Recent advances in the systemic treatment of advanced hepatocellular carcinoma (HCC) with immunotherapy have once again reignited discussion over the role of combined therapy in earlier stages. This year, different international meetings have presented recent results from clinical trials on adjuvant therapy alone (IMBrave-050) and combined with transarterial chemoembolization (EMERALD-1 and LEAP-12). Increased enthusiasm for the use of adjuvant and neoadjuvant therapy for liver transplantation, surgery, and local-regional treatment of HCC has been shown. However, the initial results from these trials should be interpreted cautiously as we wait for final analyses and effects on overall survival. In this position paper from the special interest group from the Latin American Association for the Study of Liver Diseases (ALEH), we underline the caveats of the applicability of these potential treatments in our region, explore points of agreement, and highlight areas of uncertainty. Moreover, we underscore the role of hepatologists in the clinical decision-making process and management of these patients.
Hepatocellular carcinoma comprises more than 80 % of primary liver tumors and is ranked as the fourth most frequent malignancy and fourth most common cause of cancer-related death worldwide [1]. The reported incidence rate in Latin America ranges between 5 and 7 cases per 100.000 persons/year, and 4.4 % of HCC cases worldwide are diagnosed in this region (around 39,450 cases per year) [1–3]
Over the last few years, significant progress has been made in treating locally advanced or metastatic hepatocellular carcinoma (HCC). The therapeutic approach to HCC in non-resectable Barcelona Clinic Liver Cancer A (BCLC-A), or BCLC-B stages, has evolved from the concept of transarterial chemoembolization (TACE) to other locoregional treatments associated with targeted therapies or immunotherapy, including, among others, immune checkpoint inhibitors (ICI), either alone or in combination with other systemic therapies [4].
The latest update on BCLC staging contemplates this approach, incorporating the tumor extension concept within the BCLC-B patients' framework [4]. On the one hand, there are potentially transplantable patients within composite criteria [5] or through downstaging (tumor shrinkage through locoregional or systemic treatments) [6,7]. Conversely, for patients presenting a diffuse infiltrative pattern or bilobar extension, systemic therapy is the treatment of choice [8]. Intra-arterial selective radiotherapy, or Transarterial radio embolization (SIRT), is indicated explicitly in non-operable BCLC-A patients with a single lesion tumor not surpassing 8 cm in diameter [9].
Sorafenib has been the first-line systemic treatment for advanced HCC since 2008 [10,11]. Other targeted therapies attempted to explore superior efficacy over sorafenib with sequential failures until lenvatinib, showing non-inferiority in survival benefit [12]. Second-line systemic therapies after sorafenib showed efficacy over best-supportive care with regorafenib [13], cabozantinib [14], and ramucirumab [15,16]. On the contrary, failed attempts were observed with single-agent anti-PD-1, pembrolizumab in the second line [17], or nivolumab in the first-line setting [18].
In this repeated failure scenario, several studies showed that adding targeted molecules with anti-VEGF or TKIs to ICIs could improve HCC treatment [19]. The IMbrave-150 clinical trial, which combined ICI with anti-PD-L1 atezolizumab and a monoclonal antibody against VEGF, bevacizumab [20], showed for the first time a significant improvement in overall survival (OS) and progression-free survival (PFS) compared to sorafenib. These findings led to the approval of this treatment as first-line systemic therapy in most regions of the world, including Latin America. Other combinations, including camrelizumab (anti-PD-L1) plus rivoceranib (oral anti-VEGF), showed improved efficacy in an Asian population, at the cost, however, of increased toxicity [21]. Dual immunotherapy with ICIs, including anti-PD-L1 with anti-CTLA-4 antibodies (Durvalumab and Tremelimumab) [22], has shown increased OS compared to sorafenib without significant benefit in PFS. This combination was approved in Europe, Asia, North America, and recently in some Latin American countries. More recently, results from other clinical trials in the first-line setting with nivolumab plus ipilimumab versus sorafenib or lenvatinib (the CHECKMATE-9DW trial) [23], adjuvant setting (IMBrave-050 trial) [24], and the combination of ICIs with TACE in the EMERALD-1 [25] and LEAP-12 trials [26], have been presented in different international meetings.
This manuscript reviews the data and potential approach to ICI-combination in a neoadjuvant setting and its possible applicability in Latin America. In this region, cultural heterogeneity and the different barriers to access to health care make the applicability of some therapies in HCC more complex. We have joined within the special interest group (SIG) from the Latin American Association for the Study of the Liver (ALEH) to explore points of agreement and to highlight areas of uncertainty regarding the applicability of these new therapeutic approaches. Above all, and indeed, to underline the hepatologists' role in these patients' clinical decision-making process [27].
2Insights of immunotherapy in the adjuvant or neoadjuvant settingThe development of effective systemic therapies, including ICI in advanced HCC, has not been followed by an improvement in treatment outcomes in the early stages of HCC. The most relevant end-point to be considered is the development of HCC recurrence after these curative therapies, with recurrence rates surpassing half of the population at three years of follow-up. The risk of HCC recurrence is associated with larger tumor size, microvascular invasion, or poor degree of differentiation. The underlying cause is often the unappreciated presence of occult intrahepatic micrometastases far from the resection margin. The development of perioperative neo or adjuvant treatments is thought to improve these nuances and is urgently needed [28].
There are two possible scenarios when considering immunotherapy in earlier stages: adjuvant and neoadjuvant settings. In the first scenario, the aim is to add systemic therapy to reduce the risk of recurrence. On the other hand, neoadjuvant treatment aims to reduce the risk of recurrence while attempting to bridge toward curative therapies. The theoretical basis for using ICIs at early HCC stages is that their efficacy could be more significant than in advanced stages, given a lower tumor load and a potentially reduced immune evasion mechanism [29].
The early stages' response and resistance to immunotherapy depend on the same principles as in the advanced stages. Adjuvant ICI stimulates antitumor activity against micrometastases after the primary tumor is removed, while neoadjuvant therapy uses the primary tumor as a source of antigens to elicit such a response [30]. The antitumor response depends on the interaction between T cells, antigen-presenting cells, and tumor cells. These interactions between ICI and cells will most likely occur when tumor antigens are present. This rationale probably supports greater effectiveness in the neoadjuvant rather than adjuvant setting [31]. Neoadjuvant therapies allow histological evaluation of the effect of ICI (tumor necrosis), which is conducted as a bridging treatment for radical therapies. The counterargument is that the selection of appropriate patients for neoadjuvant ICIs is challenging in those with high-risk tumors.
Studies with other tumors show that T-cell clonal activation and expansion are better achieved when ICI is administered before tumor removal. In this regard, micrometastases appear less immunogenic during adjuvant therapy than those detectable macroscopically [31]. The rationale for combining immunotherapy with locoregional therapies lies in the induction of the abscopal effect. Necrotic-treated lesions induce the exposure of neo-tumor antigens, which are recognized by dendritic cells, leading to more significant immune expansion and recognition of untreated lesions [32–35]. Nevertheless, it should be noted that until now, neither adjuvant nor neoadjuvant treatments for HCC have consistently been effective [36,37].
3Role of immunotherapy before or after surgery or local ablationLess than 10 % of the patients in the early stages of HCC receive resection or ablation as primary treatment [38]. Moreover, recurrence is an always-present menace in these individuals [28,39]. Following attempts with sorafenib [36], the use of ICIs was explored in uncontrolled, open-label trials in the neoadjuvant setting, with cemiplimab (anti-PD-1) [40], nivolumab plus cabozantinib (MET inhibitor) [41], and nivolumab plus ipilimumab [42]. Despite these exploratory phase Ib-II studies, different facts must be considered, including safety, delay in time to surgical procedure, and relevance of major pathological responses observed. Recently, D'Alessio et al. published a patient-level pooled analysis of data from 111 patients with HCC receiving ICI therapy before liver resection from 5 uncontrolled, phase I-II clinical trials. Major pathological response, defined as at least 70 % of tumor necrosis in the pathology specimen, and complete pathological response (100 % necrosis) were observed in 32 % and 18 % of the patients, respectively. The radiological overall response was associated with major pathological response, with 23 (74 %) of 31 patients with a radiological response showing major pathological response compared with 10 (14 %) of 73 patients without radiological response (p < 0.0001). Nevertheless, a very low correlation was observed between radiological response and pathological response (r = 0.43). Recurrence-free survival was significantly longer in patients presenting with major pathological response or complete tumor necrosis. The authors proposed a threshold of 90 % necrosis as the optimal cutoff of pathological tumor regression to predict improved relapse-free survival [43].
There are different phase III ongoing trials in the adjuvant setting with different ICIs, including pembrolizumab (KEYNOTE-937) [44], nivolumab (CHECKMATE-9DX) [45], durvalumab plus bevacizumab (EMERALD-2) [46], and atezolizumab plus bevacizumab (IMbrave-050) [24]. All these trials define a specific population with an estimated high risk of recurrence after surgery or ablation therapies. In all of them, the intervention is conducted over one year following surgery or ablation. All these trials, except for the IMbrave-050, are double-blinded, placebo-controlled designed trials.
The IMbrave 050 was the first phase 3, open-label, multicenter randomized controlled trial, in which the benefit on recurrence-free survival (RFS) was evaluated with the combination of atezolizumab plus bevacizumab over one year of treatment compared to active surveillance in high-risk patients undergoing surgical resection or local ablation [47]. An interim analysis showed a significantly higher 12-month RFS of 78 % in the intervention group compared to 65 % in the surveillance group, with a hazard ratio of 0.72 (95 % confidence interval 0.56–0.93). However, the Kaplan-Meier curves showed that the dynamic of events of recurrence or death (RFS) might result in the crossover of survival estimates (threatening the proportional hazard assumption). Some key points regarding this trial's design, internal validity, and generalizability should be highlighted. First, it was an open-label, non-placebo-controlled trial; it included BCLC-C patients (6 % in both treatment arms), 12 % received TACE following surgery (an unrobust evidence-based clinical-decision making), 80 % of the study population came from China, and 60 % presented hepatitis-B related etiology. Notably, the great majority of patients underwent surgical resection (88 %), and the sample size assigned to receive local ablation was smaller, with imprecise estimations within results in this group.
On the other hand, a benefit of OS should be the primary objective in the adjuvant or neoadjuvant settings. Different time co-variates might modify the effect of the random allocation (e.g., other co-interventions) that might be unbalanced between groups [28]. Second, the study does not provide enough data for a definite change in clinical practice, as the positive results are from an interim analysis [24]. On the other hand, 41 % of the patients receiving immunotherapy experienced grade 3 or higher side effects. Interestingly, antibodies against atezolizumab can develop in individuals undergoing such therapy and are associated with resistance to further immunotherapy [48]. A large proportion of patients with underlying hepatitis B undergo antiviral therapy while receiving HCC therapy, leading to a likely "controlled" underlying liver inflammation and a less tumor-friendly milieu, which might not be the case in other HCCs. It is also expected that baseline proportions of metabolic-associated steatotic liver disease (MASLD) in different regions, such as Latin America, are higher, which could impact the potential benefit of adjuvant immunotherapy [49].
There was initial enthusiasm for the potential use of adjuvant and neoadjuvant therapy for HCC within this new therapeutic landscape. However, a follow-up extension showed no benefit on RFS with atezolizumab-bevacizumab from the IMbrave-050, results updated in the European Society of Medical Oncology 2024 [50].
The most complicated aspect of neoadjuvant therapy is the correct selection of candidates. On the one hand, neoadjuvant therapy could convert large tumors into resectable tumors and even allow for more conservative surgery. On the other hand, neoadjuvant therapy carries the risk that the patient will not respond and progress to such an extent that they are no longer resectable. Likewise, the appearance of immune-mediated adverse events could also delay surgery and impact the prognosis of these patients. There is not enough evidence to indicate neoadjuvant therapy; we should wait for the results of the abovementioned trials to evaluate other ICIs and combinations for a more definitive position in adjuvant therapy for HCC [28,39].
4Role of immunotherapy concomitant with endovascular therapiesThe combination of TACE and immunotherapy is emerging as a valid therapeutic option. This approach combines the effects of tumor lysis due to ischemic necrosis with the local cytostatic impact of chemotherapy, therefore avoiding systemic toxicity due to diffusion to the whole-body circulation. Cell necrosis, or abscopal effect, induces greater tumor antigen presentation and activation of the immune system, improving the immune response to ICI [51]. On the other hand SIRT combines radiation's necrotic effect with ICIs.
TACE is the standard treatment for intermediate-stage HCC (BCLC-B). In recent years, different studies have sought to improve the efficacy of locoregional therapies in terms of PFS by adding systemic treatment. Other phase II (SPACE) [52] and III (TACE-2, TACTICS) [53,54] clinical trials evaluated the PFS superiority of the combination of TACE with Sorafenib versus TACE alone with/without placebo. Other agents have been explored, including orantinib (ORIENTAL trial [55]) or brivanib (BRISK-TA trial) [56]. The TACTICS study demonstrated that PFS was longer in patients who received sorafenib plus TACE than those receiving TACE alone [53].
In contrast, SPACE and TACE-2 trials did not find improvements in PFS [52,54]. Several reasons could explain these opposing results. First, the TACTICS study allowed patients with previous TACE or tumor vascular invasion (Vp1-Vp2). These subgroups of patients were not included in the other phase II-III studies. Second, the proportion of patients with cirrhosis and clinically significant portal hypertension may have limited the number of TACE sessions, showing different TACE protocols across studies. Finally, radiological responses or TACE-stopping rules were significantly different in the TACTICS study than in other trials. The term unTACEable progression was only applied in this trial. In contrast, in the SPACE or TACE-2 trials, objective response rates and tumor progression were defined using the modified RECIST criteria and RECIST 1.1, respectively. In contrast, the TACTICS study evaluated the response using RECICL criteria. Finally, similar comments should be considered when analyzing the results observed in the TACTICS-lenvatinib trial [57].
In other words, combining TACE with sorafenib results has been globally negative except for the TACTICS study, TACTICS-len, and the most recent LAUNCH study [58]. The LAUNCH study in which the combination of TACE plus lenvatinib was compared to lenvatinib alone in BCLC-C patients should be cautiously interpreted and needs external validation. Moreover, the definition and evaluation of local radiological effects of mRECIST or RECIST 1.1 criteria may promote an increased probability of achieving at least partial responses in the target lesions in which TACE was conducted. This may lead to overestimating radiological responses favoring the combination of locoregional therapy plus systemic treatment and an increased duration of systemic therapy. Therefore, the final effect may promote a delay in the assessment of tumor progression (mRECIST criteria). In addition, the open-label design of the study, the eligible population, and how the primary events and TACE stopping rules were defined should be considered as they might have biased the results. Thus, the validity and applicability of these trials in Latin America are questionable, limiting such therapeutic protocols.
More recently, a randomized, controlled, double-blinded, phase III clinical trial, the EMERALD-1 [25] study, including patients with unresectable BCLC A-C HCC eligible for TACE, explored the combination of durvalumab/bevacizumab plus TACE and demonstrated an increase in PFS compared to placebo plus TACE [25]. This trial included patients with BCLC B and locally advanced tumor or BCLC-C (Vp1-Vp2), excluding patients with diffuse infiltrative HCC, Vp3-Vp4 tumor vascular invasion, or previous locoregional therapy. The trial had a 2-arm intervention design (arm 1: durvalumab + bevacizumab + TACE; arm 2: durvalumab + TACE) and a control arm (TACE + placebo). The study also included two intervention phases. In the first phase, or TACE period, all arms received locoregional treatment with TACE, plus durvalumab for four weeks in arms 1 and 2 and an intravenous placebo in the control group every four weeks. In this period, a maximum of 4 TACE sessions was allowed in each arm within 16 weeks. Then, during a post-TACE period, each arm received durvalumab plus placebo every three weeks (arm 1), or durvalumab plus bevacizumab every three weeks (arm 2), and placebo plus placebo every three weeks (control arm). The study explores the superiority of any intervention arm in terms of PFS (mRECIST criteria). The study demonstrated a therapeutic benefit with durvalumab/bevacizumab plus TACE (arm 2) compared to TACE + placebo with an HR of 0.77 (95 % CI 0.61–0.98). There was no superior benefit with durvalumab alone. The median PFS increased from 8.2 months with placebo plus TACE to 15.0 months with durvalumab + bevacizumab. The number of TACE sessions was similar between arms, and there were no significant baseline differences in population characteristics between groups. Most notably, the TACE modality did not generate an effect modification, with greater therapeutic efficacy observed in the "pure" BCLC-B population without Vp1 or Vp2 tumor invasion. According to the Hepatoma Arterial Embolization prognostic (HAP) score, there were no effects between subgroups. The chance of presenting objective radiological response (ORR) was higher in both intervention arms compared to the control group (ORR arm 1 41 %, arm 2 44 %, and control 30 %), with no significant differences in disease control rate.
Although the EMERALD-1 study is likely to modify clinical practice recommendations in the intermediate stage, durvalumab + bevacizumab combination was associated with a higher incidence of serious adverse events (48 % versus 31 % in the control group), with a 25 % discontinuation rate. It is also worth noting that 16 % of patients with durvalumab plus bevacizumab developed decompensation of cirrhosis with clinical ascites, significant proteinuria induced by bevacizumab (21 % vs. durvalumab group 12 % vs. control 3 %). It is worth noting that the overall survival data has not yet been presented.
More recently, the LEAP-12 randomized clinical trial, presented at ESMO 2024, showed similar PFS benefits with the combination of TACE plus lenvatinib + pembrolizumab. Results of these trials showed a trend towards a benefit in OS, but this still needs to be robustly shown to be considered as the new standard of care. Also, hazard ratio estimates shown with PFS may have a modest correlation with a benefit in OS [26]. We recommend waiting for further evidence of the benefit in OS of this new combination treatment in the BCLC-B stage, knowing that the aim of these trials was a gain in PFS rather than on OS and that sample sizes might have been estimated for that primary event of interest. Finally, other trials are ongoing, such as the combination of nivolumab plus TACE vs. TACE (TACE-3 trial), durvalumab + tremelimumab +/-lenvatinib + TACE versus TACE (EMERALD-3), pembrolizumab + regorafenib vs TACE or SIRT (REPLACE trial), and the most challenging trial of atezolizumab plus bevacizumab versus TACE (ABC-HCC trial), in which the primary end-point would be "time-to-failure of treatment strategy," a rather innovative approach in the field (Tables 1 and 2).
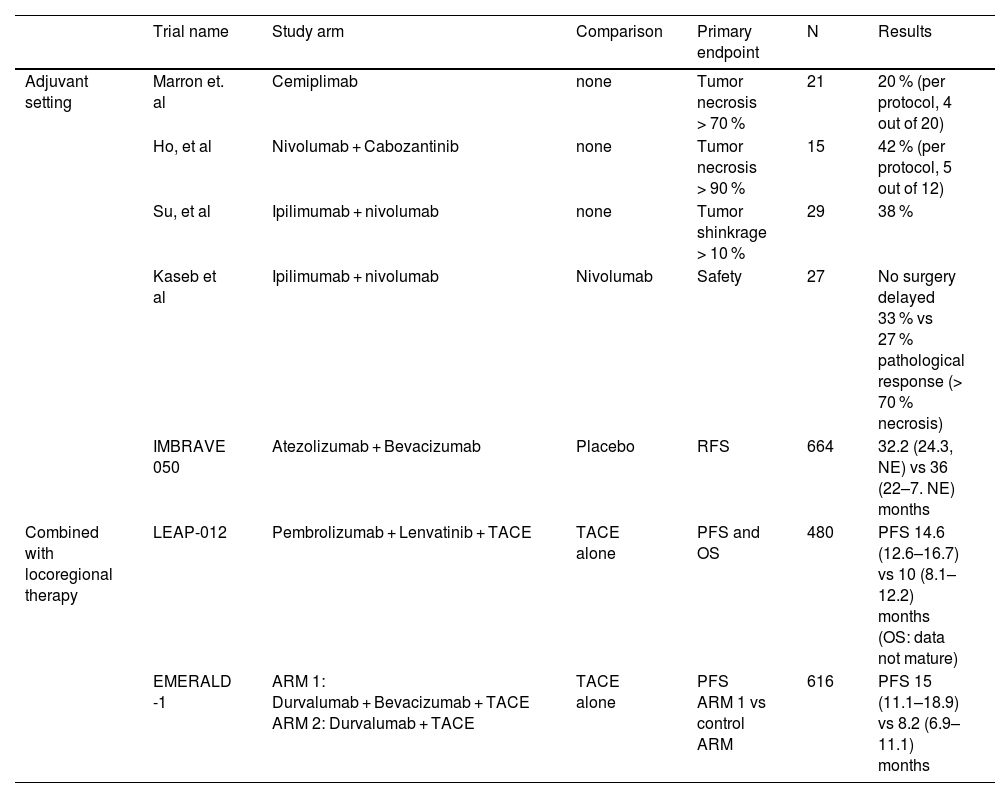
Published clinical trials investigating adjuvant ICI use.
| Trial name | Study arm | Comparison | Primary endpoint | N | Results | |
|---|---|---|---|---|---|---|
| Adjuvant setting | Marron et. al | Cemiplimab | none | Tumor necrosis > 70 % | 21 | 20 % (per protocol, 4 out of 20) |
| Ho, et al | Nivolumab + Cabozantinib | none | Tumor necrosis > 90 % | 15 | 42 % (per protocol, 5 out of 12) | |
| Su, et al | Ipilimumab + nivolumab | none | Tumor shinkrage > 10 % | 29 | 38 % | |
| Kaseb et al | Ipilimumab + nivolumab | Nivolumab | Safety | 27 | No surgery delayed 33 % vs 27 % pathological response (> 70 % necrosis) | |
| IMBRAVE 050 | Atezolizumab + Bevacizumab | Placebo | RFS | 664 | 32.2 (24.3, NE) vs 36 (22–7. NE) months | |
| Combined with locoregional therapy | LEAP-012 | Pembrolizumab + Lenvatinib + TACE | TACE alone | PFS and OS | 480 | PFS 14.6 (12.6–16.7) vs 10 (8.1–12.2) months (OS: data not mature) |
| EMERALD -1 | ARM 1: Durvalumab + Bevacizumab + TACE ARM 2: Durvalumab + TACE | TACE alone | PFS ARM 1 vs control ARM | 616 | PFS 15 (11.1–18.9) vs 8.2 (6.9–11.1) months |
ICI, immune checkpoint inhibitor; NE, not estimable / not evaluable; OS, overall survival; PFS, progression-free survival; RFS, recurrence-free survival; TACE, transarterial chemoembolization.
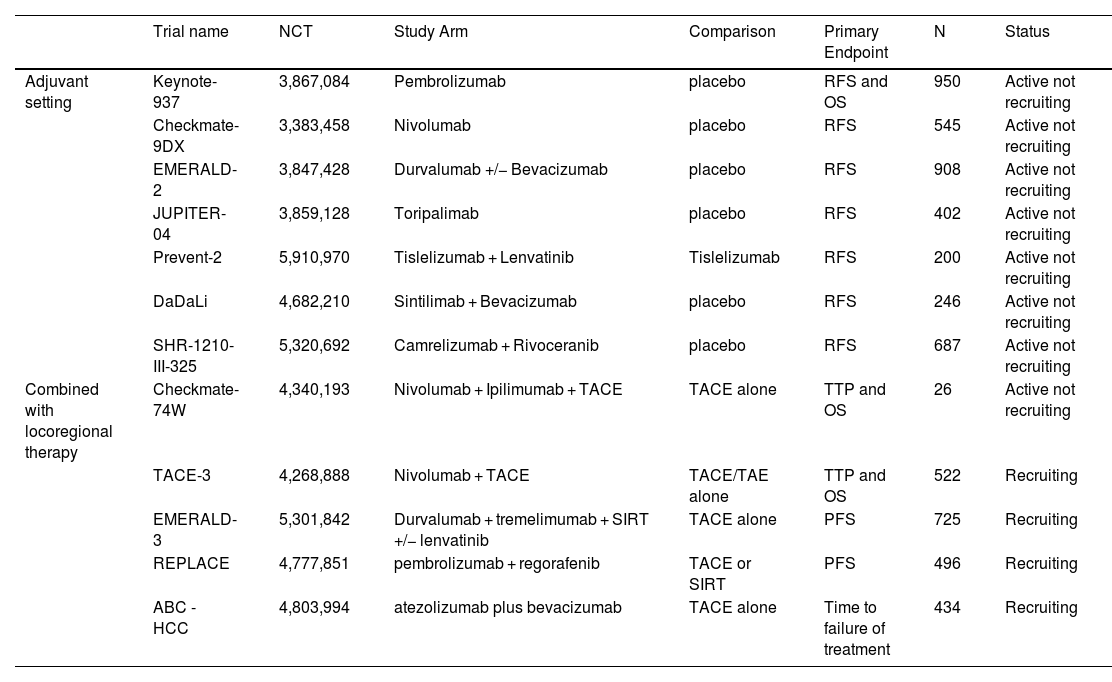
Ongoing Phase III clinical trials investigating adjuvant ICI use.
| Trial name | NCT | Study Arm | Comparison | Primary Endpoint | N | Status | |
|---|---|---|---|---|---|---|---|
| Adjuvant setting | Keynote-937 | 3,867,084 | Pembrolizumab | placebo | RFS and OS | 950 | Active not recruiting |
| Checkmate-9DX | 3,383,458 | Nivolumab | placebo | RFS | 545 | Active not recruiting | |
| EMERALD-2 | 3,847,428 | Durvalumab +/− Bevacizumab | placebo | RFS | 908 | Active not recruiting | |
| JUPITER-04 | 3,859,128 | Toripalimab | placebo | RFS | 402 | Active not recruiting | |
| Prevent-2 | 5,910,970 | Tislelizumab + Lenvatinib | Tislelizumab | RFS | 200 | Active not recruiting | |
| DaDaLi | 4,682,210 | Sintilimab + Bevacizumab | placebo | RFS | 246 | Active not recruiting | |
| SHR-1210-III-325 | 5,320,692 | Camrelizumab + Rivoceranib | placebo | RFS | 687 | Active not recruiting | |
| Combined with locoregional therapy | Checkmate-74W | 4,340,193 | Nivolumab + Ipilimumab + TACE | TACE alone | TTP and OS | 26 | Active not recruiting |
| TACE-3 | 4,268,888 | Nivolumab + TACE | TACE/TAE alone | TTP and OS | 522 | Recruiting | |
| EMERALD-3 | 5,301,842 | Durvalumab + tremelimumab + SIRT +/− lenvatinib | TACE alone | PFS | 725 | Recruiting | |
| REPLACE | 4,777,851 | pembrolizumab + regorafenib | TACE or SIRT | PFS | 496 | Recruiting | |
| ABC - HCC | 4,803,994 | atezolizumab plus bevacizumab | TACE alone | Time to failure of treatment | 434 | Recruiting |
ICI, immune checkpoint inhibitor; NCT, National Clinical Trial identifier; OS, overall survival; PFS, progression-free survival; RFS, recurrence-free survival; SIRT, selective internal radiation therapy; TACE, transarterial chemoembolization; TAE, transarterial embolization; TTP, time to progression.
Downstaging (DS) therapy is defined as a reduction in viable tumor burden with locoregional or systemic treatment to a fall in size within accepted limits for liver transplantation (LT) [59]. Large cohort studies confirmed the benefit of sustained DS in terms of overall and recurrence-free survival compared to non-transplant care [6,60]. Furthermore, complete pathological response after locoregional therapies has been associated with significantly lower 1-, 3-, and 5-year incidence of post-LT recurrence (1.3 %, 3.5 %, and 5.2 % vs 6.2 %, 13.5 %, and 16.4 %; P < 0.001) and superior overall survival (92 %, 84 %, and 75 % vs 90 %, 78 %, and 68 %; P < 0.001)[61]. A multicenter Latin American cohort showed that patients successfully downstage with the University of California-San Francisco DS protocol (UCSF-DS) have similar post-transplant outcomes compared to those within Milan criteria (MC) [62]. These results suggest expanding the selection criteria and improving the prognosis of HCC recipients beyond MC.
Conventional DS protocols are substantially heterogeneous according to predefined inclusion criteria, therapeutic options, response criteria, accepted cut-offs, observation period from DS to LT, or failure criteria [63]. Optimal DS should increase the probability of treating occult micrometastases and reduce the risk of post-transplant recurrence [63,64]. Locoregional therapies (LRT) have limited effects on undetected extrahepatic micrometastases or circulating tumor cells [64–66]. Together, they may synergize in the immunogenic microenvironment promoted by LRT [63]. Therefore, interest has arisen in using immunotherapy in a DS setting, alone or combined with LRT.
Data on the effectiveness of ICIs as DS treatment to LT is increasing. Several case reports and case series with ICIs as neoadjuvant therapy have been published. Still, results are difficult to analyze due to heterogeneous ICI protocols, clinical features, and varying time-to-LT. On the other hand, safety issues regarding ICIs and graft survival should be underlined. Although results from EMERALD-1 or LEAP-012 may support the concept of immunotherapy as an effective intervention, patients who attempted LT were not included in these trials [25]. Multiple international prospective trials investigating various ICI-containing regimens are ongoing, but randomized clinical trials, specifically in LT for HCC, are lacking. One is the XXL study, recently presented at EASL 2024, which showed promising results [67].
However, safety is a significant concern. It has been reported that the immunostimulatory effects of ICIs could induce rejection and lethal graft loss after LT [68,69]. In contrast, the available literature supports that pre-LT immunotherapy appears generally safe when a washout period is achieved, and patients have acceptable post-LT outcomes [70,71]. On the other hand, pathological tumor response is another point to explore with ICIs before LT; given the high rate of explants exceeding Milan criteria after LT and the dissociation between imaging and pathological analysis, assessing response to immunotherapy for HCC remains difficult [60]. Until reliable predictors of complete pathological response are defined, a sustained radiological response may be considered the best surrogate [71].
On the other hand, toxicity due to ICIs has to be considered not only after transplantation (risk of graft rejection), but also immune adverse events while waiting for LT. Moreover, it seems that dual ICIs such as durvalumab + tremelimumab or nivolumab + ipilimumab, may be associated with increased risk of such events. For this reason, it is important to select the ICI combination with better tolerability and safety profile if the aim is to achieve or access LT. Recently, the VITALy observational retrospective cohort study from the United States has shown that from 117 patients receiving ICI while on the waiting list (31 within and 86 beyond Milan criteria). Although the intention-to-treat overall 3-year survival was 71.1 %, only 36.7 % (95% CI 28–46 %) acceded to LT, while 50.4 % were dropped out due to tumor progression (95 % CI 41–60 %). Acute cellular rejection after transplantation occurred in 16.3 % (95 % CI 7–31 %), with 7 patients presenting rejection and 1 resulting in graft loss [72]. On the other hand, another systematic review and metanalysis of observational studies showed that the hazard of the risk of rejection reduces by 8 % for every one-week increase in ICI washout period [70]
Although immunotherapy has shown promising results, further research and more robust studies are needed to recommend it as a single or combined intervention for DS. The optimal pre-LT ICI, avoidance of dual ICIs, particularly in combination with anti-CTLA-4, definition of a washout period to ensure safety, and the best predictors of HCC tumor response to treatment are all unclear issues that need to be defined. An effort to propose and record prospective Latin American data is essential as a starting point to understand the feasibility and impact of ICIs as neoadjuvant therapy for LT for our patients and health systems.
6Role of immunotherapy after liver transplantationDue to the increased risk of graft rejection, the use of ICI after liver transplantation is still controversial. In a systematic review of case reports and case series published by Kayali et al., which included 31 publications reporting a total of 52 patients treated with ICIs after LT, acute graft rejection occurred in 15 patients (28.8 %) and seven patients (13.4 % of the total cohort) died because of graft loss. Rejection was associated with shorter overall survival (OS) (17.2 months, confidence interval [CI] 12.1–22.2 vs. 3.5 months, CI 1.6–5.4, p < 0.001) [73]. The publications on this subject are scarce and of low methodological quality. For this reason, the use of ICIs in the post-transplant setting cannot be recommended. However, a discussion of each case in the multidisciplinary team is suggested.
7Role of hepatologists in the management of these patientsEvidence suggests that managing patients with HCC in multidisciplinary care (MDC) is associated with increased receipt of curative treatment, shorter time to treatment, and improved overall survival [74–77]. In the MDC setting, hepatologists have a crucial role in all stages of BCLC [27]. Child-Pugh, MELD, or ALBI scores are valuable markers for treatment determination. Still, they do not replace the hepatologist's expertise in assessing the patient's underlying liver function, identifying portal hypertension, and stratifying overall liver-related risks based on individual and epidemiological conditions [27].
Identification and management of immune-related adverse events (irAEs), planning, anticipation, prevention, and management of liver damage in combination therapy, evaluation of efficacy, patient quality of life (QOL), and addressing the potential consequences of their use before, during and after LT are just some of the many clinical conditions that arise in ICIs landscape [78]. All these scenarios require the hepatologist's presence in the first line of decision-making to treat patients with HCC more safely and effectively [27].
Unfortunately, implementing MDC may present significant limitations for its application, especially in resource-limited settings and uneven care access, such as those of many Latin American centers (including financial and accessibility burdens) [79]. To optimize patient outcomes and associated costs, health systems and policymakers in Latin America must recognize the urgency of developing multidisciplinary groups and the central role of the hepatologist in their implementation [27].
8ConclusionsDespite current advances in the treatment of HCC, we believe that data from these studies are not yet mature enough to propose adjuvant or neoadjuvant therapy for HCC. Although it is a promising scenario, more information is needed. Cautious interpretations are mandatory when analyzing interim analyses of these trials, demanding longer follow-up or sufficient events to show significant clinical benefits. Furthermore, we underline that results of OS are still needed to recommend these ICIs combinations in the neoadjuvant, adjuvant, or with LRT. While considering these treatments in patients waiting for a LT, further safety effectiveness data is needed. This assessment becomes more relevant when evaluating the patient profile and the number of financial resources directed to health care in most Latin American countries.