST: Aspartate-aminotransferase BMI: Body mass index
Direct sequencing of the RT-PCR amplicons, corresponding to the 5’untranslated region (UTR) and to the NS5B coding region of the HCV RNA, derived from total RNA isolated from plasma allowed confirmation of the assigned HCV genotype previously established by the INNO-LIPA HCV II test. Genotype 1b was confirmed to be the most prevalent among the studied population (83%). This observation is in agreement with previous studies showing a high prevalence of genotype 1b among the Chilean population.35,36
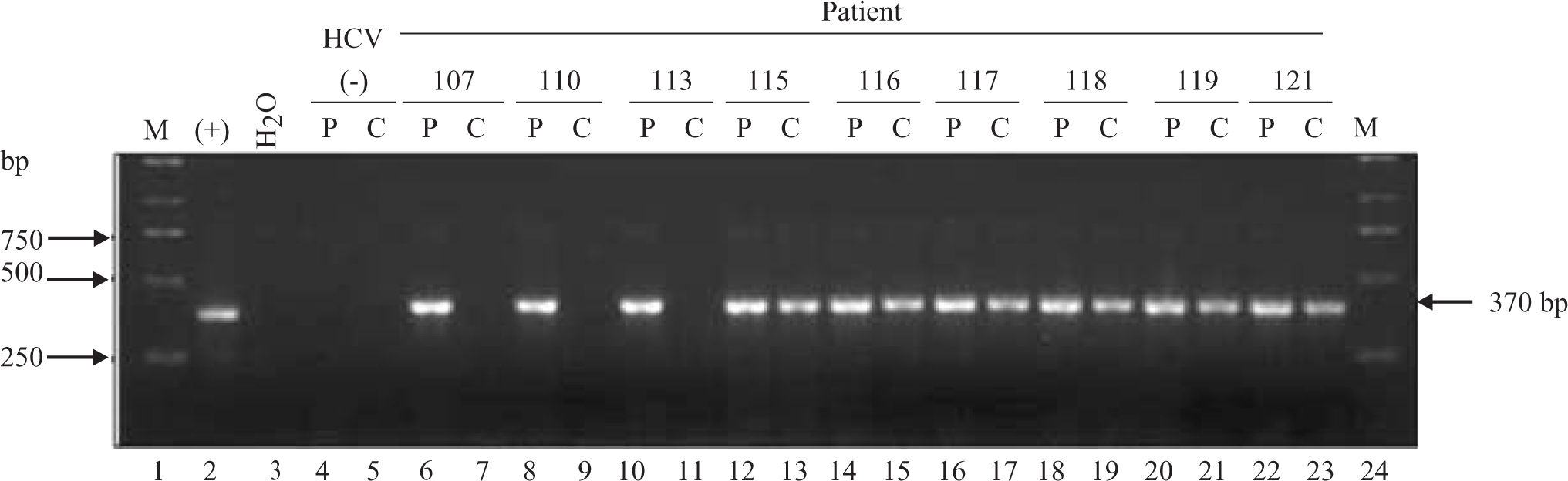
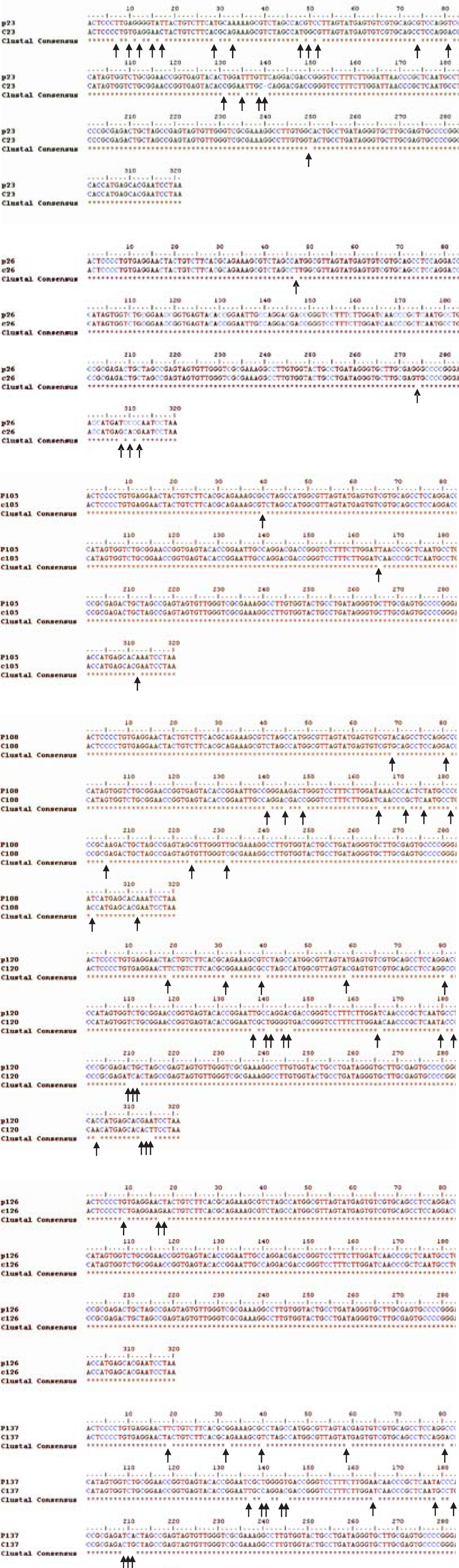
HCV RNA was detectable in PBMCs of 34 out of 47 patients (72%) (Figure 1) Sequencing of the amplicon corresponding to the 5’UTR of predominant viral quasiespecies in each biological compartment (plasma and PBMC) showed that virus present in PBMC shared the same genotype as virus in plasma, yet in most cases nucleotidic dissimilarities were found. Our findings are exemplified in Figure 2 which shows the alignment between the HCV 5’UTR sequences obtained from plasma (P) or PBMCs (C) of seven randomly selected patients, identified by their code number 23, 26, 105, 108, 120, 126, and 137.
Detection of HCV RNA in plasma (P) samples and PBMCs (C) of infected patients (lanes 6 through 23). Sample 1 lanes 4 and 5 corresponds to plasma and PBMC collected from a non-HCV infected donor (negative control). Lane 2 corresponds to the amplicon generated when in vitro transcribed HCV 1b RNA is used as template (positive control). Lane 3 corresponds to the RT-PCR water control (negative control). Lanes 1 and 24 correspond to the molecular weight marker. Numbers in the upper row represent individual patients. P: Plasma C: Cells Lane 2: Positive control (Bartenschlager’s replicon) Lane 3: Negative control (water)
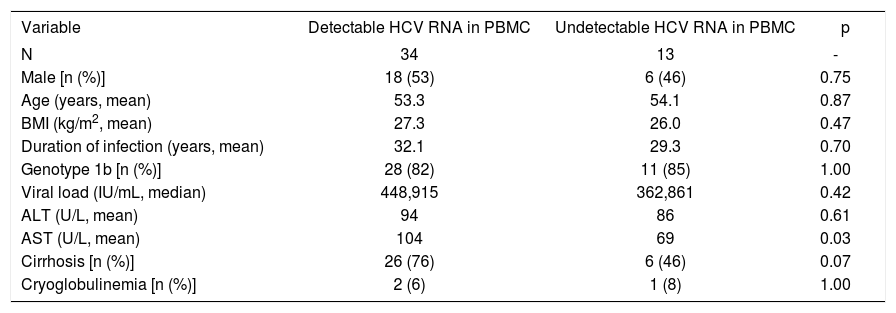
Intrigued by the fact that 72% of the studied HCV infected patients harbored detectable amounts of HCV RNA in both plasma and PBMCs we next sought to establish if they presented dissimilar clinical features to patients that showed viral RNA only in plasma (28%). Table II summarizes the clinical features of the HCV infected patients included in this study. For the sake of comparison patients are separated based on to the presence or absence of detectable viral RNA in PBMCs. Strikingly, upon analysis no statistically significant differences could be drawn when comparing the clinical features of both populations of HCV infected patients in terms of age, gender, BMI, HCV genotype, viral load or estimated duration of infection. Interestingly, however, is the observation that patients presenting detectable amounts of HCV RNA in PBMC exhibit higher AST levels (p = 0.03) and a trend to be more likely cirrhotic (76%) than those patients without detectable HCV RNA in PBMC (46%), p=0.07, OR 3.8, 95% CI: 0.98 to 14.
Comparison of patients who had detectable and undetectable HCV RNA (5’ UTR) in PBMC.
| Variable | Detectable HCV RNA in PBMC | Undetectable HCV RNA in PBMC | p |
|---|---|---|---|
| N | 34 | 13 | - |
| Male [n (%)] | 18 (53) | 6 (46) | 0.75 |
| Age (years, mean) | 53.3 | 54.1 | 0.87 |
| BMI (kg/m2, mean) | 27.3 | 26.0 | 0.47 |
| Duration of infection (years, mean) | 32.1 | 29.3 | 0.70 |
| Genotype 1b [n (%)] | 28 (82) | 11 (85) | 1.00 |
| Viral load (IU/mL, median) | 448,915 | 362,861 | 0.42 |
| ALT (U/L, mean) | 94 | 86 | 0.61 |
| AST (U/L, mean) | 104 | 69 | 0.03 |
| Cirrhosis [n (%)] | 26 (76) | 6 (46) | 0.07 |
| Cryoglobulinemia [n (%)] | 2 (6) | 1 (8) | 1.00 |
Among the studied patients recruited with cryoglobulinemia only two (2/3) had detectable HCV RNA in PBMC. Thus, no conclusion associating cryoglobulinemia with the presence of HCV RNA in PBMCs or HCV extrahepatic replication can be drawn.
DiscussionHepatitis C virus infection is a chronic disease that may take several decades to progress to clinically significant outcomes. Several viral, host and environmental factors have been described to modulate progression of the disease.5 However, up to date no report shows that infection of tissues other than the liver by HCV could accelerate the progression of fibrosis or induce a more severe form of the disease.
A high chronicity rate, a high relapse rate and the presence of extrahepatic manifestations are all pieces of the yet intriguing puzzle constituted by HCV infection. Extrahepatic replication of the virus can be associated to some or all of these manifestations, as several other studies have already suggested.8,17,37-39 A correlation between the presence of cryoglobulinemia and more advanced liver fibrosis has been described in Greek patients,40 and at least two studies show a possible association between HCV infection of PBMCs and mixed cryoglobulinemia, but a direct relationship of viral infection of extrahepatic cells with the rate of progression or severity of the disease has not been suggested previously.
It has been already shown that HCV infected PBMC often contain virus variants differing from those circulating in serum or plasma.41-47 Our results confirm these previous observations. The meaning of this divergence is still a matter of debate, but they strongly suggest a differential tropism of HCV variants in tissues.
The present study has several limitations that hamper the interpretation of the results. First, the demonstration of HCV RNA in PBMC does not prove active virus replication in these cells. It has been suggested that amplification of the negative strand of the HCV RNA, the natural RNA replication intermediate, is the gold standard for demonstrating active viral replication,48 but the technique is far from well validated and still highly controversial. Moreover, there are several arguments that support the notion that activation of PBMC is an absolute requirement for HCV replication, therefore the presence of negative strand would be expected only in mitogen activated PBMC.14,49,50 In consequence, as our study did not include the activation of PBMCs prior to total RNA isolation we would not expect to find HCV negative strand RNA within these cells. Nevertheless, the herein presented data clearly shows that HCV RNA can be readily detected in PBMC from treatment naïve HCV patients that are not injection drugs users (IDU) nor HIV-HCV coinfected, stressing that PBMC infection by HCV is not restricted to the latter populations. Additionally, we present data that favors the notion of HCV compartmentalization as plasma and PBMC harbor different virus variants belonging to different viral quasiespecies. Interestigly, even though indirect, this later finding are an indicative of HCV infection and independent replication in PBMCs.51
A second limitation of our study is the weak statistical weight of the herein driven association. We have shown a trend for a higher proportion of cirrhosis in patients who had detectable HCV RNA in PBMC with an OR 3.8 (95% CI: 0.98 to 14). This association of course does not imply causality. In fact it may well represent the opposite, that cirrhosis may be a factor predisposing to viral infection of extrahepatic sites. Progression of fibrosis is multifactorial, therefore it is important to account for possible confounding factors such as age, route of infection, genotype, viral load and others. The small sample size precluded formal multivariable analysis of data, but none of these factors were independently found to be associated to detection of HCV RNA in PBMCs in our study. A more accurate way to search for this association would be to examine the rate of progression of fibrosis, rather than the mere presence or absence of cirrhosis.
Notwithstanding the highlighted limitations of our report, this observational study does however raises a number of intriguing question that are worth further investigatation, i. e. the possible role of hepatitis C virus infection and replication in PBMC as a key factor influencing the natural history of the disease. The mechanism by which infection of PBMC could result in an accelerated progression of fibrosis remains completely speculative. It is tempting to hypothesize that lymphotropic HCV strains could derange immunologic responses of CD4 cells or other immune cells important to keep hepatic HCV replication under control.
In summary, we have shown evidence of HCV specific infection of PBMC, with detection of RNA in these cells belonging to different quasiespecies from those found in the plasma of the same patients. Moreover, our data strongly suggest that PBMC infection by HCV is not restricted, as previously suggested, to IDU, liver transplant recipients, nor HCV-HIV co-infected patients. In addition, we have also found that HCV extrahepatic infection could be associated with more advanced liver disease. In order to be confirmed, this finding needs to be addressed with further studies with higher number of patients and a prospective design.
AcknowledgementsWe thank Dr. Ralf Bartenschlager, University of Heidelberg, Germany, for kindly providing HCV related reagents. We appreciate the help of Nurse Molque Velasco during sample collection. We thank Chanell Pasquot for her administrative assistance.