Background. We compared mortality and complications of chronic hepatitis C between treated and untreated Mexican patients after long-term follow-up. We used a time-to-event analysis and identified the prognostic factors.
Material and methods. Seventy-four patients with chronic hepatitis C were studied. They were ≥ 18 years of age and had a molecular diagnosis of chronic hepatitis C and ≥ 6 months of follow-up. Patients with neoplasia or those infected with human immunodeficiency virus or hepatitis B Virus were excluded. Kaplan-Meier analysis, log-rank test, annualized incidence per 100 person-years, and stepwise discriminant analysis were used to analyse mortality and complications.
Results. The end-point of annualized incidence was lowest in sustained virological responders, intermediate in non-responders, and highest in untreated patients. The absence of treatment impacted adversely on cirrhosis development and the occurrence of portal hypertension and hepatic decompensation/hepatocellular carcinoma (log-rank, p < 0.05). Diabetes impacted adversely on liver-related death/liver transplantation among untreated patients. Stepwise discriminant analysis showed that diabetes, high blood pressure, and no retreatment predicted cirrhosis development (eigenvalue ≥ 0.8; p < 0.05). A MELD score ≥ 18 and age ≥ 50 years predicted hepatic decompensation/hepatocellular carcinoma (eigenvalue < 0.8; p < 0.05). APRI ≥ 1.5 predicted mortality/liver transplantation and liver-related death/liver transplantation (eigenvalue < 0.8; p < 0.05).
Conclusions. This is the first long-term study of chronic hepatitis C among Mexican patients. Treated patients showed less progression of liver disease. Treated patients showed less progression of liver disease; and older patients, those with metabolic comorbidities, with MELD score ≥ 18 and APRI ≥ 1.5 exhibited adverse effects.
Globally, up to 170 million people are estimated to have chronic hepatitis C virus (HCV) infection.1 Once HCV-related cirrhosis has developed, the annual incidence of clinical decompensation, death or liver transplantation (LT), and hepatocellular carcinoma (HCC) among untreated patients or non-re-sponders (NRs) to antiviral therapy (AT) with chronic hepatitis C (CHC) is ~4%, ~3%, and ~3% per year, respectively.2–7 A sustained virological response (SVR) is associated with improved overall survival,2,5 reduced risk of morbidity and liver failure, and improved quality of life.8 The relationship between AT and long-term viral suppression depends on host, viral, and on-treatment factors.9
Intermediate benefits of AT on patient outcomes and the natural history of disease are also present in NRs.5,10 Any duration of AT for HCV was associated with a lower risk of mortality in a large national sample of HCV-infected people.10 In Mexico, the CHC incidence is rising,11 and its prevalence and percentage of patients with genotype (G) 1 are 1.2-1.5%12 and ~70%,13 respectively. Hispanic patients are under-represented in longitudinal multinational studies of CHC. There is scarce information on long-term follow-up in treated Mexican patients with CHC. The aim of this study was to compare mortality and clinical complications of CHC between treated and untreated Mexican patients. We developed a time-to-event analysis and identified the prognostic factors.
Material and MethodsStudy design, participants, and settingWe conducted a dynamic, retrospective, comparative cohort study at the Liver Unit of Dr. José Eleuterio González University Hospital, Universidad Autónoma de Nuevo León (UANL), the main tertiary centre treating patients with liver diseases in north-east Mexico. Medical records of patients with CHC admitted from 1987 onwards were reviewed; 310 of > 500 patients were studied and 134/310 (43%) were eligible. Reasons for the exclusion of the remaining 176 (57%) patients were: < 6 months of follow-up (n = 131, 42%), non-molecular diagnosis of CHC (n = 21, 7%), occurrence of mortality or LT during the first 6 months of follow-up (n= 5, 2%), ALD (n = 5, 2%), neoplasia (n = 5, 2%), HIV (n = 2, 1%), HBV (n = 2, 1%), AIH ( n = 4, 1%), <18 years old at baseline (n = 1, 1%). There were two inception cohorts: patients exposed to AT (RxG) (n = 67) and those not exposed to AT (noRxG) (n = 67). Nevertheless, over the 134 eligible patients, cirrhosis and hepatic decompensation were more commonly found among noRxG than RxG patients (57%, 44% vs 40%, 13%, respectively), therefore only 74 patients were included in this study, in order to provide baseline comparability and to avoid membership bias. RxG patients were treated according to the guidelines at the time. Reasons for not fulfilling therapy were too-advanced liver disease (n = 12, 32%), medical contraindications (n = 9, 24%), refusal to receive AT (n = 12, 32%), < 12 weeks of AT because of adverse events (n = 2, 5%), and lack of financial support (n = 2, 5%). Follow-up started when RxG received their first AT and at their first medical interview for noRxG; all variables were concurrent with each start point. Inclusion criteria were aged ≥ 18 years, CHC diagnosis by HCV polymerase chain reaction (PCR) and/or HCV G, and medical management in this unit for ≥ 6 months. The exclusion criteria were coexistent malignant neoplasia, hepatitis B or HIV infection, alcoholic liver disease, autoimmune hepatitis (excluding probable and definite cases according to the revised score of the International Autoimmune Hepatitis Group [R-IAIHG]), or drug-induced liver disease. Table 1 summarizes the baseline characteristics of the two groups. The protocol was approved by the UANL Medical School Institutional Review Board, and was complied with the Helsinki Declaration.
Baseline features of the cohort.
| Total (%) | RxG (%) | noRxG (%) | p | |
|---|---|---|---|---|
| n | 74 (100) | 37 (100) | 37 (100) | - |
| Age ± SD | 49 ± 12 | 46 ± 10 | 51 ± 14 | 0.025 |
| Gender | ||||
| F/M | 44/30 (60/40) | 24/13 (65/35) | 20/17 (54/46) | 0.593 |
| Fibrosis stage | ||||
| F0-F2 | 25 (34) | 11 (30) | 14 (38) | 0.595 |
| F3 | 11 (15) | 7 (19) | 4 (11) | 0.172 |
| F4 | 38 (51) | 19 (51) | 19 (51) | 0.592 |
| D’amico, García-Tsao in cirrhotics | ||||
| Stages 1 & 2 | 23 (61) | 12 (63) | 11 (58) | 0.500 |
| Stage 3 & 4 | 15 (39) | 7 (37) | 8 (42) | |
| BMI + SD | 28 ± 9 | 27 ± 8 | 28 ± 10 | 0.926 |
| > 30 kg/m2 | 19 (28) | 10 (29) | 9 (27) | 0.618 |
| HCV RNA (UI/mL) ± SD | 842,629 ± 1,432,718 | 938,938 ± 1,879,569 | 743,310 ± 781,117 | 0.740 |
| HCV RNA (log/mL) ± SD | 5.9 ± 6.1a | 6 ± 6.2 e, b | 5.9 ± 5.9 c, d, e | |
| HCV RNA > 600,000 UI/mL | 25 (36) | 11 (31) | 14 (41) | 0.592 |
| HCV genotype | ||||
| G1 | 50 (68) | 22 (59) | 28 (76) | 0.007 |
| G 2, 3, 4 | 16 (22) | 14 (38) | 2 (5) | |
| Non available | 8 (10) | 1 (3) | 7 (19) | |
| Child-Pugh in cirrhotics | ||||
| A/B/C | 26/11/0(68/29/0)f | 14/4/0 (74/21/0)f | 12/7/0 (63/37/0) | 0.050 |
| MELD > 18 in cirrhotics | 2 (5) | 2 (11) | 0 | 0.247 |
| High blood pressure | 14 (19) | 4 (11) | 10 (27) | 0.117 |
| Diebetes mellitus | 14 (19) | 4 (11) | 10 (27) | 0.117 |
| Dyslipidemia | 7 (10) | 3 (8) | 4 (11) | 0.500 |
| Liver steatosis | 17 (23)f | 11 (31)f | 6 (16) | 0.270 |
| History of alcohol abuse | 9 (12) | 5 (14) | 4 (11) | 0.375 |
| Blood transfusion before 1992/IV | 51/6/4/26 | 22/2/3/13 | 29/4/1/13 | |
| drugs/tattoos/other | (69/8/5/35) | (59/5/8/35) | (78/11/3/5) | 0.300 |
| ALT (UI/L) | 116 ± 92g | 125 ± 104f | 107 ± 79f | 0.665 |
| AST (UI/L) | 113 ± 75g | 116 ± 73g | 109 ± 77 | 0.884 |
| Platelet count, x 109/L in cirrhotics | 150 ± 83 | 154 ± 63 | 146 ± 106 | 0.045 |
| APRI > 1.5 in cirrhotics | 24 (63) | 11 (58) | 13 (68) | 0.297 |
| Interferon α standard alone | 6 (8) | 6 (16) | - | - |
| Interferon α standard or natural Interferon + ribavirin | 11 (15) | 11 (30) | - | - |
| PegInterferon α 2a/b + ribavirin | 20 (27) | 20 (54) | - | - |
The baseline variables recorded were: demographics; AT type; comorbidities (diabetes mellitus [DM], dyslipidaemia, liver steatosis [LS]); alcohol abuse history; liver-related complications (cirrhosis, portal hypertension [PH]; gastro-oesophageal varices [GEV]; a history of ascites or gastro-intestinal bleeding secondary to PH [GIBsPH] and/or encephalopathy); body mass index (BMI); biochemical, haematological, and serological tests; α-fetoprotein (AFP) level; imaging study results; endoscopic features; and fibrosis stage (liver biopsy, non-invasive methods). In eight patients, the G was not available. Viral load (VL) values not expressed in IU/mL were converted using the conversion factor of Pawlosky, et al.14 Liver enzyme levels were expressed in IU/L and AFP in ng/mL. RxG on-treatment follow-up was performed in weeks 4, 12, 24, and 48, and then 24 weeks after AT (24postAT). Imaging studies were performed and AFP level was measured every 6 months in cirrhotic patients. AT adverse events were recorded in a predefined fashion. Follow-up times were computed as the difference in months between the cirrhosis diagnosis date and the date of attrition, end of follow-up (21 April, 2013), and/or each end-point occurrence date. For mortality/LT, liver-related death (LRD)/LT, and cirrhosis, the time-to-event analysis considered the date of occurrence minus each cohort’s follow-up starting point. Whenever a non-cirrhotic patient developed cirrhosis, a clock-reset approach was used; the follow-up was censored for non-cirrhotic status when cirrhosis was diagnosed, the follow-up of the cirrhotic patient was reset to zero, and the case was restarted as a new case. The same assessment features recorded at baseline were recorded during the last visit for a medical consultation.
Definition of end-points and baseline featuresEnd-points were primary outcomes: mortality/LT and LRD/LT, and secondary outcomes: occurrence of cirrhosis, PH, HCC and the composite event of clinical decompensation defined as D’Amico, García-Tsao stage progression (DAGTP)15 and HCC, (DAGTP/HCC).
Fibrosis stage was defined by METAVIR whenever a liver biopsy was available (n = 53) and by non-invasive tests (FibroTest, FibroScan or aspartate transaminase-to-platelet ratio index [APRI] results n = 11), and/or clinical criteria (fibrosis stage F4) (n = 10). International definitions to classify PH,16 HCC,17 ascites, HRS, SBP,18 GEV, GIBsPH,19 and encephalopathy20 were used. DAGTP from stage 1-2 to 3-4 was assessed by the date of onset of the pertinent secondary outcomes and/or jaundice (direct bilirubin ≥ 2.50 mg/dL). Patients were not censored according to previous secondary outcomes. Patients were censored if they were lost to follow-up and if they received LT before developing any end-point.
The baseline features studied included history of alcohol abuse defined as an alcohol consumption of > 60 g/day in men and > 40 g/day in women (no patient had alcohol abuse at the baseline), DM, high blood pressure (HBP), and dyslipidaemia as defined by expert committee criteria21 or mention of their diagnosis and current treatment in the patient’s history. SVR was defined as the absence of VL at 24postAT9 according to the lower limit of quantification (LLOQ) or lower limit of detection available at that time. LS was identified according to the criteria of Chalasani, et al.22 Any event that happened within the first 6 months of follow-up was framed as a baseline feature.
Statistical analysisSample size was determined in a non-probabilistic fashion. Baseline features were compared using the χ2 test (categorical) or Student’s t test (continuous). Incidence rates per 100 person-years (p100py) of primary and secondary outcomes were calculated along with 95% confidence intervals (CIs).23 Kaplan-Meier cumulative incidence analysis, grouped by end-point status (1 = present; 2 = absent), and log-rank post hoc testing were applied for the time-to-event analysis. Step-wise discriminant analysis (SDA) was performed to predict the profile and number of patients presenting with each of the 13 end-points at the end of follow-up: end-point present or end-point absent.24,25 Variables were selected for this analysis because they are alleged predictors of a favourable or unfavourable outcome of CHC. They were entered as binary predictor variables, with values corresponding to those registered at the end of follow-up. Statistical tests were done using SPSS v.19 and online resources.23
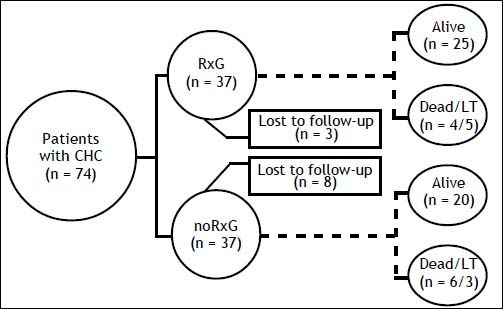
ResultsThe median follow-up was 83 (6-195) months: 82 (6-178) and 84 (10-195) months in RxG and noRxG, respectively. In RxG, AT reached 48 weeks (n = 26 G1) and 24 weeks (n = 13 non-G1). AT was not completed because of treatment failure (n = 6) and stroke at week 43 of AT (n = 1, G1). One G1 patient who completed 48 weeks of AT did not achieve 24po-stAT because of LT. SVR was achieved by 13 (35%). Despite retreatment, no treatment-experienced patient (n = 6) became HCV RNA negative. Around 15% of all patients were lost to follow-up: RxG, n = 3 (8%) and noRxG, n = 8 (21%) (Figure 1).
During follow-up, in the non-cirrhotic patients at baseline, 2/18 (11%) in RxG and 5/18 (28%) in noRxG developed cirrhosis. Annual incidence p100py was 3.3 times greater in noRxG than in NRs and 8.2 times greater in noRxG than in those with SVR (Table 2). Kaplan-Meier cumulative incidence of cirrhosis in years 6, 8, 10, and 13 remained at 13% in RxG, but increased from 25% to 75% to 100% in years 6, 8, and 11 in noRxG (log-rank p = 0.036) (Figure 2A).
End-point annualized incidence according to antiviral treatment status.
| Overall patients | RxG | noRxG | ||
|---|---|---|---|---|
| End-pointsa | SVR | NR | ||
| Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | Rate (95% CI) | |
| Mortality/LT | 6.4 (3.9-9.9) | 0.7 (0.03-3.5) | 5.5 (2.5-10.4) | 3.4 (1.7-6.1) |
| Non-LRD | 0.7 (0.1-2.3) | - | 0.6 (0.03-3.1) | 0.3 (0.02-1.7) |
| LRD/LT | 5.7 (3.3-9.1) | 0.7 (0.03-3.5) | 4.8 (2.1-9.5) | 3.1 (1.5-5.6) |
| DAGTP/HCC | 4.2 (2.3-7.2) | - | 5.2 (1.6-12.4) | 11.1 (5.1-21.1) |
| Cirrhosis | 4.2 (1.8-8.4) | 1.34 (0.06-6.6) | 3.3 (0.16-16.4) | 11.1 (4.1-24.6) |
| PHb | 9.2 (5.6-16.4) | 11.0 (1.9-38.9) | 11.0 (3.4-25.7) | 46.2 (18.7-96) |
| HCC | 3.6 (1.5-7.1) | - | 3.0 (0.5-10.0) | 4.2 (1.5-9.3) |
The time-to-event sub-analysis stratified several factors related to AT as shown in table 3 and figures 2B-2F. Only PH and DAGTP/HCC in RxG vs noRxG were significant by the log-rank test (p < 0.05).
Endpoint occurrences at the end of follow-up.
| Total, n (%) | RxG, n (%) | noRxG, n (%) | |
|---|---|---|---|
| Portal hypertension | 11/19 (58) | 5/10 (50) | 6/9 (67) |
| D’Amico, García-Tsao progression | 8/38 (21) | 3/14 (21) | 5/14 (36) |
| D’Amico, García-Tsao progression/Hepatocellular carcinomaa | 12/28 (43) | 4/14 (21) | 8/14 (57) |
| Ascites | 9/33 (28) | 4/17 (24) | 5/16 (31) |
| Gastro-intestinal bleeding secondary to portal hypertension | 5/41 (12) | 3/20 (15) | 2/21 (10) |
| Encephalopathy | 10/43 (23) | 5/21 (24) | 5/22 (23) |
| Hepatorenal syndrome | 3/45 (7) | 1/21 (5) | 2/24 (4) |
| Spontaneous bacterial peritonitis | 2/45 (4) | 1/21 (5) | 1/24 (4) |
| Hepatocellular carcinoma | 7/45 (16) | 2/21 (10) | 5/24 (21) |
| Death/liver transplantation b | 18/74 (24) | 9/37 (24) | 9/37 (24) |
The rates of PH, DAGTP/HCC, and HCC annual incidences p100py were 4.2, 1.7, and 1.4 times higher, respectively, in noRxG than in NRs (Table 2). No patients with SVR experienced any of these, except for PH (11 p100py) (Table 2).
Mortality, LRD, and LTMortality/LT reached 18/74 (24%) at the end of follow-up and was 9/37 (24%) in both groups. LRD occurred in 3/37 patients (8%) in RxG and in 5/37 patients (14%) in noRxG. Mortality/LT and LRD/LT Annual incidences p100py were more common in RxG than noRxG (Table 2). Kaplan-Meier cumulative incidence estimates were not significant for mortality/LT (p = 0.62) or LRD/LT (p = 0.35) in the RxG and noRxG (Figures 2E and 2F).
Discriminant analysisPH and DAGTP sub-analyses did not qualify for SDA (F value to enter < 3.84 or overfitting). Six variables separated the two subgroups according to their end-point occurrence (present/absent) (p < 0.05). Mortality/LT and LRD/LT were predicted by APRI ≥ 1.5; cirrhosis by DM, HBP, and no retreatment; DAGTP/HCC was predicted by model of endstage liver disease (MELD) ≥ 18 and age ≥ 50 years (Table 4). Predictor subsets with the highest power to predict prognosis had eigenvalues > 0.8. These subsets included retreatment (Wilks’ λ = 0.534; p < 0.001); HBP (Wilks’ λ = 0.643; p = 0.001); and DM (Wilks’ λ = 0.754; p = 0.002) in the cirrhosis model. Age ≥ 50 years (Wilks’ λ = 0.670; p = 0.001) and MELD ≥ 18 (Wilks’ λ = 0.839; p = 0.001) were significant in the DAGTP/HCC model. Despite a high positive predictive value (PPV) of > 80%, only the cirrhosis model had high sensitivity (> 85%) and predicted membership with classification accuracy > 25% compared with classification by chance. All other models had p < 0.05 and PPV > 80% but lower sensitivity, classification accuracy < 25%, eigenvalues < 0.80, and high variability of their predictors (Wilks’ λ > 0.2).
Discriminant models for the presence/absence of each end-point according to concurrent predictors present at the end of the follow-up.
| End-point occurrence | Discriminant analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| End-point^ | Predictor | Wilks’ λ | F | Discriminant fun ction | Eigen value | Misclassified cases | CCC (%) | ||
| Sen (%) | PPV (%) | MPP (%) | |||||||
| Cirrhosis development | Retreatment ** | 0.534 | 6.5 | −0.721 | 0.872 | 86 | 86 | 68 | 94.4 |
| HBP ** | 0.643 | 11.5 | 0.911 | ||||||
| DM ** | 0.754 | 17.2 | 0.948 | ||||||
| DAGTP/HCCa | Age ≥50 years**,b | 0.670 | 5.5 | 0.644 | 0.823 | 42 | 100 | 51 | 75.0 |
| MELD ≥ 18 **,c | 0.839 | 13.2 | 0.890 | ||||||
| LRD/LT | APRI ≥ 1.5 **,d | 0.819 | 12.8 | 1 | 0.221 | 25 | 40 | 76 | 85.9 |
| Mortality/LT | APRI ≥ 1.5 **, d | 0.888 | 9.0 | 1 | 0.126 | 30 | 60 | 76 | 86.5 |
Sen: sensitivity. MPP: membership prior probability. CCC: correctly classified cases.
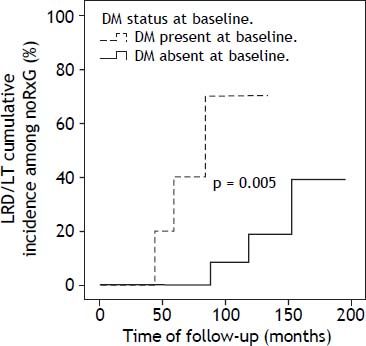
DM was present in 14/74 patients at baseline: RxG n = 4, noRxG n = 10. At end of follow-up, two non-diabetics (n = 1/25 [4%] NR, n = 1/28 [3.6%] noRxG) developed DM. The clock-reset approach introduced 16 newly diagnosed patients into the time-to-event analysis. The median follow-up for diabetics and non-diabetics was 52 (17-167) and 88 (6-195) months, respectively. The percentage of patients with SVR was lower in diabetics (20%) than in non-diabetics (36%). Because of the low DM prevalence, only the LRD/LT comparison in noRxG with and without DM is presented here (p < 0.05) (Figure 3).
DiscussionThis is the first study of Mexican patients with CHC to evaluate the effects of AT given before the protease-inhibitor era and assessed after long-term follow-up. As expected, the end-point annual incidence was lowest among those with SVR (4.4 to 11.1 times less than in the noRxG), intermediate in NRs (1.3 to 4.2 times less) and highest among the noRxG (0.3-46.2 p100py). The prognostic factors identified by SDA showed that MELD ≥ 18 and age ≥ 50 years predicted DAGTP/HCC and that APRI ≥ 1.5 predicted mortality/LT and LRD/LT. The log-rank test showed that the absence of AT impacted adversely on cirrhosis, PH, and DAGTP/HCC cumulative incidences in noRxG vs RxG even after > 10 years of follow-up.
Our comparison of the AT effect vs the noRxG agrees with that in a previous report showing that the rates of hepatic decompensation/HCC and mortality were higher in untreated vs. treated patients26 during a similar follow-up (50 vs. 13% and 45 vs. 13%, respectively). A higher percentage of noRxG had G1, Child-Pugh score ≥ 6, and thrombocytopenia than RxG (p < 0.05), although mortality/LT did not differ significantly between these groups. The higher mortality/LT ratio among the noRxG (6/3) vs. the RxG (4/5) group, alongside the non-probabilistic nature of the present sample-size and the high proportion of patients with advanced liver disease at baseline, could have overcome the benefit among the RxG that the improvement in fibrosis progression and cirrhosis complications should have had on their mortality/LT. When SVR is reached, DM may not have a negative impact on LRD/LT.2 There were few RxG diabetics in this cohort, and any adverse influence of DM on LRD/LT was confirmed only in the noRxG (p < 0.05).
The treated CHC patients had increased endpoints of cumulative incidences for mortality/LT at 6 and 10 years compared with Italian27 and US26 cohorts, and for HCC compared with Taiwanese3 and Italian-Argentine28 cohorts. The cumulative incidence of DAGTP/HCC was also higher here than in US patients26 during a similar follow-up. The lower cumulative incidence in non-Latino cohorts could reflect different AT schemes or G distribution, lower percentage of patients with cirrhosis (≤ 36% in other studies, ≥ 51% in our study), and higher percentage of an SVR (67% in Taiwanese patients,3 36% in our study). Studies from other countries have demonstrated diverse CHC complication burdens in treated and untreated patients; notably, the baseline distribution of features and cohort data collection designs were not uniform.3–7,26,28–33
Mortality/LT cumulative incidence among noRxG CHC patients here and in a Japanese cohort6 were similar after 6 years of follow-up and lower than in the US (26) (~18%, ~20%, and ~28%, respectively). However, the cumulative incidence in both studies6,26 increased steadily thereafter, as opposed to the value in these Mexican patients, which remained lower at 10 years (~35%, ~45%, and ~50%, respectively). The HCC cumulative incidence here is similar to that in Italian-Argentine patients28 (~16% at 6 years and ~36% at 10 years) but is lower than that reported in two large Japanese cohorts (≥ 28% at 6 years and ≥ 55% at 10 years).6,33 Mortality here and in the Japanese study6 was mostly liver related, whereas ~50% of mortality in the US patients was not.26 Older age and male sex were prognostic factors for mortality/LT and HCC by multivariate analysis in noRxG CHC cohorts.6,26,28,33 History of alcohol abuse was also a significant factor for HCC in US26 and Japanese6 patients.
Cirrhosis annual incidence among our NR CHC non-cirrhotic patients (3.5 p100py) was lower than that in a US cohort (9.9 p100py),32 although the US cohort had a higher incidence of BMI ≥ 30, G1 (100%), and DM (26%), possibly accounting for these differences.32 Longer follow-up of noRxG and the baseline occurrence of DM (27%), overweight (BMI = 28), hepatic decompensation (22%), and G1 (74%) may explain the higher frequency of cirrhosis and HCC annual incidence compared with Asian3,31 populations.
End-point annual incidence values among NR CHC compensated cirrhotic patients were similar to those reported for Canadian-European2 patients for DAGTP and LRD/LT, and higher than in North American,2 European,2,5 and/or Taiwanese3 cohorts for HCC. However, the HCC annual incidence is lower here than in Japanese patients.33
The annual incidence in SVR compensated CHC cirrhotic patients here was nil for DAGTP suggesting that a favourable response to treatment affected the CHC natural history. This was reported in similar cohorts from North America,2 South America,30 and Europe,2,5 although the annual incidence of HCC among Canadian-European,2 Italian,5 and Japanese33 patients was slightly higher than in our patients with SVR (0.55, 0.66, 0.49, and 0 p100py, respectively).
In our non-cirrhotic CHC cohort, the cirrhosis annual incidence was 4.2 p100py, whereas fibrosis/ year progression was 0.04-0.12 METAVIR units in an Argentinean retrospective study.7 DM, HBP, and no retreatment were negative prognostic factors in our patients, whereas in the Argentinean study7 alcohol abuse predicted fibrosis/year progression. Annual incidence values for HCC and mortality/LT in compensated CHC cirrhotics were higher here than those reported in other Latin-American populations (3.4 and 6.4 vs. ≤3.0 and ≤ 3.5 p100py, respectively).4,29 Annual incidence for hepatic decompensation in Cubans4 was double that in our patients, possibly because of higher G1 and NR/noRxG prevalence in the Cubans (96 and 92% vs. 68 and 82%, respectively). The reasons for these differences between ethnic groups are unclear.
This study has some limitations. It was a retrospective study of a sample of the Mexican population. Because of the non-probabilistic sample size, log-rank differences and SDA findings were not evident for all end-points. Fifteen per cent of the overall cohort abandoned the follow-up, and noRxG may have been susceptible to attrition bias (21%). We tried to reduce potential bias by studying secondary outcomes only among cirrhotic patients fulfilling similar inclusion/exclusion criteria.
ConclusionWe found that interferon-based therapy beneficially affected HCV-related complications among Mexican patients with CHC after a long-term follow-up. The prognostic factors were metabolic co-morbidities and no retreatment for cirrhosis development; APRI ≥ 1.5 for mortality/LT and LRD/LT; and older age and MELD ≥ 18 for DAGTP/HCC. DM was confirmed to impact adversely on LRD/LT in noRxG. Noteworthy, the high proportion of patients with advanced liver disease at baseline in this study could have overcome the benefit among the RxG that the improvement in fibrosis progression and cirrhosis complications should have had on their mortality/LT.
Abbreviations- •
24postAT: 24 weeks after antiviral therapy.
- •
AFP: α-fetoprotein.
- •
APRI: aspartate transaminase-to-platelet ratio index.
- •
AT: antiviral therapy.
- •
BMI: body mass index.
- •
CHC: chronic hepatitis C.
- •
CI: confidence interval.
- •
DAGTP: D’Amico, García-Tsao progression.
- •
DM: diabetes mellitus.
- •
G: genotype.
- •
GEV: gastroesophageal varices.
- •
GIBsPH: gastro-intestinal bleeding secondary to portal hypertension.
- •
HBP: high blood pressure.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
HRS: hepatorenal syndrome.
- •
LLOQ: lower limit of quantification.
- •
LRD: liver-related death.
- •
LS: liver steatosis.
- •
LT: liver transplantation.
- •
MELD: model of end-stage liver disease.
- •
noRxG: group of patients not exposed to antiviral therapy.
- •
NR: non-responder to antiviral therapy.
- •
p100py: per 100 person-years.
- •
PCR: polymerase chain reaction.
- •
PH: portal hypertension.
- •
PPV: positive predictive value.
- •
RxG: group of patients exposed to antiviral therapy.
- •
SBP: spontaneous bacterial peritonitis.
- •
SDA: stepwise discriminant analysis.
- •
SVR: sustained virological response.
- •
VL: viral load.
The authors who have taken part in this study declare that they do not have anything to disclose regarding funding or conflicts of interest with respect to this manuscript.
AcknowledgementsWe thank Jesús Adrián Guerra-Rivera and Mariana Arroyo-Tiburcio for their support in data collection and Ana María Rivas-Estilla and Neri Alejandro Álvarez-Villalobos for their critical discussion of the manuscript. This project was partially supported by CONACYT-GRANT 162077 LEME.