Acute viral hepatitis is the most common cause of jaundice in pregnancy. The course of most viral hepatitis infections (e.g., hepatitis A, B, C and D) is unaffected by pregnancy, however, a more severe course of viral hepatitis in pregnancy has been observed in patients with hepatitis E. Notwithstanding, opinions differ over the maternal and fetal outcome of pregnancy associated with viral hepatitis. While some authors reported that acute viral hepatitis carries a high risk for both mother and fetus others conclude that non-fulminant viral hepatitis did not influence the course of pregnancy or fetal well-being. Rate of transmission of the virus during pregnancy depends on the virus. For instance, intra-utero transmission of hepatitis A virus is very rare, but perinatal transmission could occur. Conversely sixty percent of pregnant women who acquire acute HBV infections at or near delivery will transmit the HBV virus to their offspring and mother to child transmission of hepatitis E virus infection was established between 33.3 and 50%. Breast-feeding is not contra-indicated in women infected with the hepatitis A, E or C. However, for acute hepatitis B, with appropriate immunoprophylaxis, including hepatitis B immune globulin and hepatitis B vaccine, breast-feeding of infants of HBV infected mother’s poses no additional risk for the transmission of the hepatitis B virus. Finally, whether live or inactivated vaccines are used, vaccination of pregnant women should be considered on the basis of risks versus benefits. Pregnant women who think they may have been exposed to hepatitis B may be given and hepatitis B immunoglobulin (ideally within 72 hours of exposure), as well as the hepatitis B vaccine.
List of Abbreviations
Hepatitis B virus
HBV
Hepatitis C virus
HCV
Hepatitis A virus
HAV
Hepatitis E virus
HEV
Human immunodeficiency virus
HIV
Hepatitis B Immunoglobulin
HBIG
Immunization Practices Advisory Committee
ACIP
Fulminant liver failure
FLF
Acute viral hepatitis is the most common cause of jaundice in pregnancy.1,2 The course of most viral hepatitis infections (e.g., hepatitis A, B, C and D) is unaffected by pregnancy, however, a more severe course of viral hepatitis in pregnancy has been observed in patients with hepatitis E.2
Notwithstanding, opinions differ over the maternal and fetal outcome of pregnancy associated with viral hepatitis. While some authors reported that acute viral hepatitis carries a high risk for both mother and fetus3 others conclude that non-fulminant viral hepatitis did not influence the course of pregnancy or fetal well-being.
Besides, grater mortality and morbidity has been noted during epidemics of viral hepatitis, particularly in developing countries.3,4 This may indicates that the state of nutrition might make the difference.
Otherwise, the newborn babies do not tend to have any health concerns if their mother has hepatitis. However, it is sometimes possible for the baby to become infected with the virus around the time of birth or during their early childhood years, particularly with hepatitis B and C. Transmission of the virus during pregnancy does not usually happen, but the risk for this can be increased if the mother first becomes infected just before she conceives or during her pregnancy (this mainly relates to virus causing chronic hepatitis).
Lastly, most women with hepatitis will have a normal pregnancy, but the physical process of pregnancy may cause some problems on a woman’s liver. About 6% of women with hepatitis can develop gallstones (or ‘cholelithiasis’) during their pregnancy.5
This paper plans to review and update the current literature on acute viral hepatitis in pregnancy, outlining the management and prevention recommendations.
Hepatitis AIf a pregnant woman becomes infected with hepatitis A, generally her baby is not affected. Intra-utero transmission of hepatitis A virus (HAV) is very rare,6 but perinatal transmission could occur.7,8 In fact, there are just a few reported cases of vertical transmission of hepatitis A virus infection.9,10
It is important to note that healthcare professionals should not treat women with acute hepatitis A in a different way during their pregnancy, labor, birth or postnatal recovery.
However, a note of caution should be added since a recent study evaluating the impact of acute hepatitis A on pregnancy over consecutive admissions of 79.458 pregnant females during a 25-year period, reported that acute HAV infection during pregnancy was associated with high risk of maternal complications and preterm labor.11 Interestingly, the authors evaluated thirteen cases of second and third trimester HAV infection, and several complications were found, for instance, 9 of the 13 patients (69%) developed gestational complications, including premature contractions (n = 4), placental separation (n = 2), premature rupture of membranes (n = 2), and vaginal bleeding (n = 1).
Finally, Hepatitis A virus has rarely been implicated in congenital infections. However, it was described a case of maternal hepatitis A at 13 weeks’ gestation associated with fetal ascites and meconium peritonitis in which, after delivery, a perforated distal ileum was resected in the newborn.12
Prevention and vaccinationMaternal immunization embraces the concepts that vaccines given to pregnant women enhance their resistance to vaccine-preventable diseases and passive antibodies that cross the placenta protect the neonate for the first 3 to 6 months of life.13
Generally, live-virus vaccines are contraindicated for pregnant women because of the theoretical risk of transmission of the vaccine virus to the fetus. Whether live or inactivated vaccines are used, vaccination of pregnant women should be considered on the basis of risks versus benefits-i.e., the risk of the vaccination versus the benefits of protection in a particular circumstance.
The safety of hepatitis A vaccination during pregnancy has not been determined; however, because hepatitis A vaccine is produced from inactivated hepatitis A virus, the theoretical risk to the developing fetus is expected to be low. The risk associated with vaccination should be weighed against the risk for hepatitis A in women who may be at high risk for exposure to hepatitis A virus (Guidelines for Vaccinating Pregnant Women from Recommendations of the Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Department of Health and Human Services (DHHS). October 1998 (updated July 2005).14
Finally, the effect of maternal antibody on hepatitis A vaccine immunogenicity in infants has been recently study and it was observed that passively acquired maternal anti-HAV resulted in a significantly lower final antibody response when infants were administered hepatitis A vaccine15 concluding that maternal antibodies interfere with hepatitis A vaccination in young infants. Also, it is proposed that all women be screened at delivery for anti-HAV antibodies and children born to anti-HAV-negative mothers could be vaccinated early during the first year of life, whereas vaccination could be postponed in children born to anti-HAV-positive mothers, if necessary.16
As a final point, because hepatitis A is a viral infection, many women worry about transmitting the illness through their breast milk. According to the American Academy of Pediatrics Work Group on Breastfeeding, Hepatitis A, even during the acute infectious period, is not a contraindication to breastfeeding. As there is no evidence for transmission via breast milk, breastfeeding women infected with the hepatitis A virus are encouraged to breast-feed.17
Hepatitis BHepatitis B virus (HBV) has a high rate of vertical transmission causing fetal and neonatal hepatitis. Additionally, maternally and neonatally transmitted HBV infection predisposes to carriage, liver cirrhosis and hepato-cellular carcinoma in young adults. Thus, acute hepatitis B involves a particular risk not only for the mother but also for the newborn.
Some particular characteristics of HBV should be remembered in order to understand the behavior of acute hepatitis B. Hepatitis B virus is a double-stranded DNA virus in the Hepadnaviridae family which incubation period from time of exposure to onset of symptoms is 6 weeks to 6 months. HBV is found in highest concentrations in the blood, and lower concentrations in semen, vaginal secretions, and wound exudates.
Perinatal transmission of hepatitis B virus occurs if the mother has had acute HBV infection during late pregnancy or in the first months postpartum, or if the mother is a chronic HBsAg carrier. Sixty percent of pregnant women who acquire acute HBV infections at or near delivery will transmit the HB virus to their offspring.18
Furthermore, intrauterine HBV infection has been suggested to be caused by transplacental transmission that cannot be blocked by hepatitis B vaccine. A case-control study including 402 newborn infants from 402 HBsAg positive pregnant women showed that there is a significant association between intrauterine HBV transmission and HBV infection in villous capillary endothelial cells in the placenta (OR = 18.46, P = 0.0002).19 In fact the authors state the main risk factors for intrauterine HBV infection are maternal serum HBeAg positivity, history of threatened preterm labor, and HBV in the placenta especially the villous capillary endothelial cells.
Hepatitis in pregnancy is not associated with increased abortion rate, stillbirth, or congenital malformation. However, prematurity seems to be increased if hepatitis is acquired in the last trimester.20
Prevention and vaccinationSeveral aspects should be considered when given universal recommendations for acute viral hepatitis B in pregnancy. First of all, it is already known that testing for the hepatitis B virus is generally a standard, routine test performed on all pregnant women at or before their first pregnancy visit, (usually before about 12 to 14 weeks of the pregnancy).21
Second, pregnant women who think they may have been exposed to hepatitis B may be given and injection of hepatitis B immunoglobulin (HBIG) against the virus (ideally within 72 hours of exposure), as well as the hepatitis B vaccine (within 7 days of exposure). A second hepatitis B vaccination needs to be given about 1 month later with a 3rd injection at around 6 months after the first vaccination (or 5 months after the second vaccination).
For instance, pregnant women who are identified as being at risk for HBV infection during pregnancy (e.g., having more than one sex partner during the previous 6 months, been evaluated or treated for an sexual transmitted disease, recent or current injection-drug use, or having had an HBsAg-positive sex partner) should be vaccinated.22
At present it is thought that giving the hepatitis B vaccine to pregnant women is relatively safe, even though there is not sufficient research evidence to completely confirm its safety for the unborn baby. However, the risks of a woman becoming infected with the hepatitis B virus and possibly infecting her baby are thought to outweigh the possible small risks from the vaccination.18 Besides, limited data indicate no apparent risk for adverse events to developing fetuses when hepatitis B vaccine is administered to pregnant women. Additionally, current vaccines contain noninfectious HBsAg and should cause no risk to the fetus.22
Pregnant women at risk for HBV infection during pregnancy should be counseled concerning other methods to prevent HBV infection.22
Breast-feeding recommendation: mothers who carry the hepatitis B virus are encouraged to breastfeed their babies.23 It is recommended that the baby breastfeeds after the administration of the HBIG but not necessarily before the first hepatitis B vaccination. The hepatitis B vaccination can be delayed more than 24 hours after the baby’s birth but definitely needs to be given before the baby is 7 days old (Centers for Disease Control. Protection against viral hepatitis. Recommendations of the Immunization Practices Advisory Committee (ACIP).14 Caution should be added to the above-mentioned recommendations since transmission of HBV through breast milk has been reported in several studies.24,25
As a final point, HBV infection does not appear to be teratogenic. However, there appears to be a higher incidence of low birth weight among infants born to mothers with acute HBV infection during pregnancy.26-28
Hepatitis CTransmission risk of hepatitis C virus (HCV) is much lesser, since it is about 5% for a woman who is positive for viral RNA at the end of her pregnancy, and at least 10% if the woman is moreover positive for the human immunodeficiency virus (HIV).29,30
Clinically acute hepatitis C is a uncommon event to see for the practitioners, even during pregnancy. Besides, diagnosis of asymptomatic forms is extremely difficult. That is the reason why most of the studies reporting data about vertical HCV transmission are based on woman who are chronic carriers of the virus.
However, it was recently reported a case report about the occurrence of acute hepatitis C during pregnancy in a women with 32 weeks of amenorrhea and jaundice. Interestingly, the authors observed that the disease seems to increase the risk of premature delivery, but not that of vertical transmission.31
Another particular issue is about the effect of viral interaction between HIV and hepatitis C virus after HCV superinfection, particularly during pregnancy. We reported the case of a 16 year-old pregnant woman who was evaluated because of icteric acute hepatitis. Surprisingly, we observed that HCV superinfection temporarily interfered with HIV replication since the HIV levels were undetectable during the course of acute HCV infection.32
Prevention strategiesIn recent years, the testing for Hepatitis C (anti-HCV) during pregnancy has become increasingly accepted as ‘routine’ by many maternity caregivers in different countries, along with testing for hepatitis B (HBsAg).33,34 The main reason to do that is because most infected women are asymptomatic and unaware of their infection, so routine prenatal testing is needed to fully meet that goal. Conversely, other authors do not believe that current data justify universal testing, and instead they recommend that it is time for all obstetricians to test selectively based on risk factors.35 In this regards, a recent cost-effectiveness analysis about routine hepatitis C virus screening in pregnancy showed that when compared with no screening, the marginal cost and effectiveness of screening, treatment, and cesarean delivery was 117 US dollars and 0.00010 quality-adjusted life years, respectively, which yields a cost-effectiveness ratio of 1,170,000 US dollars per quality-adjusted life year. In conclusion the authors demonstrated that the screening of asymptomatic pregnant women for hepatitis C virus infection is not cost-effective.36
Regarding to breast-feeding recommendation, both anti-HCV antibody and HCV-RNA were present in colostral samples but in significantly lower levels in a study that explored the role of breast-feeding in transmission of HCV to infants of HCV-infected mothers.37,38 For that reason, some authors recommend that among asymptomatic mothers breast-feeding seems safe, however, women with high viral loads, should not breast-feed to avoid the risk of viral transmission through breast-feeding.37
Hepatitis EHepatitis E is a self-limited enterically transmitted acute viral hepatitis that occurs frequently in epidemic outbreaks and as sporadic hepatitis in the Indian sub-continent, Southeast and Central Asia, the Middle East, parts of Africa, and Mexico.39 Endemic hepatitis E virus (HEV) transmission has not been recognized in Western Europe or in the United States; the prevalence in developing countries ranges from 7.2 to 24.5%.39
Transmission of HEV is by fecal-oral route and clinical illness is similar to other forms of viral hepatitis except in pregnant women, in whom the illness is particularly severe with mortality as high as 25%.39,40 It was shown that one-third of the pregnant women with HEV infection had a severe form of hepatitis in the third trimester of pregnancy, i.e. fulminate hepatic failure.40
The exact mechanism for the severe outcome in pregnant women is unknown. Nevertheless, Pal et al. found an increased concentration of Th2 cytokines in pregnant women with acute hepatitis E41 assuming that this Th2 bias may have a role in the greater severity of hepatitis E among pregnant women.
Besides, hepatitis E in pregnancy is associated with high rates of preterm labor and mortality.40
Lastly, death of the mother and fetus, abortion, premature delivery, or deaths of a live-born baby soon after birth all are common complications of hepatitis E infection during pregnancy.42
Moreover the HEV is essentially dangerous for the mother, maternal-fetal transmission of hepatitis E virus has been reported. In fact, HEV RNA was detected by PCR in cord or birth blood samples of infants born from acute HEV infected mothers concluding that hepatitis E virus is commonly transmitted from infected mothers to their babies with significant perinatal morbidity and mortality.43 In two prospective studies conducted in India, mother to child transmission of hepatitis E virus infection was established between 33.3 and 50%.40,44
Prevention strategiesThere is no current evidence regarding the transmissibility of HEV through breast milk, or regarding the consequences of its transmission for the infant.
In an elegant study encompassing ninety-three infected pregnant mothers in the third trimester of pregnancy of which 36 were positive for anti-HEV antibodies and 57 for HEV RNA, despite that anti-HEV antibody and HEV-RNA were present in the colostrums of HEV infected mothers, breast-feeding appeared to be safe for the infants.45
In conclusion, at the present time, it appears that it would be especially important to continue breastfeeding during epidemics of HEV in underdeveloped, endemic areas to prevent a greater risk of infant mortality from other infectious diseases.
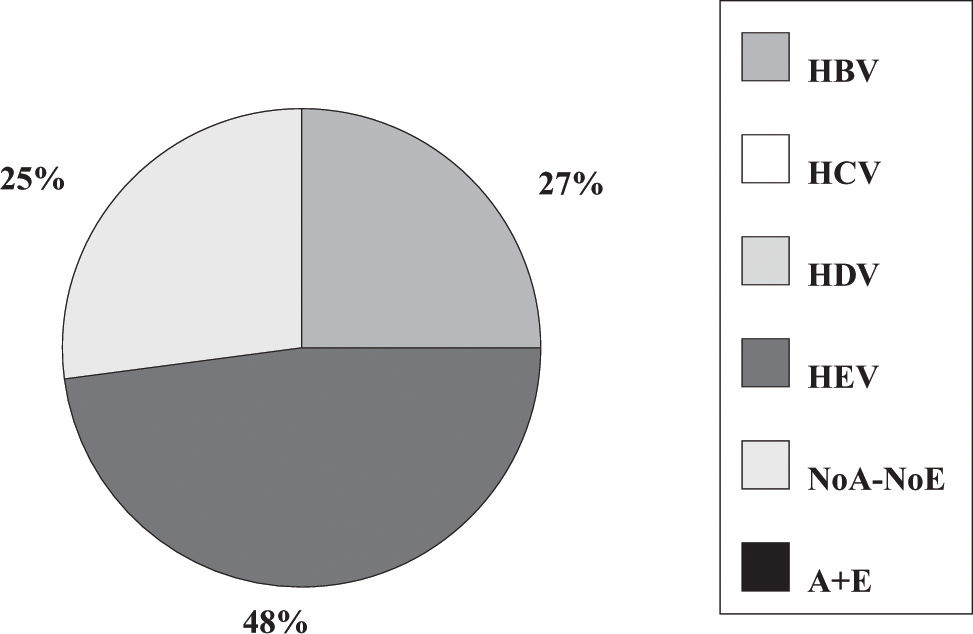
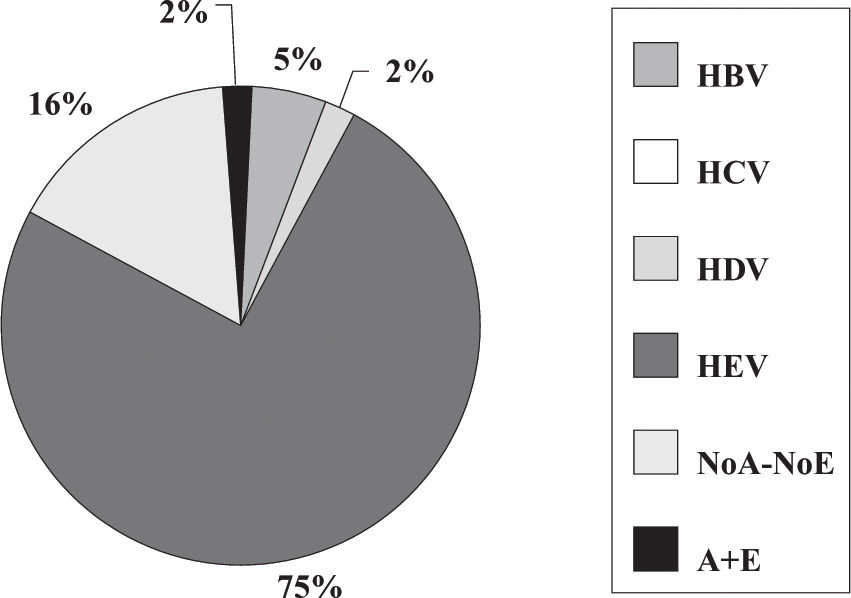
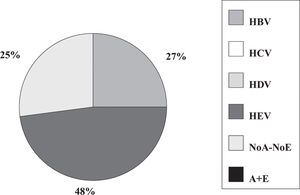
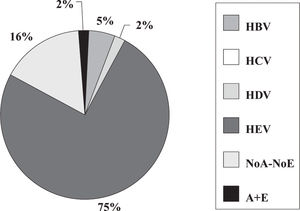
Fulminant liver failure (FLF) following acute viral hepatitis in pregnancySome studies in India and Pakistan reported that hepatitis E is the most frequent cause of acute liver failure in pregnant women46,47 being the etiological agent associated with higher morbidity and mortality (Figures 1and2).
Incidence of hepatitis viruses among 83 pregnant women with acute viral hepatitis. Adapted from Jaiswal et al. 2001.47
Incidence of hepatitis viruses among 44 pregnant women with fulminant liver failure (FLF). Adapted from Jaiswal et al. 2001.47
Some authors said that sometimes, clinical and laboratory features do not permit accurate distinction between acute HEV infection and acute fatty liver of pregnancy.46 Also, in a prospective study encompassing 62 pregnant women having jaundice in the third trimester of pregnancy, 45.2% had HEV infection and nine developed FLF. Eighty-one percent of FLF cases and 37.25% of acute viral hepatitis cases were caused by HEV.40
On the contrary, in Chinese people, infection with HBV during pregnancy is more likely to lead to FLF with a high mortality rate (43-80%) and fast progression, being most common during late pregnancy.48 Certainly, the reason could be related to the fact that hepatitis B virus infection is very common in China.
Besides the high maternal mortality rate, the main dangers of FLF are fetal malformation, preterm labor, abortion, dead fetus in uterus and stillbirth.48
Finally, decisions regarding delivery and liver transplantation must be made if severe hepatic failure develops. In fact, successful liver transplantation during the mid trimester of pregnancy was reported, showing that pregnancy itself is not a contraindication to liver transplantation with life-threatening illness.49,50
To conclude, in most cases the pregnancy itself will not affect the severity of the hepatitis infection for the woman, or the long term course of the hepatitis disease, unless it is found to be hepatitis E or ‘HEV’, which can become worse during pregnancy.