Cholangiocarcinomas are a heterogeneous group of tumors that can be classified into three clinically distinct types of cancers, intrahepatic, perihilar and distal cholangiocarcinoma. The inconsistent use of nomenclature for these cancers has obscured a true knowledge of the epidemiology, natural history and response to therapy of these cancers. Our aims were to define demographic characteristics, management and outcomes of these three distinct cancer types.
Materials and methodsA retrospective study of patients enrolled in an institutional cancer registry from 1992 to 2010. Median survival was compared between different treatment modalities over three time periods for the three types of cholangiocarcinoma at different stages of the disease using Kaplan Meyer analysis.
Results242 patients were identified. All cases were reviewed and classified into intrahepatic (90 patients), distal (48 patients) or perihilar (104 patients) cholangiocarcinomas. These cancers differed in median age of onset, gender distribution, median survival and stage. 13.8% of patients presented with stage I, 5.8% with stage II, 9.6% with stage III, 28% with stage IV, with 41.8% having unknown stage. The overall median survival was 15.8 months, and was 23, 25, 14, and 4.5 months for stages I, II, III, and IV respectively. Surgery improved survival in both early and advanced stages. Multimodality therapies further improved outcomes, particularly for perihilar cholangiocarcinoma.
ConclusionPerihilar, distal and intrahepatic cholangiocarcinoma vary in their presentation, natural history and therapeutic approach to management. A consistently applied classification is essential for meaningful interpretation of studies of these cancers.
Cholangiocarcinomas (CCAs) are a heterogeneous group of tumors that arise from the biliary tract epithelia, and account for ~3% of all gastrointestinal tumors.1 The epidemiology of these cancers has been poorly understood. The management of these uncommon cancers has been challenging, in part because of the lack of effective treatments. The absence of a consistent nomenclature and the propensity to consider different tumor types together in many published reports have contributed to a lack of clarity regarding the natural history, epidemiology and optimal approaches to the management of these cancers.
Recent studies have focused on the recognition of three types of cholangiocarcinoma that can be described on the basis of their anatomic location, clinical presentation, and molecular features; intrahepatic CCA (iCCA), perihilar CCA (pCCA), or distal CCA (dCCA).2 These three types of CCA are distinct in their presentation and natural history, as well as the approach to diagnosis and management. However, the characteristics of the different types of tumors are not accurately reflected in the literature. For example, studies suggest that iCCA comprise of only 5-10% of all CCA, but these are not borne out by observations in clinical practice.3,4 Data from epidemiological studies such as from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) database have increased recognition of the incidence of these cancers.5 However, these data have been compromised by inconsistencies in coding as intrahepatic and extrahepatic cholangiocarcinoma.6-8 In particular, pCCA have been variably and inconsistently coded as either intrahepatic or ex-trahepatic. An emerging appreciation of the differences between these different types of tumors is reflected in the recent TNM 7 staging system from the AJCC now includes separate staging systems for all three types of cholangiocarcinoma. Most therapeutic trials of patients with these cancers have grouped together all types of biliary tract cancers, and several have also included gallbladder cancer. Tumors classified as intrahepatic or extra-hepatic differ in response to combination therapy with gemcitabine-cisplatin in the ABC-01 and ABC-02 stud-ies,9,10 although the responsiveness of perihilar cancers has not been specifically established. Given the implications of inaccurate classification in determining optimal therapeutic strategies, we sought to systematically define the different types within a cohort of cholangiocarcinomas, and to analyze their management and outcomes.
Material and MethodsIdentification of patientsPatients seen at the Mayo Clinic in Florida from 1992 to 2010 and enrolled in the Mayo Clinic Cancer registry with a relevant diagnosis were identified. We included all patients with ICD-O-3 codes of C22.1, C 24.0, 24.8, 24.9, and 23.9. All patients enrolled in the Registry were followed on an annual basis. The following definitions were used. For intrahepatic cholangiocarcinoma, a topography code of c22.1 (intrahepatic bile duct) and histology codes 8140, 8160, 8161, 8020 and 8010 were used. For extrahepat-ic cholangiocarcinoma, a topography code of C24.0 was used along with histology codes 8010, 8020, 8041, 8070, 8140, 8144, 8160, 8161, 8260, 8310, 8480, 8490 and 8560. Patients with gall-bladder cancer were identified using a topography code of c23.9 but were not analyzed further as these have generally been characterized separately from the other types of cholangiocarcinoma. The study was reviewed by the Institutional IRB and noted to be exempt from IRB review.
Classification and stagingChart review was performed and all patients were classified into intrahepatic, perihilar or distal cholangiocarci-noma based on conventional classifications.2,11,12 Re-classification was verified by an independent observer to ensure validity and accuracy. Staging was performed based on the TNM 7 staging system on either clinical or pathological criteria and where all necessary information to assign a stage was available.13-15 The vital status at the most recent follow-up was verified.
Data collectionPatient demographics, pathology, and the nature of any treatments received were documented. Treatments were categorized as surgery (which included surgical resection or transplantation), systemic chemotherapy, or radiation therapy (which also included intrabiliary brachytherapy). Locoregional therapies were not considered as a separate category because these were inconsistently used during the study period. Data on patient survival and disease recurrence were obtained annually by cancer registry staff from the time of enrollment to the time of last follow-up. Patients were grouped into three multi-year eras in order to examine temporal trends in management and their outcomes. Survival curves for each of the three types of cholangiocarcinoma were generated and temporal trends and outcomes of single or multimodality treatment were determined.
Statistical analysisFor analysis of trends for categorical data, the Mantel-Haenszel χ2 test was used. For continuous data, one-way analysis of variance was used to test the null hypothesis that multiple population mean values are all equal. Kaplan Meier plots were used to assess patients’ survival. To test median values across multiple groups, p values were computed using the nonparametric t test. All statistical analyses were performed using SAS 6.12, SAS Institute, Cary, North Carolina.
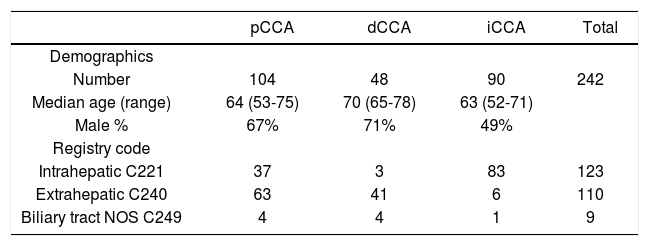
ResultsA total of 242 patients with a diagnosis of cholangiocar-cinoma encountered at a single institution between 1992 and 2010 were identified using a cancer registry. 123 patients had been classified as intrahepatic, 110 as extrahepat-ic, and 9 as non-specified bile duct cancer. Follow-up was available for all patients for a median of 11.6 months (range 3.1-24.3 months). All cases were re-classified into iCCA, pCCA or dCCA by two independent observers with 100% concordance. Overall, there were 90 iCCA, 104 pCCA and 48 dCCA. The mean age at diagnosis was 63 years (range 22 - 91) with only 18 patients (7.4%) less than forty years of age at the time of diagnosis. Some demographic differences were noted. Compared to either pCCA or iCCA, dCCA presented at an older median age. There were gender differences noted with 49% of iCCA, 67% of pCCA and 71% of dCCA being male.
Amongst those patients originally classified as intrahe-patic, 37 patients (30%) were reclassified as pCCA, and 3 patients (2.4%) as dCCA (Table 1). Similarly, differences were also noted between the registry coded diagnoses of extrahepatic cholangiocarcinoma, six of which were re-classified as iCCA (5.4%). The remainder of the patients coded as extrahepatic were reclassified into pCCA (63 patients) or dCCA (41 patients). Moreover, nine patients who were coded as biliary cancer with no specified location could be reclassified into four pCCA, four dCCA and one iCCA. These data indicate the high potential for mis-classification of these cancers even in carefully annotated datasets from trained cancer registrars. They highlight and emphasize a major limitation in epidemiological studies that are based on inconsistent classifications.
Re-classification of cancer registry coded cholangiocarcinomas.
| pCCA | dCCA | iCCA | Total | |
|---|---|---|---|---|
| Demographics | ||||
| Number | 104 | 48 | 90 | 242 |
| Median age (range) | 64 (53-75) | 70 (65-78) | 63 (52-71) | |
| Male % | 67% | 71% | 49% | |
| Registry code | ||||
| Intrahepatic C221 | 37 | 3 | 83 | 123 |
| Extrahepatic C240 | 63 | 41 | 6 | 110 |
| Biliary tract NOS C249 | 4 | 4 | 1 | 9 |
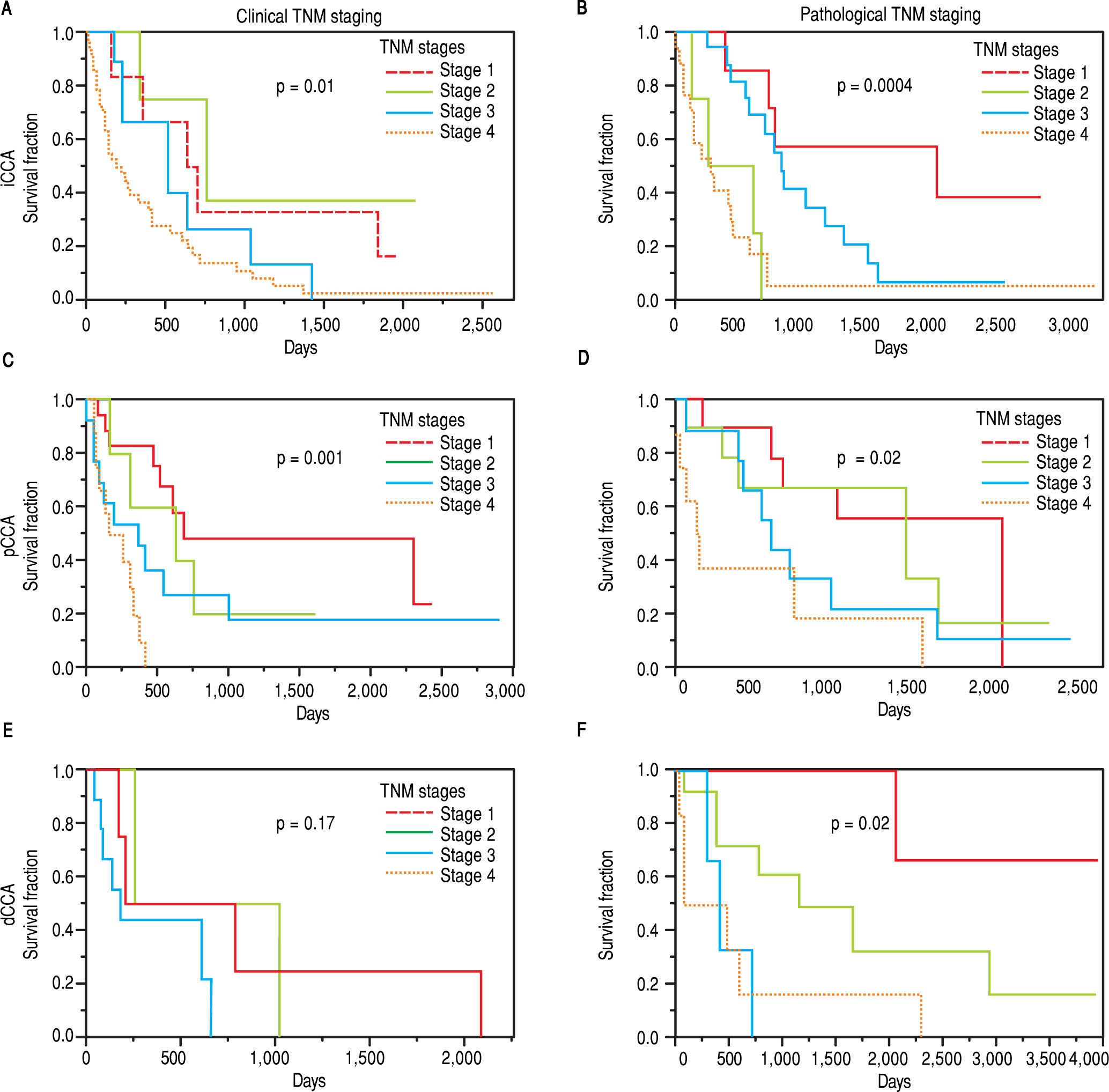
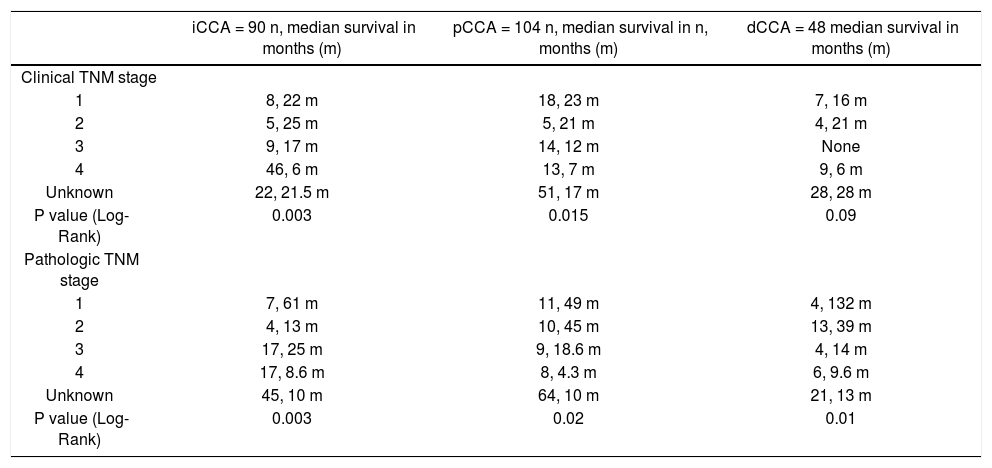
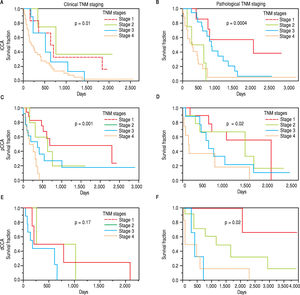
The clinical and pathological TNM staging at presentation for each of the three types of CCA is shown in Figure 1. Tumor staging could not be described for all patients because of inadequate data. Overall, 13.8% of patients presented with stage I, 5.8% with stage II, 9.6% with stage III, 28% with stage IV, and 41.8% were unknown. The overall median survival (MS) of all patients was 15.8 months. As expected, patients with early stage disease had a higher median survival compared to those presenting at later stages with median survivals of 23, 25, 14, and 4.5 months for stages I, II, III, and IV respectively. More patients with iCCA presented with advanced disease at stage IV (51%) as compared to either pCCA (12%), or dCCA (18%). In contrast, patients with pCCA presented more frequently with stage I disease. The median survival for unstaged pCCA, iCCa and dCCA was 17, 21.5 and 28 months respectively, as shown in table 2.
TNM staging and median survival.
| iCCA = 90 n, median survival in months (m) | pCCA = 104 n, median survival in n, months (m) | dCCA = 48 median survival in months (m) | |
|---|---|---|---|
| Clinical TNM stage | |||
| 1 | 8, 22 m | 18, 23 m | 7, 16 m |
| 2 | 5, 25 m | 5, 21 m | 4, 21 m |
| 3 | 9, 17 m | 14, 12 m | None |
| 4 | 46, 6 m | 13, 7 m | 9, 6 m |
| Unknown | 22, 21.5 m | 51, 17 m | 28, 28 m |
| P value (Log-Rank) | 0.003 | 0.015 | 0.09 |
| Pathologic TNM stage | |||
| 1 | 7, 61 m | 11, 49 m | 4, 132 m |
| 2 | 4, 13 m | 10, 45 m | 13, 39 m |
| 3 | 17, 25 m | 9, 18.6 m | 4, 14 m |
| 4 | 17, 8.6 m | 8, 4.3 m | 6, 9.6 m |
| Unknown | 45, 10 m | 64, 10 m | 21, 13 m |
| P value (Log-Rank) | 0.003 | 0.02 | 0.01 |
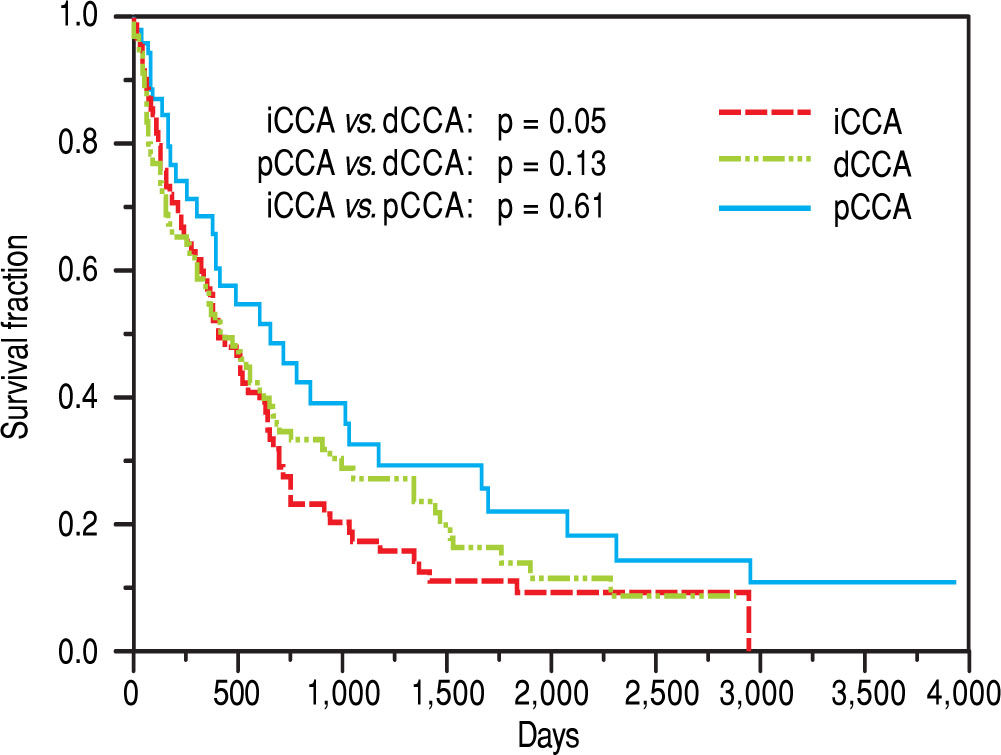
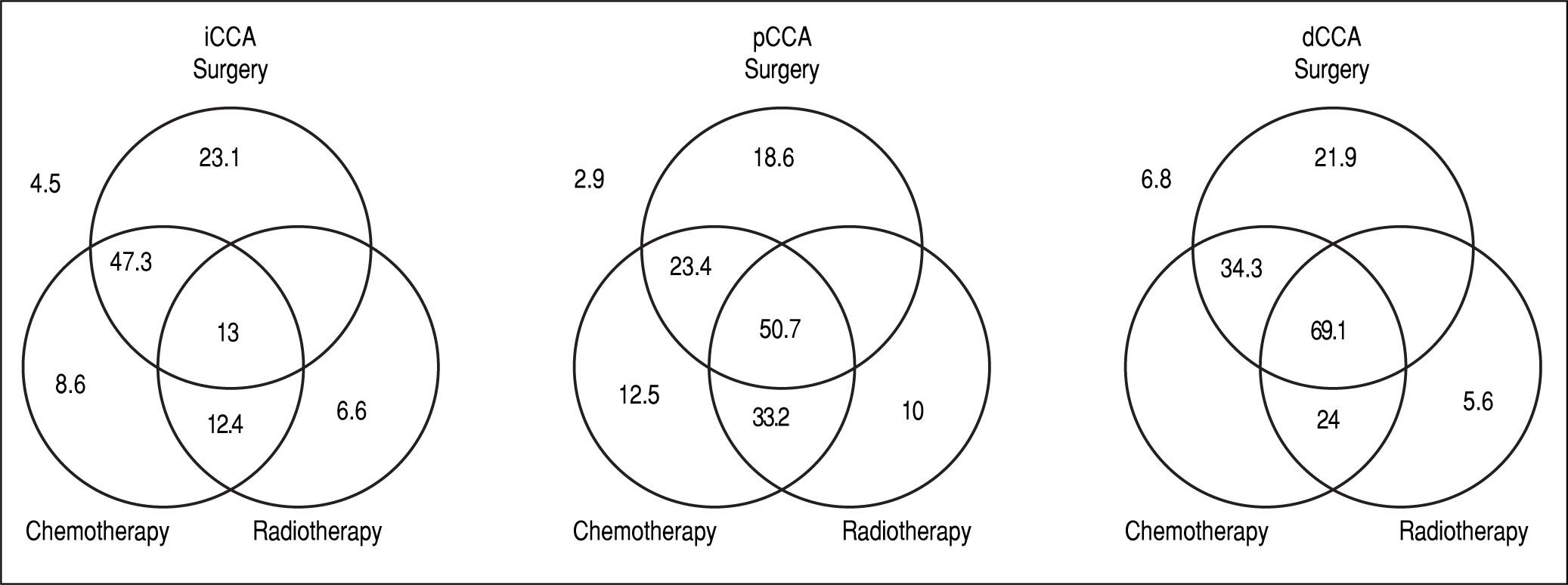
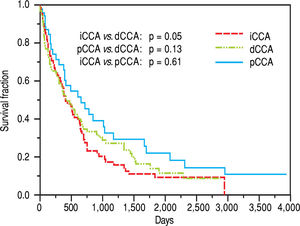
The median survival for iCCA (13.5 months) was similar to that for pCCA (13.9 months), but was lower than dCCA (22 months). However, overall survival was not significantly different between the three types (Figure 2). Median survival was significantly higher for patients with pCCA or dCCA who received treatment compared to those who did not receive any treatment. However, this was not observed for patients with iCCA and could reflect more advanced stage at time of presentation. 156 patients [62 iCCA, 63 pCCA, and 31 dCCA] underwent surgery, chemotherapy, radiotherapy, or a combination of these. Surgery with or without chemoradiation provided an overall survival benefit (25 vs. 7.5 months, p < 0.0001). The median survival of patients with each of the three types of CCA who underwent different treatment modalities is illustrated in Figure 3.
Management and outcomes. The median survival in months of patients undergoing either single modality (surgery, chemotherapy or radiation), or multimodality treatment (two or more modalities). Median survival for patients who did not undergo any treatment is shown outside the Venn diagram.
To examine the temporal trends in management, patients were grouped into one of three eras based on their date of diagnosis, namely pre 2001, 2001-2005, and 2005-2010. In the most recent era (2005-2010), median survival ranged from 20-54 months for patients who had surgery compared to 3-7 months for those who did not have surgery, Similar results were observed for other eras examined. These findings could potentially arise from more advanced disease in non-surgical patients.
For patients with iCCA, the presence of underlying diabetes mellitus, liver cirrhosis, primary sclerosing cholan-gitis or a history of smoking, alcohol use, or inflammatory bowel disease did not significantly alter median survival. Surgical resection was performed in 39% of the patients either as a single modality intervention (22%) or combined with chemotherapy (10%), radiation therapy (1.1%), or chemo-radiation therapy (5.5%). Twenty one percent of patients with iCCA received only chemotherapy, 2.2% received only radiation therapy, and 6.6% received chemo-radiation in combination. The highest median survival with any single modality was noted with surgery, whereas multimodality therapy of surgery combined with chemotherapy achieved the highest median survival.
For patients with pCCA, 62% underwent surgical resection either as a single intervention (15.3%) or combined with chemotherapy (1.9%), radiation therapy (1%), and chemo-radiation therapy (20.1%). 3.8% received only chemotherapy, 1.9% received only radiation therapy, and 16.3% received combination chemo-radiation therapy. The median survival of patients who underwent any multimodali-ty treatment was significantly higher than with any single treatment modality. As with iCCA, the highest median survival was observed for patients who underwent a combination of surgery and chemoradiation when compared to other single or multimodality treatments. A multi-modality approach of chemoradiation followed by liver transplantation has been used to treat patients in this group, and the improved survival of this intervention has been reported.16-18
For patients with dCCA, 87% underwent surgical resection either as single modality (52%) or combined with chemotherapy (3%) or chemo-radiation therapy (32%). 7% received only chemotherapy, 3% received only radiation therapy, and 3% received combined chemo-radiation therapy. Patients who underwent multimodality treatment had higher median survival compared with those who underwent surgery alone or with any other single modality treatment (P = 0.0009). Likewise, surgery and chemoradi-ation therapy resulted in a higher median survival compared to other single modality treatments, although not statistically significant, likely due to the small number of patients in the dCCA cohort.
DiscussionA recognition of the distinctive nature of different types of cholangiocarcinoma is now emerging. In this study, patients with cholangiocarcinoma were reviewed, reclassified and systematically analyzed to derive a detailed and accurate description of the frequency, management and outcomes of the three different types of cholangiocarcinoma. This study thus provides a relevant snapshot of contemporary management and outcomes of patients with these cancers by eliminating the confounding effects of inconsistencies that arise from inaccurate coding.
The optimal choice of individual or multimodality therapeutic strategies for cholangiocarcinoma remains obscure, and the management of these cancers continues to evolve. Median survival was increased for all patients who underwent surgery, regardless of resection margin. Since CCA usually presents in late stages, R1 resections are frequent even when surgery is performed with curative in-tent.19 Adjuvant therapy is not routinely used, although chemotherapy and/or radiotherapy can improve survival following R1 resections compared to those patients who do not undergo surgery.20-23 Irrespective of the type of cholangiocarcinoma, surgical resection along with chemo-radiation therapy may have the greatest impact on survival for patients with resectable disease.22
Many patients are not eligible for curative surgical resection due to advanced disease at diagnosis. The median survival for patients who did not undergo any surgery ranged from 5-12 months for all three types of cholangi-ocarcinoma. For unresectable lesions, a multimodality treatment approach could be considered.24 Higher survival rates were observed with the use of multimodality treatment compared to the use of single modality treatment with surgery, chemotherapy, or radiation therapy at all disease stages.25,26 This was particularly true for pCCA, where multi-modality strategies have been most extensively used. These cancers present at an earlier stage but have a poor survival, justifying an aggressive approach. In contrast to pCCA, median survival was greater for iCCA where surgery alone or with chemotherapy was performed. A potential reason is that smaller lesions that are amenable to complete resection may have a greater chance of curative resection, or have a lower risk for invasion or recurrence. These cancers often present at a later stage and effective strategies for earlier detection are needed.
Compared to other types of cholangiocarcinoma, patients with dCCA had the highest median survival, although the number of patients in this group was small. For this group, surgical approaches offer the best outcomes when combined with chemotherapy or radiation. Our observations regarding improved prognosis of dCCA are consistent with reports in extrahepatic CCA. The median survival reported by DeOliveira, et al.12 for iCCA were greater (28 vs. 13.5 months), whereas the median survival for dCCA were lower (18 vs. 22 months) than those in our cohort. We speculate that these differences could reflect regional variations in management or in risk factors for these cancers.
There are limitations to this study, as with any other retrospective analyses. In particular, data regarding variables that can influence choice of therapy or outcomes including performance status and underlying organ function were not available and thus could not be incorporated in the analysis. Other factors such as patient desire, access to therapies and physician and institutional expertise in managing these conditions are mitigated in part by restricting this analysis to a single institution that offers all conventional therapeutic options for the management of these cancers, including liver transplantation for pCCA. These studies thereby provide a more accurate practice-based and temporal representation of trends in the management and outcomes of each of the three different types of cholangi-ocarcinoma.
Abbreviations- •
CI: confidence intervals.
- •
dCCA: distal cholangiocarcinoma.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
HCV: hepatitis C virus.
- •
iCCA: intrahepatic cholangiocarcinoma.
- •
ICD: international classification of disease.
- •
OR: odds ratio.
- •
pCCA: perihilar cholnagiocarcinoma.
There are no conflicts of interest to disclose.
AcknowledgementsWe acknowledge, with gratitude, the assistance provided by Nicholas Figura and Abigail Trotter in data collection.
Financial SupportSupported in part by NIH grant DK 069370 (TP).