Intrahepatic cholestasis of pregnancy (ICP) is a disease characterized by generalized pruritus and biochemical cholestasis that appears typically during the last trimester of gestation. The most predictive and accurate markers for diagnosis and follow-up of ICP are increased total bile acid levels (above 11,0 μmol/L), enhanced cholic acid percentage (above 42%) and decreased glycine/taurine bile acid ratio (below 1.0). Although essentially benign for the mother, evidence associates ICP with fetal poor prognosis resulting from increased transfer of bile acids from mother to fetus, who showed reduced ability to eliminate bile acids across the placenta. Those conditions lead to an accumulation of bile acids in the cord blood serum, meconium and amniotic fluid that may account for a diminished fetal well-being and sudden intra-uterine death by ICP. Ursodeoxycholic acid (UDCA) treatment was shown to reduce the bile acid content in the fetal compartment, while restoring the ability of the placenta to carry out vectorial transfer of these compounds towards the mother, decreasing bile acid levels in maternal serum and its passage to the fetus. In addition, UDCA administered to the mother also lowers the amount of bile acids present in colostrum without either increasing the UDCA concentration or causing major changes in lithocholic acid levels, further supporting the safety of UDCA in late pregnancy. Therefore, it is tempting to indicate UDCA as a first choice therapy for ICP as much as relevant aspects of fetal outcome may also be improved. This review focuses on the altered bile acid profiles in maternal and fetal compartments during ICP and its recovery by UDCA administration. Further elucidation of the precise mechanisms of action of UDCA and its therapeutic potential in improving fetal prognosis could result in the approval of UDCA for ICP treatment.
ICP, intrahepatic cholestasis of pregnancy; UDCA, ursodeoxycholic acid
IntroductionCholestasis, whether resulting from hereditary or acquired liver diseases, is one of the most common manifestations of impaired bile flow. Intrahepatic cholestasis of pregnancy (ICP) is a condition of unknown etiology characterized by generalized pruritus and biochemical cholestasis, which occurs predominantly during the last trimester of pregnancy.1,2 However, appearance of ICP as early as the 10th week of pregnancy has been reported.3Pruritus generally starts in palms and soles. A mild jaundice may be noticed in 20% of patients after the onset of itching4 while a slight elevation in the concentration of conjugated bilirubin (17.1 ± 1.7 μmol/L) is observed in 90% of ICP women.5 Among serum liver enzymes, alanine aminotransferase is the most sensitive test,6,7 with 2 to 10 times increased values8 and its efficiency was referred to be over 90%,5 while γ-glutamiltranspeptidase is the least elevated. A more specific biochemical parameter of ICP is the rise of serum bile acids1,6,9 with levels comprised between 12.4 and 219.4 μjmol/L5 as compared to the upper normal limit in late gestation of 11 μJ.mol/L.10 Shortly after delivery, symptoms disappear and biochemical parameters of liver cell function return to normal.11
The incidence of ICP is high in Chile (14%)4 and Bolivia (9.2%),12 but less common in Europe where prevalence rates of 1 to 1.5% have been described for Portugal, Sweden, Poland and Finland.12,13 Prevalence is higher in twin than in single pregnancies and several clinical and experimental observations evidenced a primary role of estrogens12,14,15 and progesterones7,16,17 in ICP. Genetic predisposition also plays a key role in the pathogenesis of ICP and incidence increases within the same family.4 Current research indicates that mutations of the MDR3 gene encoding the canalicular phosphatidylcholine translocase (Figure 1) may in some cases predispose to ICP,18 justifying the raised γ-glutamiltranspeptidase in 20 to 40% of the patients.19,20 However, other characteristics of ICP suggest that exogenous factors may superimpose on the hormonal and genetic factors. This is indicated by seasonal changes in the appearance of the disease, with a higher number of cases in January, pointing to a greater incidence during the winter.5,21 Recent studies also link ICP to low serum selenium levels.22,23 In conclusion, ICP seems to result from combined and multivariate effects.
Hepatocanalicular transport systems. This schematic representation shows two adjacent hepatocytes comprising a bile canaliculus. Bile salts are excreted into the bile via a bile salt export pump (BSEP). The canalicular conjugate export pump (MRP2) appears to be responsible for ATP-dependent elimination of amphipathic anionic conjugates and reduced glutathione (GSH). The MDR1 gene product (MDR1) is responsible for elimination of hydrophobic cationic compounds while MDR3 P-glycoprotein (MDR3) mediates canalicular secretion of phosphatidylcholine. Women with mutations of the MDR3 gene may in some cases be predisposed to the development of intrahepatic cholestasis of pregnancy (ICP). A P-type adenosine triphosphatase (FIC1) is putatively involved in aminophospholipid translocation (PE, phosphatidylethanolamine; PS, phosphatidylserine). Adapted from.102-104
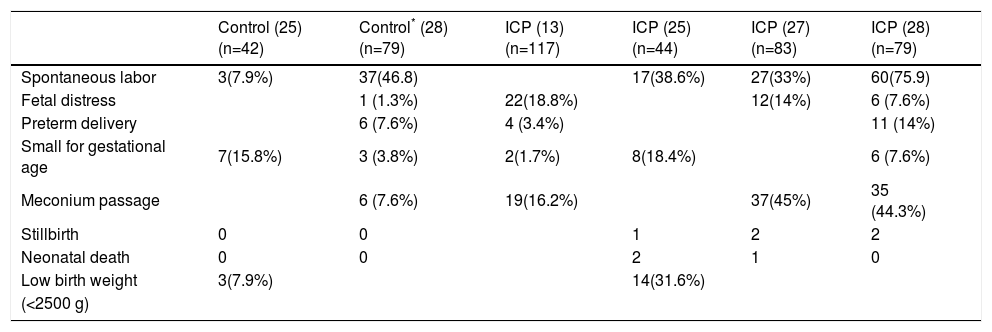
Although essentially benign to the mother, quality of life can be impaired due to itching, and urinary tract infection, postpartum hemorrhage, and fat and vitamin K malabsorption are common complications during ICP.11,24,25 The most serious consequences of ICP are increased fetal distress, premature deliveries and perinatal mortality and morbidity24,26,27(Table I). Meconium staining of amniotic fluid is considered a sign of poor prognosis for the fetus.27,28 Interruption of pregnancy is considered mandatory and urgent, mainly in an already mature fetus, when fetal distress is detected and there is risk of sudden death in utero.4 Therefore, ICP should be considered a high-risk condition, and an early and accurate identification of high-risk pregnancies together with an appropriate medical intervention might improve fetal outcome.
Perinatal outcome in both control and cholestatic pregnancies.
| Control (25) (n=42) | Control* (28) (n=79) | ICP (13) (n=117) | ICP (25) (n=44) | ICP (27) (n=83) | ICP (28) (n=79) | |
|---|---|---|---|---|---|---|
| Spontaneous labor | 3(7.9%) | 37(46.8) | 17(38.6%) | 27(33%) | 60(75.9) | |
| Fetal distress | 1 (1.3%) | 22(18.8%) | 12(14%) | 6 (7.6%) | ||
| Preterm delivery | 6 (7.6%) | 4 (3.4%) | 11 (14%) | |||
| Small for gestational age | 7(15.8%) | 3 (3.8%) | 2(1.7%) | 8(18.4%) | 6 (7.6%) | |
| Meconium passage | 6 (7.6%) | 19(16.2%) | 37(45%) | 35 (44.3%) | ||
| Stillbirth | 0 | 0 | 1 | 2 | 2 | |
| Neonatal death | 0 | 0 | 2 | 1 | 0 | |
| Low birth weight | 3(7.9%) | 14(31.6%) | ||||
| (<2500 g) |
Several treatments, such as cholestyramine, phenobarbital, dexamethasone, S-adenosyl-L-methionine, and epomediol failed to improve pruritus, biochemical abnormalities, or fetal prognosis during ICP.29-35 The most efficacious current medical management that improves maternal condition and might prevent the perinatal complications of ICP is ursodeoxycholic acid (UDCA) administration.36-42 Thus, as soon as ICP is diagnosed, UDCA administration coupled with close maternal-fetal surveillance is indicated.
Bile acids in fetal pathophysiologyBile acids easily go through the placenta to fetal compartments and also to the amniotic fluid. Usually, there are higher concentrations of bile acids in fetal than in maternal serum and the main transfer of bile acids across the placenta occurs towards the mother43,44(Figure 2). B. During ICP, in addition to the increased levels of bile acids, the efficiency of the ATP-independent transport mechanisms is enhanced (Figure 2). Provided they are not unidirectional, both changes may facilitate the passage of bile acids from the mother to the fetus, and hence counterbalance or even overcome bile acid flux across the placenta in the physiological direction, which is mediated in part by ATP-independent systems and whose efficiency is reduced during ICP.45 Furthermore, the same authors have shown that vectorial bile acid transfer from fetus-to-mother is also impaired, thus contributing to an accumulation of bile acids in the fetal compartment. This accumulation may be associated with the occurrence of increased fetal distress by ICP. Actually, Laatikainen and Tulenheimo46 found a correlation between total serum bile acids and the incidence of meconium and fetal distress. In contrast, others were unable to find a direct correlation between bile acids in any compartment and fetal distress.47 Nevertheless, total bile acids in maternal serum greater than 50 μmol/L, or superior to 25 μmol/L in fetal cord serum, are associated with diminished well-being47 and levels particularly elevated are referred to cause sudden fetal death.7
Transport systems of bile acids across the trophoblast plasma membrane. The anion exchanger system involved in bile acid transport against an inversely directed flux of bicarbonate is localized in the fetal-facing basal trophoblast plasma membrane. This system is seriously impaired in intrahepatic cholestasis of pregnancy, but evidence shows that it can be corrected by ursodeoxycholic acid treatment. The kinetic data regarding the diffusion of bile acids across the placenta in the fetal-maternal direction did not show any changes in patients, regardless of whether they were receiving ursodeoxycholic acid or not. The transfer of bile acids across the maternal-facing plasma membrane of the trophoblast is mediated by an ATP-dependent transport system, which appears to also work in the absence of ATP. While the efficiency of the former is reduced during ICP, that of the later is greatly enhanced. The two components showed to be restored in patients treated with ursodeoxycholic acid. Adapted from.45,105
Autopsy specimens in cases of intrauterine fetal loss from ICP are consistent with death from acute intrauterine anoxia.27 Meconium and bile acids, especially cholic acid, have been indicated to induce vasoconstriction of human placental chorionic veins in vitro,48 as well as causing acute umbilical vein constriction.49,50 Thus, there is some experimental evidence that bile acids are implicated in the mechanisms triggering fetal asphyxia in pregnancies complicated by ICP. In addition, it was recently shown that taurocholate (0.3 and 3 mM), the main bile acid during ICP, causes a decrease in the rate of contraction of rat cardiomyocytes and loss of synchronous beating.51 This data corroborates a direct role of bile acids in the sudden intrauterine death by ICP.
Therefore, the decrease in bile acid levels induced by UDCA therapy in ICP patients, besides reversing maternal symptoms, may also improve fetal outcome. Palma et al.52 confirmed that patients who received UDCA had had their deliveries closer to term and less frequent fetal distress as compared to non-treated patients.
Relevance of UDCA as a therapeutic option for ICPDuring the three last decades, several clinical studies have established UDCA as a promising therapeutic option in a variety of cholestatic liver diseases, including primary biliary cirrhosis53 benign recurrent intrahepatic cholestasis54 and progressive familial intrahepatic cholestasis;55 improvements in clinical symptoms and biochemical indices of liver function consistently occur following UDCA administration. Although not fully understood, the mechanisms of action of UDCA include protection against injury of bile ducts by hydrophobic bile acids,12 replacement of hepatotoxic bile acids,56 immune modulation,57 cytoprotective mechanisms by preventing apoptosis,58,59 choleretic activity and stimulated secretion of potentially hepatotoxic compounds by the liver.60 It also inhibits intestinal absorption of more cytotoxic bile acids.61,62 It was proposed that the mechanisms of action of UDCA in ICP were similar to those observed in other cholestatic liver diseases but protection against estrogen-induced cholestasis in rats couldn't be attributed to choleretic activity or lipid membrane protection.63 Beneficial effect may be partly due to stimulation of bile secretory function, as also occurs with sulphated progesterone metabolites in ICP women during UDCA therapy.64 Recently, it was proposed that taurineconjugated UDCA stimulates organic anion secretion of cholestatic hepatocytes by inducing hepatobiliary exocytosis and insertion of the transport protein MRP2 in the canalicular membrane65(Figure 1). In addition to the beneficial effect on the functionality of the maternal liver, UDCA therapy restores the ability of the placenta to carry out vectorial bile acid transfer,45 thus contributing to prevent an excessive accumulation of bile acids in the fetal compartment during ICP.
This highly hydrophilic bile acid reduced pruritus, aminotransferases, and serum levels of total bile acids when administered to patients with ICP.3,36,66,67 Patients usually receive UDCA at oral applications of 450 mg/day,12,66 12-16 mg/kg/day36,64 or 1.5 to 2 g/day (20-25 mg/kg/day),42 in three or four daily divided doses. The drug is well tolerated and seems to be completely safe for the mothers or their babies.42,52 Pregnancy outcome and perinatal prognosis were improved in ICP patients treated with UDCA compared to placebo.41,52
UDCA normalizes bile acid profile in maternal serumThe most specific and sensitive biochemical test of ICP is serum bile acid levels, which may reach values 100 times above normal.1 Concentrations of total bile acids in normal pregnant women are consistently lower than 11.0 μmol/L,10 while values from 12.3 to 219.4 μmol/L5 or even 290 mmol/L7 are encountered in women with ICP. Evaluation of serum total bile acids is recommended, not only to diagnose but also to monitor ICP.1,7,9,68 The upper reference limits for both cholic and chenodeoxycholic acids in healthy pregnant women vary from 1.5 μmol/L6 to 4.2 μmol/L.5 In contrast, cholic acid values may increase up to 176 and 170 μmol/L5 during ICP, while chenodeoxycholic acid represent 3 to 4 times less (Table II). Taurocholic acid is the predominant species presenting values between 2.4 and 119.8 μmol/L, accounting for 38.1 ± 1.9% of the total bile acids as compared to 23.6 ± 1.4% for glycocholic acid (concentrations from 2.0 to 55.8 μmol/L).5 This shift towards a more extensive conjugation with taurine (glycine/taurine bile acid ratio of 0.8 ± 0.1, as compared to 1.4 ± 0.1 in normal pregnancies) is pointed as a marker of ICP.5,69 In healthy women, either pregnant or not, cholic acid is never higher than 45% of serum total bile acids, but accounts for 60 to 70% during cholestasis.5,70 Therefore cholic/chenodeoxycholic acid ratio is always greater than one.6,16,33,67,71
Concentrations of primary bile acids in maternal serum, amniotic fluid, umbilical cord serum, colostrum and meconium from both control and cholestatic pregnant women and their babies, when untreated or treated with ursodeoxycholic acid.
| Control | Intrahepatic cholestasis of pregnancy | ||||
|---|---|---|---|---|---|
| Untreated‡ (42) | Treated‡** (42) | Untreated | Treated | ||
| Maternal serum (at diagnosis) | (n=10) | (n=20) | (n=15) (67) | ||
| Cholic | 20.0 ± 3.1 | 18.5 ± 1.9 | 45.9 ± 11.4 | ||
| Quenodeoxycholic | (n=38)* | 5.6 ± 0.6 | 5.8 ± 0.8 | 14.7 ± 3.7 | |
| Maternal serum (at delivery) | (n=10) | (n=20) | (n=39)* | (n=15) (67) | |
| Cholic | 2.2 ± 0.2 | 20.3 ± 2.3 | 10.5 ± 1.9º£ | 42.7 ± 6.2† | 6.4 ± 1.8º£ |
| Quenodeoxycholic | 2.2 ± 0.1 | 5.4 ± 0.5 | 3.0 ± 0.7º£ | 11.3 ± 1.6† | 4.8 ± 1.3ºƒ |
| Amniotic fluid | (n=17)* | (n=9) | (n=15) | (n=9)* | (n=17)* |
| Cholic | 3.9 ± 1.3 | 17.9 ± 27.5 | 4.9 ± 12.4£ | 12.5 ± 4.7§ | 8.2 ± 2.1¥ |
| Quenodeoxycholic | 0.4 ± 0.2 | 18.5 ± 20.9 | 4.8 ±7.7£ | 1.9 ± 0.7 | 1.1 ± 0.4 |
| Umbilical cord serum | (n=17)* | (n=9) | (n=20) | (n=9)* | (n=7)* |
| Cholic | 3.1 ± 0.8 | 21.9 ± 5.6 | 6.0 ± 0.9£ | 14.0 ± 2.9¥ | 5.9 ± 1.1ƒ |
| Quenodeoxycholic | 3.3 ± 0.4 | 18.9 ± 2.1 | 5.2 ± 0.95£ | 6.9 ± 3.7 | 3.7 ± 0.5 ƒ |
| Colostrum | (n=5) (79) | (n=9) (79) | (n=7) (79) | ||
| Cholic | 0.6 ± 0.2 | 19.0 ± 13.1† | 3.6 ± 1.5¥ | ||
| Quenodeoxycholic | 0.19 ± 0.02 | 2.7 ± 2.1 | 1.5 ± 0.8 | ||
| Meconium | (n=8) (83) | (n=8) (83) | (n=8) (83) | ||
| Cholic | 0.8 ± 0.3 | 8.4 ± 4.1† | 6.1 ± 2.2† | ||
| Quenodeoxycholic | 0.3 ± 0.1 | 1.0 ± 0.3¥ | 0.6 ± 0.1¥ | ||
Data are μmol/g for meconium and μmol/L for the other biological fluids;
As previously noticed the beneficial effect of UDCA therapy may be associated with its ability to promote changes in the hydrophobic-hydrophilic balance of the bile acid pool, by increasing hydrophilicity.72 In a study with 15 ICP patients treated with UDCA (14 mg/kg/day), the concentration of total bile acids decreased significantly (P<0.01) from 68.4 ± 16.1 μmol/L at baseline to 20.8 ± 5.1 μmol/L during therapy,67 in agreement with reports by others.3,37,52,66 Individually, the most significant alteration is the reduction (P<0.01) in serum cholic acid concentration from 45.9 ±11. 4μmol/L at baseline to 6.4 ± 1.8 μmol/L with UDCA administration (67) (Table II). The decrease in cholic acid may be clinically relevant since it has been reported to cause fetal distress,48,73 as previously mentioned.
This change in the composition of the bile acid pool is accompanied by a significant increase in the concentration and proportion of UDCA from 0.6 ± 0.2 μmol/L and 1.4 ± 0.6% to 5.9 ± 1.9 μmol/L and 24.7 ± 2.3%, respectively, at baseline and during treatment.67 These results resembled those of 0.1-4.8 μmol/L obtained in another set of patients under similar dosage (12-16 mg/kg/day).64 However, serum conjugated UDCA levels may reach concentrations as high as 16.5 ± 1.8 μmol/L when dosages of 1.5 to 2g/day are used.42 Experimental studies have not shown any teratogenic or adverse effects of UDCA on pre-or post-natal development in rats.40,74
Only a small proportion of total bile acids is detected in the unconjugated fraction,70 even during UDCA administration, despite its increase from 7.2 ± 1.3% at baseline to 12.8 ± 1.6% during treatment.67 Since UDCA may be converted to lithocholic acid, known to induce growth retardation and malformations in rat embryos,75 some concern has been expressed regarding its safety in pregnancy and the treatment is contraindicated in France.7 Nevertheless, data has shown that the serum lithocholic acid concentration is maintained during UDCA administration (1.7 ± 0.5 μmol/L at baseline, and 1.2 ± 0.2 μmol/L during therapy), despite an increase in its proportion (7.4 ± 1.3% vs 3.3 ± 0.5%, P<0.01).67 Therefore, although under continued evaluation, UDCA might be the first-line therapy for ICP.
Passage of bile acids into colostrum decreases following UDCA administrationInfant exposure to high levels of bile acids may be particularly risky, since enterohepatic circulation is immature at birth,76 ileal and hepatocyte transport mechanisms are impaired, and serum bile acid concentrations are increased,77-79 conditions that may aggravate neonatal complications in ICP. Excretion of bile acids in colostrum is enhanced following ICP. In fact, when bile acid excretion in colostrum collected from 16 lacting ICP women, within 72 h postpartum was compared to five lacting healthy women, elevation of total bile acids levels (23.3 ± 14.8 μmol/L vs 0.7 ± 0.2 μmol/L, P<0.01) and of cholic acid concentrations (19.0 ± 13.1 μmol/L vs 0.6 ± 0.2 μmol/L, P<0.01) were found (Table II).80 The low levels of cholic acid in colostrum from control lacting women are similar to those reported in other studies, even for breast milk.81,82 Cholic acid predominates either in colostrum from normal or ICP lacting women.80,81 It is worthwhile to mention, however, that values of total bile acids as higher as 100 μ mol/L may arise in colostrum of ICP patients and be absorbed by breast-feeding infants.80 Therapy diminishes the excretion of total bile acids in colostrum to 5.7 ± 2.5 μmol/L and cholic acid is the most reduced one (3.6 ± 1.5 μmol/L). Accumulations of UDCA (0.3 ± 0.2 μmol/L) and lithocholic acid (0.01 ± 0.01 μmol/ L) in colostrum are irrelevant and toxicity is not expected to occur in nursing infants.
Altered patterns of bile acids in meconium are unchanged by UDCA therapyData on meconium bile acid composition in neonates from ICP patients is scant. In a recent study it was reported a considerable meconium elevation of total bile acids (13.5 ± 5.1 μmol/g vs 2.0 ± 0.5 μmol/g) and cholic acid (8.4 ± 4.1 μmol/g vs 0.8 ± 0.3 μmol/g)83 in newborns from women with ICP, indicating placental transfer of these compounds from mother-to-fetus (Table II). This is reinforced by the presence of deoxycholic and lithocholic acids in meconium, which are supposed to be of maternal origin since they are products of bacterial metabolism and fetal colon is sterile. Thus, these secondary bile acids must reach the fetus by transfer across the placenta.84,85 Continued ingestion by the fetus of amniotic fluid may also contribute to the increase of bile acids in meconium, due to its high content in cholic acid.6,47,86
In addition to cholic, chenodeoxycholic, deoxycholic and lithocholic acids, significant amounts of unusual bile acids were equally identified,83,87,88 resembling composition of fetal gallbladder bile.89,90 Their increased levels in meconium during ICP, besides reflecting hepatic immaturity and/or activation of liver enzymes less important in later life, may be caused by swallow of amniotic fluid enriched in bile acids as a consequence of ICP. Since the presence of these unusual bile acids is scarce in the maternal blood, it is assumed that they derive from the fetus.91 Interestingly, during ICP there is a decrease in the rate of the chenodeoxycholic acid (from about 30% to less than 20%) and in that of the hyocholic acid (from 17% to 7%),83 which is the predominant unusual bile acid in meconium.87,92
In contrast to the preferential conjugation with taurine in normal meconium,92,93 an equal representation of glycine and taurine conjugates occur in ICP, which may be due to a restrained maternal-to-fetus transport across the placenta for taurine conjugates.83 However, this finding does not take place in amniotic fluid or blood cord serum (see below). Regarding the sulphate conjugated fraction, and similarly to normal pregnancies, it also represents a large proportion of the total bile acids in meconium of babies from ICP women.83,94
Most importantly, UDCA administration appears not to influence major bile acid concentrations in meconium including that of its toxic metabolite, lithocholic acid. Cholic acid percentage, instead of decreasing, is slightly increased in meconium (79.2 ± 4.1% vs 74.0% ± 4.4%), following UDCA therapy.83 The accumulation of cholic acid in meconium may result both from the inability of the fetus to excrete meconium (in normal conditions) and the diminished intestinal absorption, greatly reducing the beneficial effects of the therapy at this level. Nevertheless, it must be emphasized that in order to restrain the placental passage of bile acids and the accumulation of these compounds in meconium, it is important to decrease bile acid levels in maternal serum and thus UDCA treatment must be initiated as soon as the diagnosis of ICP is made.
UDCA reduces the levels of bile acids in amniotic fluidBile acids in amniotic fluid may originate from the mother, the fetus, or both.86 Levels of primary bile acids are dramatically elevated in the amniotic fluid during ICP, indicating the maternal compartment as the first source. In addition, the presence of unusual bile acids, from which the more abundant are polyhydroxylated bile acids, suggest that fetal liver under conditions of higher bile acid level attempts to excrete bile acids into urine by increasing their polarity.91
Whether bile acids reach the amniotic fluid by diffusion through umbilical cord or placental membranes or by fetal urine and meconium remains to be established. Interestingly, both cholic and chenodeoxycholic acids are more elevated in the amniotic fluid from normal pregnancies than in the maternal serum.47
During ICP, cholic acid elevation is the main contributor to the increased content of amniotic fluid in primary bile acids (Table II) with mean values from 12.5 ± 4.7 μmol/L (95) to 49.9 ± 19.0 μmol/L,86 4 to 70 times higher than those in the controls (3.9 ± 1.3 μmol/L and 0.58 ± 0.17, respectively). Contribution of meconium contamination may justify the highest values reported. It is possible that cholic acid is carried more easily than chenodeoxycholic acid to the amniotic fluid,47 although chenodeoxycholic, deoxycholic and even lithocholic acid concentrations also increase by 3-to 5-fold (former) by ICP.95
In a previous study (Brites D, unpublished data), treatment with UDCA did not induce any significant changes in concentration of all the major bile acids in amniotic fluid, despite the decrease in cholic acid (12.5 ± 4.7 μmol/L to 8.2 ± 2.1 μmol/L) (Table II). UDCA concentration in the amniotic fluid enhanced significantly (4.5 ± 1.4 μmol/ L, after treatment; 0.49 ± 0.25 μmol/L, controls; 0.37 ± 0.13 μmol/L, ICP women; P<0.01). In addition, in an unusual case of severe cholestasis of pregnancy,3 administration of 1g/day UDCA originated an elevation in the proportion of UDCA that reached 30.4% of total bile acids, whereas lithocholic acid did not surpass 4%. Total bile acid concentration in amniotic fluid of 27.3 μmol/L must represent a beneficial consequence of UDCA therapy, since the patient presented at entrance 198.0 μmol/L of serum total bile acids. Other reports42 using dosages of 1.5 to 2g/day of UDCA obtained a significant reduction in conjugated cholic acid concentration from 17.9 ± 27.5 μmol/L in untreated patients to 4.9 ± 12.4 μmol/L in treated ones (Table II). In contrast to previous works, the level of 0.8 ± 2.4 μmol/L found for conjugated UDCA in amniotic fluid taken from patients treated with such high doses, immediately before or at the time of delivery, agrees with the existence of only 0.1 μmol/L obtained in another study38 after a week of treatment (600 mg daily in two divided doses) and indicate that accumulation of UDCA probably depends on the severity of each case. Additionally, cholic acid percentage that reveals to be similar in both control and non-treated patients (78.0 ± 4.1% and 76.9 ± 5.1%, respectively) shows a marked decrease during therapy (56.2 ± 4.4%, P<0.01).96 The rise in chenodeoxycholic acid proportion from non-treated ICP patients relatively to control (14.1 ± 3.7% vs 6.8 ± 1.2%) seems to be adjusted by treatment (7.1 ± 0.7%). Lithocholic acid proportion is maintained but, although not significant, 2-fold elevated concentrations are observed by UDCA therapy (0.51 ± 0.23 μmol/L vs 0.25 ± 0.08 μmol/L in non-treated ICP patients).95 The beneficial effects expected from the decrease in cholic and chenodeox-ycholic acids and the increase in UDCA levels probably overcome the potential toxic effect due to the moderate elevation of lithocholic acid concentration.
Bile acid levels diminish in umbilical cord blood when UDCA is given to ICP womenConcentrations of total bile acids in umbilical cord serum of premature and term neonates range from slightly to 2-3 times higher than in maternal blood69,89,97-99 and increase even more in fetus of an early gestational age.100 Primary bile acids predominate and the cholic/chenodeoxycholic acid ratio is close to 1.0.71,97 The elevation of both cholic and chenodeoxycholic acids in the fetal blood as compared to maternal serum suggests immaturity of liver function during the fetal period,47 as seen during the first months after birth.100 On the other hand, the placenta also seems to maintain this concentration difference.47
Levels of primary bile acids increase up to 2 and 5 times in the cord blood serum during ICP, although elevation in maternal serum is even more notorious.47,97 Mean ratio between levels of cholic and chenodeoxycholic acids augment to 2.097 reflecting, although to a less extent, the typical elevation of this ratio in the ICP women. Serum total bile acids are greatly increased in babies from ICP complicated gestations (26.0 ± 12.2 μmol/L, P<0,01) when compared with values from those of healthy mothers (11.8 ± 4.7 μmol/L).101 Cholic acid levels of 14.0 ± 2.9 μmol/L obtained in the same study are close to the value of 21.9 ± 5.9 μmol/L reported for another group of 9 babies42(Table II). The higher cholic acid proportion (51.8 ± 7.8%), in contrast with that of chenodeoxycholic acid (25.2 ± 8.3%), appears to result from its greater distribution in maternal serum, which is considered a characteristic feature in ICP patients. The diminished glycine/taurine ratio found in the fetal bile acid pool (0.8 ± 0.2) also seems to be determined by an equivalent ratio in maternal serum.101
UDCA therapy induces an increase in the fetal serum proportion of this bile acid (from 1.9 ± 2.0% to 12.5 ± 8.4%, P<0.05), accompanied by a significant decrease in total bile acid concentration (14.8 ± 2.7 μmol/L, P<0.05), and in cholic acid proportion (39.4 ± 13.3%, P<0.05), and by a trend to normalization in the glycine/taurine ratio (1.2 ± 0.4, P<0.10).99 UDCA concentration increases from 0.57 μmol/L in control, and from 0.40 μmol/L in nontreated patients, to 1.83 μmol/L in ICP women receiving UDCA. In contrast, a more recent report42 indicates a lower accumulation of conjugated UDCA (0.9 ± 0.14 μmol/ L), despite the high-dosage of 1.5 to 2.0 g/day used. The authors justify the differences based on an increase in the mother’s bile acid secretion by such high-dose UDCA treatment strategy. Both studies show that important improvements are achieved for cholic and chenodeoxycholic acids (Table II). Therefore, improved fetal prognosis during maternal treatment with UDCA, in the course of ICP, is probably related with the UDCA capability to normalize the bile acid profile in the umbilical cord serum.
ConclusionDuring ICP high bile acid levels reach the fetal compartment having maternal serum as the first source. Increased bile acid concentrations in serum and colostrum of ICP patients determine an enhanced flux of bile acids from mother to fetus and its absorption by breast-feeding infants, respectively, with cholic acid representing the major species. Whether bile acids reach the fetus through umbilical cord or placental membranes, remains to be established. To the accumulation of bile acids in fetal serum, meconium and amniotic fluid contribute the enhanced transplancental systems of bile acid transport towards the fetus on one hand, and the reduced ability of the fetus to eliminate bile acids on the other hand. UDCA therapy restores the maternal-fetal bile acid balance during ICP, is not harmful to the fetus and above all may hamper stillbirths and preterm labors. It is hoped that research in this field yields insights that will result in the approval of UDCA as the first line treatment option for ICP patients.
Acknowledgements:The author wishes to express his appreciation to Drs. Joaquina Poeiras, Nuno Oliveira, M Conceição Cardoso, Cecília MP Rodrigues, JJG Marín, MA Serrano, MY El-Mir, Helena van-Zeller, Alexandra Brito and Rui Silva who participated in the realization of the publications in which the findings are described in this review. Studies from the author’s research Unit were funded by Fundação para a Ciência e Tecnologia (Portugal).