Background and rationale of the study. Effect of Long-term nucleoside/nucleotide (NUC) on hepatocellular carcinoma (HCC) incidence in a population of HBeAg-negative genotype D patients has not been adequately studied in real-life cohorts. Our aim was to evaluate the impact of liver fibrosis and other variables on HCC incidence in this population of patients. Of 745 patients with chronic hepatitis B (CHB), 306 HBeAg-negative genotype D were selected and included in this study. All patients received treatment with NUC for at least 18 months. Patients with CHB or compensated cirrhosis were included. Patients with HCC diagnosed before or during the first 18 months of NUC therapy were excluded.
Results. HCC was diagnosed in 2 CHB patients (1.0%) and 23 cirrhosis patients (20%) (OR = 24.41, 95% CI 5.40 < OR < 153.2; p < 0.0001). Multivariate analysis revealed that HCC risk was independently associated with age ≥ 60 years (OR = 6.45, 95% CI 1.22 to 34.0; p = 0.02) and liver cirrhosis (OR = 12.1, 95% CI 1.39 to 106.2; p = 0.02), but not with virological response (VR), and previous resistance to NUC, or rescue therapy. Multivariate analysis in cirrhosis patients revealed that only age ≥ 60 years was an independent risk factor associated with HCC (p = 0.003). Conclusions. Liver cirrhosis and age > 60 years are the stronger risk factors for HCC in genotype D HBeAnegative patients. Previous resistance to NUC in patients that achieved a VR after rescue therapy was not a predictive factor regarding HCC. VR does not appear to significantly reduce the overall incidence of HCC when a patient has already progressed to liver cirrhosis.
Hepatocellular carcinoma usually develops in patients with chronic liver disease, particularly patients with liver cirrhosis.1,2 Chronic hepatitis B (CHB) is one of the most frequent underlying causes of HCC. Several studies have demonstrated that variations in the hepatitis B virus (HBV) genotype have different effects on HCC.3,4 HBV genotypes C and D had lower responses to interferon-based therapy and higher frequencies of basal core promoter mutations than genotypes A and B.4 For this reason, HBV genotypes C and D seem to lead to more severe liver disease, including cirrhosis, compared with the other HBV genotypes. Because liver cirrhosis is one of the strongest HCC risk factors in CHB patients, antiviral therapy may prevent the development of liver complications such as HCC.5–7 The aim of this study is to evaluate the impact of liver fibrosis and other variables, such as age, sex, VR, and resistance to NUC therapy, in a population of genotype D HBeAg-negative CHB patients treated with long-term NUC therapy.
Material and MethodsPatient populationsFrom January 1998 to December 2012, 745 HBV-infected patients were included in the CLEO Group database. Of these, 438 were excluded: 226 did not fulfil the diagnosis of CHB, 75 had HBeAg-positive CHB, 20 had received NUC for < 18 months, 26 had HCC diagnosed before or within the first 18 months of therapy, and 61 presented a different HBV genotype. Thirty patients had decompensated cirrhosis. A total of 306 HBeAg-negative genotype D naive patients were selected and included in this study. Patients were treated with different NUCs. From 1999 lamivudine (LAM) (Glaxo Ltd Greenford UK) was the only NUC available in Italy while in 2003 adefovir dipivoxil (ADV) (Gilead Science Cambridge UK) was approved for HBV treatment with preferential indication in lamivudine-resistant or naïve patients. In 2007, entecavir (ETV) (Bristol Mayers Squibb Uxbridge UK), telbivudine (LdT), and later tenofovir disoproxil fumarate (TDF) (Gilead Science Cambridge UK) were selected as first-line therapy in naïve patients or as rescue therapy in patients resistant to LAM and/or ADV. Starting NUC in 306 naïve patients analyzed was: LAM in 111, ETV in 126, ADV in 7, LAM associated to ADV in 32, TDF in 21 and LdT in 9. All patients were followed-up at the centres of the CLEO Group. All of the patients’ data were recorded on the EpiInfo central database. The patients were included in this study if they were ≥ 18 years old and had received treatment with NUC for a period of at least 18 months. Patients with CHB or compensated cirrhosis were included, while patients with decompensated cirrhosis were excluded because of the low number of cases observed. Patients with HCC diagnosed before or during the first 18 months of NUC therapy, as well as patients coinfected with hepatitis D, hepatitis C, or HIV, were excluded. Some patients enrolled in the study received an antecedent treatment with interferon.
Follow-upAll patients were treated and followed at participating centres of the CLEO Group in accordance with the guidelines of the European Association for the Study of the Liver (EASL) for the treatment of chronic viral hepatitis.8 Every patient treated with NUC underwent a clinical examination, as well as routine laboratory and viral testing at least every 4 months. HBV DNA levels were detected every 3-5 months using different PCR real time assays with different sensitivities, and the results were converted to IU/mL and expressed as log10.9 A value of HBVDNA < 20 IU/mL was considered, during therapy, as maintained virological response. HBV genotypes were determined using a line probe assay (INNO-LiPA HBV genotyping assay; Innogenetics NV, Ghent, Belgium). Patients with liver cirrhosis, underwent ultrasonography and measurement of alpha-fetoprotein (AFP) every 6 months, while ultrasonography was performed every year in CHB patients. Computed tomography (CT) or nuclear magnetic resonance (NMR) of the liver was employed when required.
Definition of cirrhosisDiagnosis of CHB and liver cirrhosis was based on liver biopsy features according to Ishak Score. The diagnosis of liver cirrhosis was based on histological criteria (Ishak stages 5 and 6).10 In patients that did not undergo liver biopsy, cirrhosis was diagnosed on the basis of other criteria, such as ultrasound signs (spleen size > 12 cm, coarse nodular eco-pattern in the hepatic parenchyma), and/or transient elastometry value (FibroScan®) > 12.5 KPa and/or endoscopic findings compatible with cirrhosis (oesophageal varices, portal gastropathy), and/or platelet count < 100,000 mm3.11
Definition of virological responseVR during treatment was defined as undetectable HBV DNA in serum during therapy. Partial virological response (PVR) was defined as an HBV DNA decrease of > 1 log10 IU/mL, but detectable HBV DNA after at least 6 months of therapy in treatment-compliant patients. Virological non-response (VNR) was defined as HBV DNA undetectability never achieved. Virological breakthrough (VB) was defined as > 1 log10 increase in HBV DNA in serum compared with the nadir value (lowest value) during treatment. HBV resistance to NUC therapy was defined by selection of HBV variants that confer reduced susceptibility to the administered NUC.8 Resistance to NUC therapy was defined as the presence of one or more viral mutations.
Definition of rescue therapyRescue therapy was defined as a switch to another not resistance sharing NUC or add on of the latter NUC to the starting NUC in patients with proven resistance or PVR.
Definition of hepatocellular carcinomaHCC was defined by histological findings or by one or two concordant imaging results (CT or NMR) compatible with HCC depending on the dimensions of the nodule.12
Definition of follow-upThe date of entry into the study protocol was defined as the date of NUC initiation. Follow-up was the time interval between study entry and December 2012 in patients that did not develop HCC, or the interval between study entry and definite HCC diagnosis.
EndpointsThe primary endpoint of the study was the development of HCC. We assessed the risk of development of HCC according to liver status, viral response to treatment, and the presence of previous resistance to NUC therapy.
Statistical analysissAll data were entered into and centralized in the EpiInfo program, and then analysed using the statistical package IBM SPSS (Version 20). Median values are presented for continuous variables. The non-parametric Kruskal-Wallis test or Fisher’s exact test were used for comparisons. Multivariate Cox proportional hazards regression analysis included all variables used in the univariate analysis. Odds ratios (ORs) with 95% confidence intervals (CIs) and p-values from the Wald test are presented. Values of p<0.05 were considered statistically significant. The cumulative incidence for HCC was calculated by the Kaplan-Meier method and was stratified by hepatitis B status (CHB and cirrhosis). The Kaplan-Meier product-limit method was used to compute non-parametric estimates of survival.
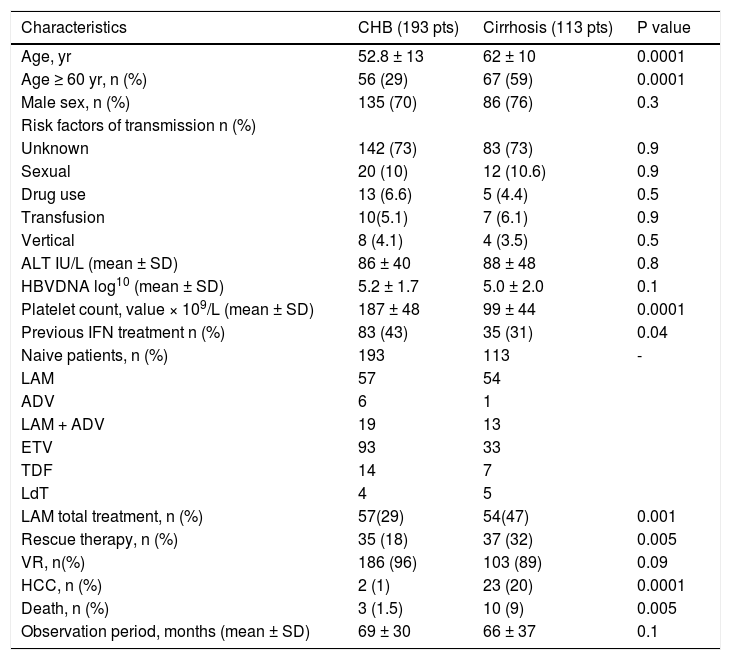
ResultsPatients’ characteristicsWe studied 306 genotype D HBeAg-negative naive patients affected by CHB and treated with NUC’s: 193 (63%) had CHB and 113 (37%) had compensated cirrhosis. Diagnosis of compensated cirrhosis was based on histological features in 48 patients (42%); on fibroscan with a value > 12.5 KPa in 20 patients (17%); and on ultrasonography findings, endoscopic findings, and platelet count in the remaining 45 patients (40%). Patients with decompensated cirrhosis were not included in the study because of the small number of cases. The characteristics of 306 patients are reported in table 1.
Baseline characteristics of 306 patients with genotype D HBeAg negative chronic hepatitis B treated with NUC in relation to severity of the liver disease.
| Characteristics | CHB (193 pts) | Cirrhosis (113 pts) | P value |
|---|---|---|---|
| Age, yr | 52.8 ± 13 | 62 ± 10 | 0.0001 |
| Age ≥ 60 yr, n (%) | 56 (29) | 67 (59) | 0.0001 |
| Male sex, n (%) | 135 (70) | 86 (76) | 0.3 |
| Risk factors of transmission n (%) | |||
| Unknown | 142 (73) | 83 (73) | 0.9 |
| Sexual | 20 (10) | 12 (10.6) | 0.9 |
| Drug use | 13 (6.6) | 5 (4.4) | 0.5 |
| Transfusion | 10(5.1) | 7 (6.1) | 0.9 |
| Vertical | 8 (4.1) | 4 (3.5) | 0.5 |
| ALT IU/L (mean ± SD) | 86 ± 40 | 88 ± 48 | 0.8 |
| HBVDNA log10 (mean ± SD) | 5.2 ± 1.7 | 5.0 ± 2.0 | 0.1 |
| Platelet count, value × 109/L (mean ± SD) | 187 ± 48 | 99 ± 44 | 0.0001 |
| Previous IFN treatment n (%) | 83 (43) | 35 (31) | 0.04 |
| Naive patients, n (%) | 193 | 113 | - |
| LAM | 57 | 54 | |
| ADV | 6 | 1 | |
| LAM + ADV | 19 | 13 | |
| ETV | 93 | 33 | |
| TDF | 14 | 7 | |
| LdT | 4 | 5 | |
| LAM total treatment, n (%) | 57(29) | 54(47) | 0.001 |
| Rescue therapy, n (%) | 35 (18) | 37 (32) | 0.005 |
| VR, n(%) | 186 (96) | 103 (89) | 0.09 |
| HCC, n (%) | 2 (1) | 23 (20) | 0.0001 |
| Death, n (%) | 3 (1.5) | 10 (9) | 0.005 |
| Observation period, months (mean ± SD) | 69 ± 30 | 66 ± 37 | 0.1 |
CHB: chronic hepatitis B. yr: years. PVR: partial virological response. IFN: interferon. LAM: lamivudine. VR: virological response. HCC: hepatocellular carcinoma.
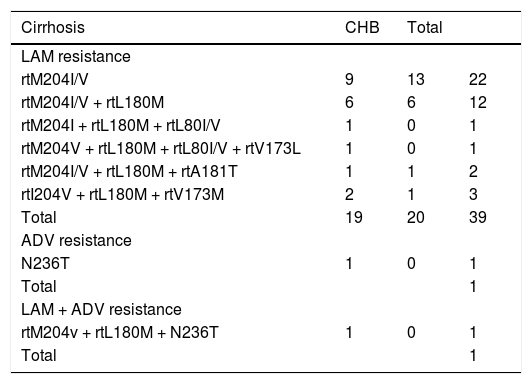
A total of 306 naive patients were treated with NUC therapy for at least 18 months. Starting treatment were ETV (n = 126 patients), TDF (n = 21), LAM (n = 111), Ldt (n = 9), ADV (n = 7), LAM + ADV (n = 32). During the time 72 out of 306 patients (23.5%) received a rescue therapy due to developing of a resistance or PVR to starting NUC. Forty-one patients (21 cirrhotic and 20 CHB) developed a resistant to LAM (LAM-R); 26 (14 cirrhotic and 12 CHB) had a PVR to LAM; 2 cirrhosis patients developed a resistance to ADV and to a combination of LAM + ADV, respectively; 3 CHB patients presented a PVR to ETV, TDF, and ADV, respectively. Rescue therapy was started at a median of 6.36 ± 2.08 months in all patients that developed a virological resistance (detected by INNO-LiPA assay or sequencing) or after detection of a PVR. Resistance to NUCs are reported in table 2.
HBV resistance to NUC in cirrhotic and CHB patients.
| Cirrhosis | CHB | Total | |
|---|---|---|---|
| LAM resistance | |||
| rtM204I/V | 9 | 13 | 22 |
| rtM204I/V + rtL180M | 6 | 6 | 12 |
| rtM204I + rtL180M + rtL80I/V | 1 | 0 | 1 |
| rtM204V + rtL180M + rtL80I/V + rtV173L | 1 | 0 | 1 |
| rtM204I/V + rtL180M + rtA181T | 1 | 1 | 2 |
| rtI204V + rtL180M + rtV173M | 2 | 1 | 3 |
| Total | 19 | 20 | 39 |
| ADV resistance | |||
| N236T | 1 | 0 | 1 |
| Total | 1 | ||
| LAM + ADV resistance | |||
| rtM204v + rtL180M + N236T | 1 | 0 | 1 |
| Total | 1 |
In 2 patients it was not described resistance to LAM. CHB: chronic hepatitis B.
A total of 111 patients started LAM treatment; of these, 67 (60%) received a rescue therapy. In 41 patients with LAM resistance, rescue therapies were ETV (n = 12), TDV (n = 17), LAM + ADV (n = 11), TDF + LAM (n = 1). In 26 patients with PVR to LAM, rescue therapies were ETV (n = 2), TDF (n = 10), ADV + LAM (n = 13), LdT (n = 1), and ADV + ETV (n = 1). Forty-four (39%) LAM patients with persistent undetectable HBV DNA levels remained on treatment.
Rescue therapy in patients treated with other NUCsFive of 195 patients (2.5%) that started treatment with a NUC other than LAM received a rescue therapy. One patient treated with ETV and 1 treated with TDF had a PVR and switched to TDF and ETV, respectively; 1 patient treated with LAM + ADV developed a resistance (Table 2) and switched to ETV + TDF; and 2 ADV patients (1 with resistance and 1 with PVR) switched to ETV (Table 2).
Virological outcomesVR was obtained in 186 of 193 (96%) CHB patients and in 103 of 113 (89%) cirrhosis patients. VR was disclosed in 223 of 234 (95) naïve patients, and in 66 of 72 (91%) patients with rescue therapy for resistance or PVR to the initial NUC respectively. VNR was observed in 11 (5.6%) naïve patients (n = 6 with cirrhosis and n = 5 with CHB) and in 6 patients (6.1%) that switched to another NUC (n = 4 with cirrhosis and n = 2 with CHB) (OR = 1.08, 95% CI 0.39 < OR < 3.04; p = 0.9), respectively. In the 67 patients with resistance or PVR to LAM, that switched to another NUC as rescue therapy, VR was reached in 61 patients (91%), whereas VR was reached in all 5 of the remaining patients with resistance or PVR to another NUC. In naïve patients, PVR was observed in 10 patients treated with ETV (n = 8), TDF (n = 1), and LAM + ADV (n = 1), respectively.
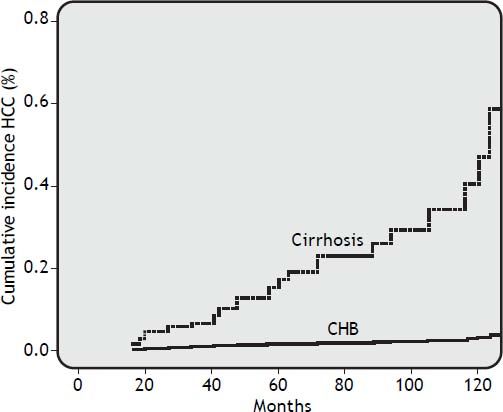
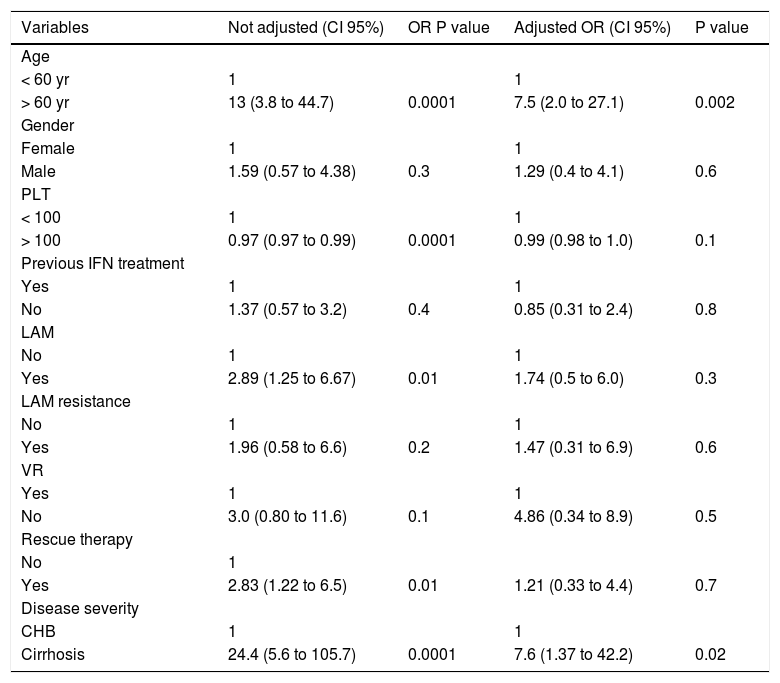
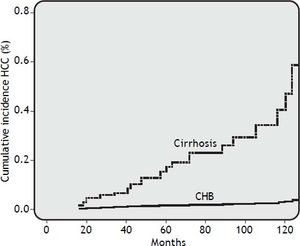
Risk factors for HCCDuring a median follow-up of 62.5 months (range, 18 to 112 months), HCC was diagnosed in 25 of 306 patients (8.2%). HCC was diagnosed in 2 out of 193 CHB patients (1.0%) and in 23 out of 113 cirrhosis patients (20%) (OR 24.41, 95% CI 5.40 < OR < 153.2; p = 0.0001). In all, 60% of patients developed HCC after more than 48 months of NUC therapy. The cumulative HCC incidence was significantly higher in patients with cirrhosis than in patients with CHB. The cumulative HCC incidence in cirrhosis patients was 4.2%, 6.2%, 15%, 22%, and 43% at 2, 3, 5, 7, and 10 years respectively, whereas was 0.5% at 10 years in CHB patients (Figure 1). The HCC incidence per 100 person year in LAM, LAM-R and ETV cirrhotic patients was respectively 0.14, 0.10, and 0.12. While HCC incidence per 100 person year in LAM CHB patients was 0.001. Univariate Cox regression analysis indicated that the HCC risk was significantly higher in patients: older than 60 years, with PLT count < 100,000/mm3 at baseline, with liver cirrhosis, or that underwent a rescue therapy. Previous resistance or PVR to LAM were not factors predictive of HCC. All univariate Cox regression analysis results are reported in table 3. Regarding age, HCC developed in 18% (22/123) of patients older than 60 years and in 1.6% (3/183) of patients younger than 60 years (p = 0.0001), respectively. In cirrhotic patients diagnosis of HCC was also performed in 31% (21/67) of patients older than 60 years and in 4.3% (2/44) of patients younger than 60 years, respectively (OR = 9.59% CI 1.98 < OR < 63; p = 0.001). HCC was disclosed in 7% (20/289) of patients with VR and in 29% (5/17) of patients without VR, respectively (OR=0.18, 95% CI 0.05 < OR < 0.65; p = 0.004). Furthermore, HCC was diagnosed in 17% (18/103) of cirrhosis patients with VR and 50% (5/10) of cirrhosis patients without VR (OR = 0.21 95%CI 0.21 < OR < 0.96 p = 0.02). HCC was revealed in 1% (2/186) of CHB patients with VR and 0% (0/7) of CHB patients without VR. Onset of HCC was revealed after a mean of 65 ± 38 months in patients with VR and at a mean of 52 ± 34 months in patients without a VR. HCC was disclosed in 15% (11/72) of patients who received rescue therapy, with respect to 14/234 naive patients (5.9%) who remained on first treatment (OR = 0.35 95%CI 0.14 < OR < 0.89 p = 0.01). In cirrhotic patients, HCC developed in 27% (10/37) of patients who underwent a rescue therapy and in 17% (13/76) of those who remained on first line treatment, respectively (OR = 0.56, 95% CI 0.20 < OR < 1.59; p = 0.3). Multivariate Cox regression analysis including all variables reported in univariate analysis showed that only patient age older than 60 years and the presence of liver cirrhosis were independently associated with HCC (Table 3).
Risk factors for the development of HCC in 306 patients with genotype D HBeAg negative chronic hepatitis B treated with NUC. Results of Univariate and Multivariate Cox regression analysis.
| Variables | Not adjusted (CI 95%) | OR P value | Adjusted OR (CI 95%) | P value |
|---|---|---|---|---|
| Age | ||||
| < 60 yr | 1 | 1 | ||
| > 60 yr | 13 (3.8 to 44.7) | 0.0001 | 7.5 (2.0 to 27.1) | 0.002 |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 1.59 (0.57 to 4.38) | 0.3 | 1.29 (0.4 to 4.1) | 0.6 |
| PLT | ||||
| < 100 | 1 | 1 | ||
| > 100 | 0.97 (0.97 to 0.99) | 0.0001 | 0.99 (0.98 to 1.0) | 0.1 |
| Previous IFN treatment | ||||
| Yes | 1 | 1 | ||
| No | 1.37 (0.57 to 3.2) | 0.4 | 0.85 (0.31 to 2.4) | 0.8 |
| LAM | ||||
| No | 1 | 1 | ||
| Yes | 2.89 (1.25 to 6.67) | 0.01 | 1.74 (0.5 to 6.0) | 0.3 |
| LAM resistance | ||||
| No | 1 | 1 | ||
| Yes | 1.96 (0.58 to 6.6) | 0.2 | 1.47 (0.31 to 6.9) | 0.6 |
| VR | ||||
| Yes | 1 | 1 | ||
| No | 3.0 (0.80 to 11.6) | 0.1 | 4.86 (0.34 to 8.9) | 0.5 |
| Rescue therapy | ||||
| No | 1 | |||
| Yes | 2.83 (1.22 to 6.5) | 0.01 | 1.21 (0.33 to 4.4) | 0.7 |
| Disease severity | ||||
| CHB | 1 | 1 | ||
| Cirrhosis | 24.4 (5.6 to 105.7) | 0.0001 | 7.6 (1.37 to 42.2) | 0.02 |
PLT: platelets. VR: virological response. CHB: chronic hepatitis B. LAM: lamivudine. OR: odds ratio.
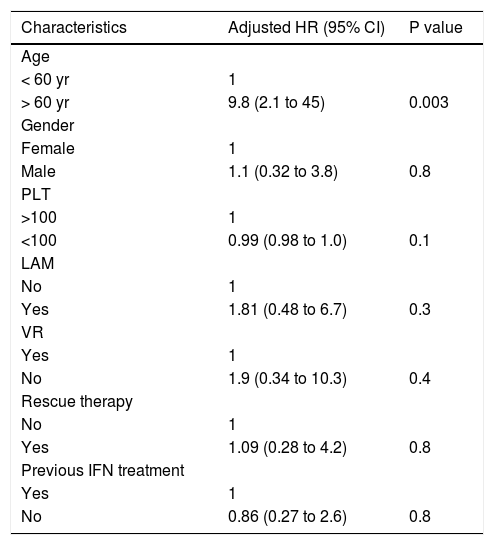
Multivariate Cox regression analysis focused on only cirrhosis patients revealed that biological sex, induction and maintenance of VR, previous resistance or PVR in response to LAM, and rescue therapy were not associated with the risk for HCC, while age older than 60 years was an independent risk factor associated with HCC (p = 0.003) (Table 4).
Risk factors for the development of HCC in 113 genotype D HBeAg negative cirrhotic patients treated with NUC Results of Multivariate Cox regression analysis.
| Characteristics | Adjusted HR (95% CI) | P value |
|---|---|---|
| Age | ||
| < 60 yr | 1 | |
| > 60 yr | 9.8 (2.1 to 45) | 0.003 |
| Gender | ||
| Female | 1 | |
| Male | 1.1 (0.32 to 3.8) | 0.8 |
| PLT | ||
| >100 | 1 | |
| <100 | 0.99 (0.98 to 1.0) | 0.1 |
| LAM | ||
| No | 1 | |
| Yes | 1.81 (0.48 to 6.7) | 0.3 |
| VR | ||
| Yes | 1 | |
| No | 1.9 (0.34 to 10.3) | 0.4 |
| Rescue therapy | ||
| No | 1 | |
| Yes | 1.09 (0.28 to 4.2) | 0.8 |
| Previous IFN treatment | ||
| Yes | 1 | |
| No | 0.86 (0.27 to 2.6) | 0.8 |
PLT: platelets. LAM: lamivudine. VR: virological response. IFN: interferon.
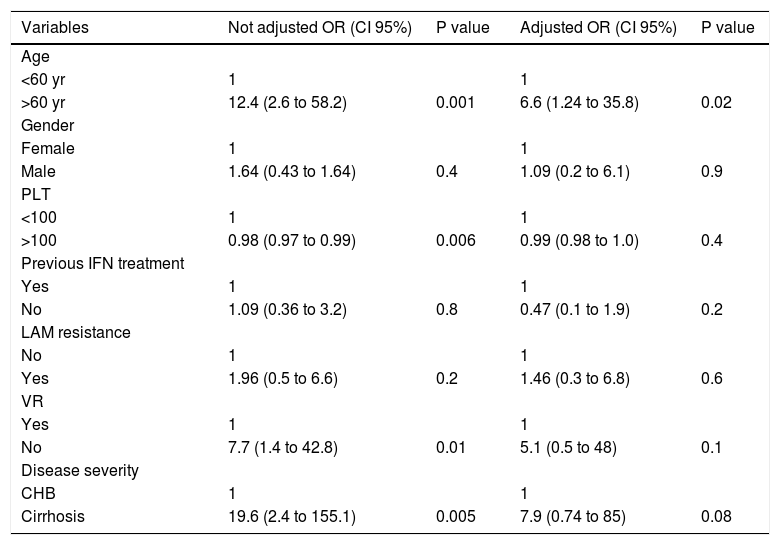
Regarding patients that started treatment with LAM, HCC was revealed in 16% (11/67) of patients with resistance or PVR to LAM, and in 9% (4/44) of those without resistance (OR = 1.96 95%CI 0.52 < OR < 7.95 p = 0.4). Univariate Cox regression analysis in patients treated with LAM indicated that the HCC risk was significantly higher in patients: older than 60 years, with PLT count < 100,000/mm3 at baseline and with liver cirrhosis; while presence of resistence or PVR to LAM and rescue therapy were not correlated with an increase risk to develop HCC. Multivariate Cox regression analysis showed that in patients treated with LAM, an age older than 60 years but not the presence of resistance or PVR to LAM was associated with the risk to develop HCC, liver cirrhosis exhibited a trend toward being an independent risk factor associated with HCC (Table 5).
Risk factors for the development of HCC in 111 patients with genotype D HBeAg negative chronic hepatitis B treated with LAM. Results of Univariate and Multivariate Cox regression analysis.
| Variables | Not adjusted OR (CI 95%) | P value | Adjusted OR (CI 95%) | P value |
|---|---|---|---|---|
| Age | ||||
| <60 yr | 1 | 1 | ||
| >60 yr | 12.4 (2.6 to 58.2) | 0.001 | 6.6 (1.24 to 35.8) | 0.02 |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 1.64 (0.43 to 1.64) | 0.4 | 1.09 (0.2 to 6.1) | 0.9 |
| PLT | ||||
| <100 | 1 | 1 | ||
| >100 | 0.98 (0.97 to 0.99) | 0.006 | 0.99 (0.98 to 1.0) | 0.4 |
| Previous IFN treatment | ||||
| Yes | 1 | 1 | ||
| No | 1.09 (0.36 to 3.2) | 0.8 | 0.47 (0.1 to 1.9) | 0.2 |
| LAM resistance | ||||
| No | 1 | 1 | ||
| Yes | 1.96 (0.5 to 6.6) | 0.2 | 1.46 (0.3 to 6.8) | 0.6 |
| VR | ||||
| Yes | 1 | 1 | ||
| No | 7.7 (1.4 to 42.8) | 0.01 | 5.1 (0.5 to 48) | 0.1 |
| Disease severity | ||||
| CHB | 1 | 1 | ||
| Cirrhosis | 19.6 (2.4 to 155.1) | 0.005 | 7.9 (0.74 to 85) | 0.08 |
PLT: platelets. IFN: interferon. LAM: lamivudine. VR: virological resistance. CHB: chronic hepatitis B.
After a mean follow-up of 62 ± 37 months, 13 patients (n = 10 with cirrhosis, n = 3 with CHB) had died. Causes of death in cirrhosis patients were multifocal HCC (n = 6 patients), infiltrating HCC (n = 1), myocardial infarction (n = 1), liver failure (n = 1), and upper gastrointestinal bleeding (n = 1). Causes of death in CHB patients were myocardial infarction (n = 1 patient), multifocal HCC (n = 1), and bowel cancer (n = 1). Patients with baseline liver cirrhosis were more likely than those without to die from liver-related causes (RR = 15.3, 95% CI 1.97 < RR < 119; p = 0.0007) or from HCC only (RR = 11.96, 95% CI 11.9 < RR < 95.3; p = 0.004), although the death rate from causes unrelated to the liver was similar (RR = 0.85, 95% CI 0.08 < RR < 9.31; p = 0.6). The remaining 294 patients are still alive. The 10-year event-free survival rate was 86% for patients with compensated liver cirrhosis and 93% for patients with CHB (p = 0.007 according to log rank test).
DiscussionOur study demonstrates that in a Caucasian population of genotype D HBeAg-negative CHB patients risk for HCC remains high over time, particularly in patients with liver cirrhosis and age older than 60 years, despite long-term NUC treatment. Unlike other studies, our experience in these patients (caucasian, genotype D, HBeAg negative ), shows that previous resistance to or PVR to first generation NUC’s and a further rescue therapy do not appear to increase the risk for HCC. These results could be due to an effective VR after a rescue therapy in patients who exhibited resistance or PVR to first generation NUC (LAM, ADV), mainly with the more potent third generation NUC’s as ETV and TDF. Furthermore, maintenance of VR during therapy reduced but not eliminated completely the HCC risk in our population. In clinical studies that included untreated historical controls, it was demonstrated that NUC treatment could reduce but not eliminate HCC risk compared with untreated patients. A milestone study by Liaw et al. demonstrated that patients treated with LAM had a reduced risk of developing HCC compared with untreated patients. In this study, the emergence of YMDD mutations, in a subpopulation of patients, reduced the HCC-preventative benefit of LAM in that subpopulation compared with patients that lacked the mutations.13 A recent study of Kumada et al. used a propensity analysis to show that there was a reduced risk of HCC in a group of patients that received different NA therapies (LAM or LAM + ADV or ETV) compared with untreated controls. In this study, high serum levels of HBV core-related antigen (HBcrAg) and basal core promoter (BPC) mutations were associated with progression to HCC independent of NA therapy.14 A study of Di Marco et al. demonstrated that LAM-treated patients that achieved a VR had a reduced risk for HCC compared with LAM-R patients.15 In this study, cirrhotic patients presented a higher incidence of HCC after emergence of LAM resistance with respect to patients without LAM resistance and with maintained VR (17 vs. 10 cases, respectively) (OR = 5.37, 95% CI 2.28 < OR < 12.82; p < 0.00001). Upon this study, the likelihood of developing HCC in cirrhotic patients was significantly lower in patients with VR with respect to LAM-R patients, but, at that time (1995-2002), a rescue therapy for LAM-R patients with alternative NUC’s was not available. This might explain the high incidence of HCC in LAM-R cirrhosis patients observed in the study. We strongly believe that the great effectiveness of rescue therapy with third generation high genetic barrier NUC’s has deeply modified the importance of LAM-R on onset of HCC. Accordingly with our findings, Eun, et al. demonstrated that LAM therapy reduced the incidence of HCC in cirrhosis patients when VR was present.16 In this study, HCC incidence was higher among cirrhosis patients with LAM-R or PVR to LAM with respect to LAM patients with complete VR. In contrast, the HCC incidence was lower in CHB patients, independent of the presence or absence of LAM-R or VR. In the same study, when a rescue therapy was applied to LAM-R patients with cirrhosis, the incidence of HCC was reduced. In particular, HCC risk was lower in LAM-R cirrhosis patients that used ADV as rescue therapy. A recent study by Yang, et al. demonstrated that in patients that were resistant to LAM or ADV, a rescue therapy with ETV was associated with a reduced risk of HCC.17 Compared with other studies that reported a high incidence of HCC in patients with resistance to or PVR to LAM performed when an effective rescue therapy was not available,13,15,16 in our study, a rescue therapy based on switch to a single third generation high genetic barrier NUC or towards a combination of NUC’s, was promptly started to LAM-treated patients that experienced resistance or PVR. In our experience HCC incidence in cirrhotic patients that underwent an effective rescue therapy was similar to HCC incidence in naive cirrhosis patients with maintained VR. The introduction of newer NUC agents has changed the risk of developing HCC in LAM-resistant patients. We demonstrate upon our experience that LAM-R is not a per se condition associated with a higher risk of developing HCC when an efficacy rescue therapy is promptly applied. Papatheodoridis, et al., in a population of genotype D HBeAg-negative patients, showed that factors as aged over 60, male gender, and presence of liver cirrhosis were each one independently associated with HCC risk, while a maintained VR did not appear to significantly reduce the overall incidence of HCC.18 The Authors stated that VR might reduce, but do not eliminate the HCC risk, possibly owing to the integration of HBV DNA into the host genome before the beginning of treatment with further genome instability. This study analysed patients treated with NUC’s therapy for a period ≥ 12 months and in LAM-R patients, a rescue therapy was started after a median of 1.4 years from the observed resistance. In our study, we have enrolled patients treated with NUC therapy for longer than 18 months, and rescue therapy was started promptly within a median of 6.2 months after the evidence of resistance or PVR to LAM. A delay in rescue therapy in the study by Papatheodoridis et al. might have facilitated the direct oncogenetic role of HBV. Indeed, in the same study, in patients with VR, HCC was diagnosed at a median of 15 months (range, 7-30 months) after the initiation of therapy. In our study, HCC was diagnosed in patients with VR at a median of 57 months (range, 18-119 months) after the initiation of therapy. The oncogenetic mechanism of HBV infection in our study seems to be strictly more dependent on the presence of liver cirrhosis (indirect oncogenetic mechanism), while the short interval between the start of NUC therapy and the development of HCC reported by Papathedoridis, et al. might be due to a previous integration of HBV into the host cell genome (direct oncogenetic mechanism). Lampertico, et al. reported that HCC developed in 11% of NUC-naïve cirrhosis patients treated with ETV, even though they experienced a VR to ETV. The study concluded that Entecavir monotherapy in naïve patients with liver cirrhosis, not fully prevent HCC.19 In our study, maintenance of VR was not associated with a reduced risk of HCC. We demonstrated that VR did not eliminate the risk of HCC in patients that had already progressed to cirrhosis. Multivariate analysis indicated that liver cirrhosis and an age > 60 remain the most important risk factors for HCC. Older age is a risk factor for HCC, and, in our population this factor might be related to the frequent presence of cirrhosis in HBeAg-negative genotype D patients older than 60 years. Some studies report that genotype D cause more frequent progression to liver cirrhosis20,21 and hence age older than 60 years might be a surrogate for old HBV infection and liver cirrhosis. For this reason, we believe that NUC therapy must be prescribed for HBV patients early, before they progress to liver cirrhosis. One limitation of the present study is due to variability of HBV-DNA quantification method used. The use of different tests with a range of variability in sensitivity from different laboratories scattered throughout Italy could partially have been a source of potential error that could be over-come at least partially by the large cohort size. Another limitation of this study is represented by the absence of a historical untreated controls for comparison, although several studies have shown unequivocally that untreated HBV infected patients had an higher incidence of HCC respect to treated patients.13,16
In conclusion, in our study we point up the attention on the importance of liver cirrhosis and old age as the most important factors for the development of HCC in HBeAg-negative genotype D patients. Our findings suggest that, in genotype D HBeAg-negative patients with resistance to or PVR to starting NUC’s (LAM, ADV) and promptly treated with an effective rescue therapy, HCC incidence is similar to the HCC incidence in naive patients with maintained VR. Induction and maintenance of VR does not appear to significantly reduce the overall incidence of HCC in patients that have already progressed to liver cirrhosis. Furthermore, sub-analysis of cirrhosis patients shows that only age older than 60 years remains a risk factor predictive of HCC.
Abbreviations- •
ADV: adefovir dipivoxil.
- •
AFP: alpha-fetoprotein.
- •
CHB: chronic hepatitis B.
- •
CLEO: club epatologi ospedalieri.
- •
CT: computed tomography.
- •
EASL: European Association for the Study of the Liver.
- •
ETV: entecavir.
- •
HBV: hepatitis B virus.
- •
HCC: hepatocellular carcinoma.
- •
LAM: lamivudine.
- •
NMR: nuclear magnetic resonance.
- •
NUC: nucleoside/ide.
- •
ORs: odds ratios.
- •
PVR: partial virological response.
- •
TDF: tenofovir disoproxil fumarate.
- •
VNR: virological no response.
- •
VR: virological response.
No financial supports have been received for this paper.
Conflict of InterestThe authors declare that there is not a conflict of interest about this paper.
For all the authors.
Adriano M. Pellicelli, M.D.
The Authors declare that part of the data of this paper were presented as Oral communication at The Liver Meeting 2012 AASLD 63rd Annual Meeting Abstrat 21 Presented November 11, 2012.