The mechanism of damage of the biliary epithelium remains partially unexplored. However, recently many works have offered new evidence regarding the cholangiocytes' damage process, which is the main target in a broad spectrum of pathologies ranging from acute cholestasis, cholangiopathies to cholangiocarcinoma. This is encouraging since some works addressed this epithelium's relevance in health and disease until a few years ago. The biliary tree in the liver, comprised of cholangiocytes, is a pipeline for bile flow and regulates key hepatic processes such as proliferation, regeneration, immune response, and signaling. This review aimed to compile the most recent advances on the mechanisms of cholangiocellular damage during cholestasis, which, although it is present in many cholangiopathies, is not necessarily a common or conserved process in all of them, having a relevant role cAMP and PKA during obstructive cholestasis, as well as Ca2+-dependent PKC in functional cholestasis. Cholangiocellular damage could vary according to the type of cholestasis, the aggressor, or the bile ducts' location where it develops and what kind of damage can favor cholangiocellular carcinoma development.
Liver diseases remain a significant health problem worldwide due to profound changes in modern lifestyle sustained by the unmoving remarkable alcohol consumption, hepatotropic virus infection, and the increased ingestion of lipid- and carbohydrate-enriched diets, in addition to a sedentary lifestyle. The increment of non-alcoholic fatty liver disease (NAFLD) has emerged as a critical driver and promoter of more aggressive liver pathologies such as non-alcoholic steatohepatitis (NASH), cholestasis, and even hepatocellular carcinoma (HCC) [1].
Cholestatic diseases continue as poorly studied liver disorders, perhaps due to an apparent low incidence compared to those widespread liver diseases. Definitively, knowledge of hepatocyte physiology and pathophysiology compared with cholangiocyte biology could represent one of the main reasons for the limited comprehension of cholestasis and its therapeutic approach.
In the present review, we were focused on presenting a comprehensive examination of the state-of-the-art mechanism of cholangiocyte damage in cholestasis diseases to provide a clear landscape of the problem that could set a pipeline to the development of new therapeutic approaches in cholestasis.
2Cholestasis and cholangiopathiesCholestasis is a persistent liver disorder defined by the decrement of the bile flow that reaches the duodenum, either due to mechanical impairment obstructing the bile transit (obstructive cholestasis) or due to functional alterations in the ability of the hepatocytes to generate the bile (hepatocellular or functional cholestasis) [2]. The consequent intracellular retention of toxic bile components induces parenchyma damage, mainly by mechanisms mediated by oxidative stress [3, 4].
Hepatocytes and cholangiocytes represent the main targets of damage in cholestasis[2]; depending on the severity of the disease, the damage could be differentiated regarding the primary cell type involved [5]; Hepatocellular damage leads to inflammation, fibrosis, and hepatic dysfunction [6] however, when the damage is cholangiocellular, disease presentation is different, leading to well-differentiated cholangiopathy.
Cholangiopathies are a group of hepatic diseases characterized by direct damage of the biliary epithelium, mainly associated with an inflammatory response [7, 8], such as primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), biliary atresia (BA), cystic fibrosis (CF), drug-induced cholangiopathies among others [2]. Cholangiopathies are the primary diseases resolved by liver transplantation [9, 10].
Cholangiocyte proliferation is fundamental in chronic cholestasis to maintain the homeostasis and the structure of the biliary system [11, 12], and failure in this compensatory mechanism has been associated with initiation and worsening of diverse biliary diseases such as cholestasis, PSC, biliary fibrosis, PBC among others [13, 14].
3The cholangiocyteCholangiocytes are epithelial cells that line the bile ducts (intrahepatic and extrahepatic bile duct network)[8], they represent 3-5 % of the cell types in the liver [12]. Cholangiocytes form a complex network of (the biliary tree) tubular ducts of various sizes and properties extending from the canals of Hering in the liver to the duodenum [6]. The cholangiocytes' size and certain functions depending on their location in the biliary tree [15]. As in any other epithelial cell, the cholangiocyte is polarized, with well-defined apical and basolateral membranes. The main functions of the cholangiocytes reside in the apical side. It contains a fascinating organelle-like specialized membrane extension named primary cilium, which encloses multiple receptors and channels that efficiently sense the quality of the bile and drives its modulation, particularly in terms of osmolarity, playing notable roles in the secretion of ions and water [8], through the apical and basolateral faces of the cholangiocyte [16].
Bile is the product of the transport and secretion of bile acids, phospholipids, bilirubin, cholesterol, electrolytes, proteins, endo, and xenobiotic detoxification products and water by the hepatocyte to a greater extent, but the cholangiocyte also plays a vital role in bile formation [6]; for this reason, it has a higher proportion of actin cytoskeleton, which allows it to carry out vesicular transport, necessary for maintaining polarity and proper protein function, as well as promoting bile flow [15].
Cholangiocytes are the main cell type affected in cholangiopathies. Consequently, cytokine and growth factors burst mediates cell proliferation, fibrosis, and apoptosis [17, 18]. Cholangiocytes act as a "pinch-hitter" in the liver regeneration process when hepatocytes lose this capacity [19].
As previously mentioned, cholangiocytes' size and location define their function and response [17, 20, 21]. The cholangiocytes are classified into small and large, considering as small those that form the intrahepatic branches of the biliary tree (4 to 5 cells or 15 mm thick tubular) and large ones those of the most prominent biliary structures (10 to 12 cells or up to 415 mm), adopting a columnar type structure [17, 22-24].
Large and small cholangiocytes are different in size but also differentially express proteins and carry out various processes. For example, large cholangiocytes express the machinery necessary for cAMP-dependent ductular secretion, such as secretin (SR) and somatostatin receptors (SSTR2), cystic fibrosis transmembrane conductance regulator (CFTR), and Cl−/HCO3– anion exchanger 2 (AE2) [24, 25]. In contrast, small cholangiocytes' secretion process is Ca2+ dependent. This variance explains why each cholangiocyte's damage mechanisms in both kinds of cholestasis [17, 26].
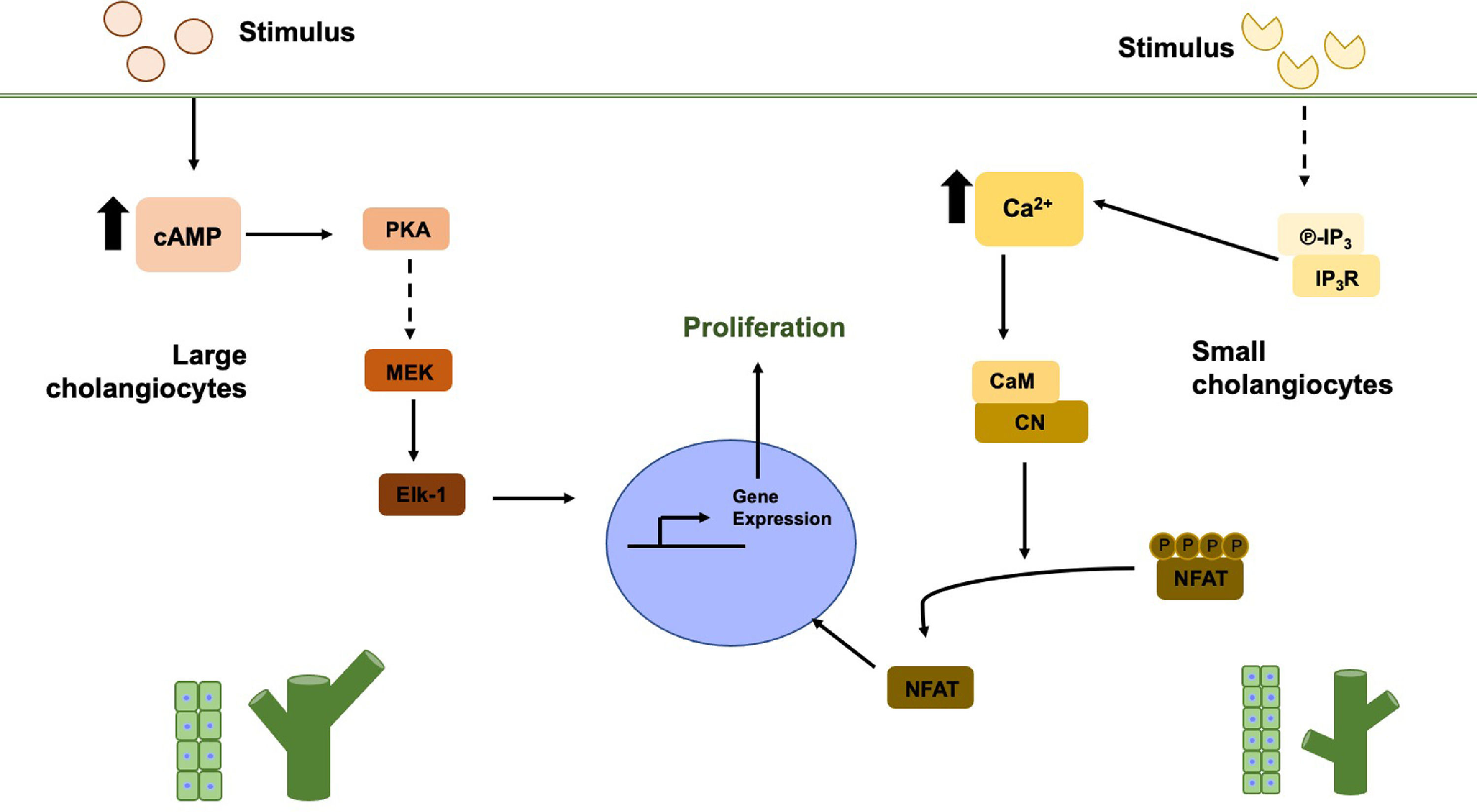
4Cholestasis damageThe responsiveness of cholangiocytes to the damage is dependent on the type of aggression as observed in experimental models of bile duct ligation (BDL), partial hepatectomy, CCl4 toxicity, bile acids, and α-naphthylisothiocyanate (ANIT) treatment [17]. Most of these experimental models favor cell proliferation preferentially in large cholangiocytes [8], as observed in a BDL model in rats where expansion occurred exclusively in the bile ducts of larger size due to the increase in the expression of SR in the cholangiocytes. Therefore secretin (SCT) binds to SR [27], inducing cAMP levels that favor the PKA/ERK/Elk-1 pathway (Fig. 1, left side) [17, 28, 29], leading to proliferation, in addition to increasing ATP levels through CFTR and thereby promoting the secretion of HCO3− in the bile [30].
Large and small cholangiocytes proliferation pathways. In large cholangiocytes, the stimulus is mainly hormonal, which increases cAMP levels, activating PKA and through several steps MEK, ERK1/2 and finally Elk-1 are activated within the nucleus. In small cholangiocytes, when receiving the stimulus, IP3 levels increase and binds to IP3R, which induces the release of Ca2+. The increase in Ca2+ levels induces the activation of CN and CaM, finally these two participate in the dephosphorylation of NFAT to enter the nucleus
The specific proliferation of large cholangiocytes observed in the BDL model may be due to the peribiliary vascular plexus (PBVP) near the large bile ducts. PBVP is a vascular network of capillaries that run from the hepatic artery branches and crosses through the sinusoid [31]. The PBVP unfolds around the large bile ducts, and this number decreases until reaching a capillary in the small bile ducts [12]. In PBVP, the blood flow is opposite to that of bile flow. That is, the bloodstream goes from large to small ducts. This is why cholangiocytes (the large and the small ones) differentially proliferate, leading to ductular activation, and, at the same time, explains the reason for damage susceptibility among cholangiocytes [23].
Interestingly, some reports provide evidence suggesting that when large cholangiocytes are affected, the small ones are responsible for the regeneration and repopulation of damaged bile structures, acquiring a phenotype similar to large cholangiocytes. Ca2+-dependent signaling conducts the process, and the activation, in small cholangiocytes, of the inositol triphosphate (IP3) receptor, which induces the release of Ca2+ and the activation of calcineurin (CN) and calmodulin (CaM), leading to the activation of the nuclear factor of activated T-cells (NFAT) proteins and CaM-dependent Kinase (CaMK) (Fig. 1, right side) [26, 32, 33]. Furthermore, large cholangiocytes in the BDL model decrease their IP3R expression [34]. These studies show that small cholangiocytes differentiate into large ones due to their stemness characteristics [17, 27, 35].
However, some cholestatic models do not generate differential cholangiocyte responses, such as ANIT administration in rats. This chemical affects the cholangiocytes equally, increasing apoptosis and proliferation in both kinds of cholangiocytes [36].
5Mechanism of damage and repair in cholangiocytesWe will revise some of the main mechanisms of cholangiocellular damage and repair, particularly in obstructive and functional cholestasis.
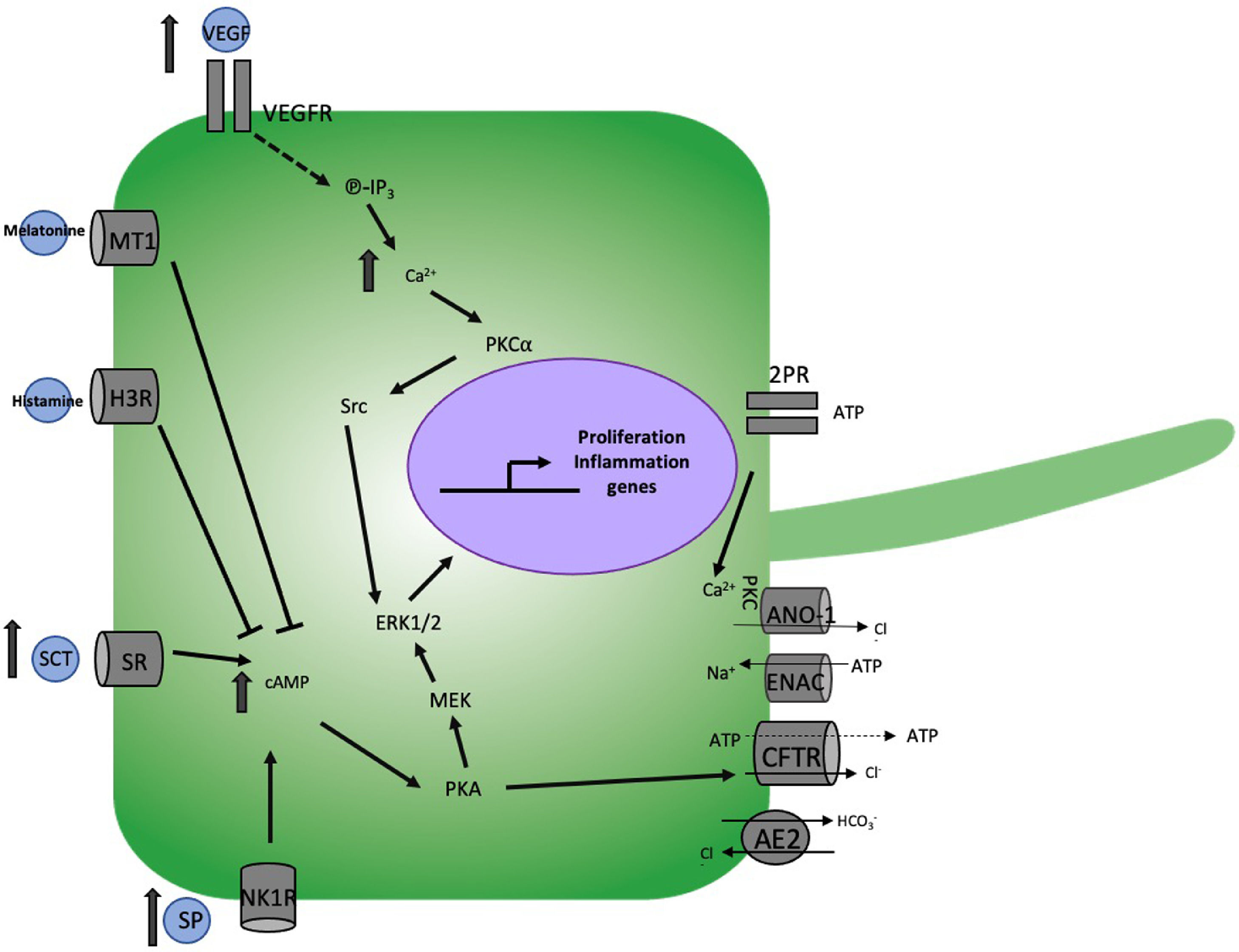
6Obstructive cholestasis6.1The role of secretin and its receptorSecretin is a gastrointestinal hormone secreted by S cells in the duodenum during the postprandial period [37, 38]. Secretin carries its effects out by the binding and activation of its receptor (SR) in the cholangiocytes, stimulating the intracellular increment of cAMP [24] inducing, as a consequence, the activation of protein kinase A (PKA) [39]. Once active, PKA phosphorylates the CFTR causing a conformational change permitting its activation and Cl− release [40], which leads to the activation of AE2 [37, 41, 42], stimulating the secretion of bicarbonate (HCO3−) into the bile and generating the so-called "bicarbonate umbrella" (Fig. 2) [2].
Mechanism of cholangiocellular damage in obstructive cholestasis. The bile duct ligation (BDL) model induces the increase of Secretin (SCT) and substance P (SP) which increase intracellular levels of cAMP activating protein kinase A (PKA) and this, on the one hand cystic fibrosis transmembrane conductance regulator (CFTR) to regulates diverse apical membrane activities such as Na+ channels, ATP release mechanisms, bile flow by Cl−/HCO3− exchangers, Ca2+ activated Cl− and on the other hand the activation of MEK and ERK1/2 resulting in cell proliferation. Similarly, BDL increases to VEGF which activates IP3, which induces an increase in Ca2+ that activates PCK⍺ and this to Src and finally ERK1/2. The binding of melatonin and histamine with their MT1 and H2K receptors respectively induces a decrease in cAMP, preventing ductular hyperplasia.
The SCT/SR axis has been associated with cholestatic disease since in rats subjected to BDL, the expression of SR increases significantly [43]; even more, this increment also favors fibrosis development [44]. Similarly, in BDL, the increase in SR promoted large cholangiocytes' proliferation [43, 45]. This was corroborated in SCT−/− mice where hyperplasia decreased after BDL [46], proliferation, as well as levels of cAMP, proliferating cell nuclear antigen (PCNA), and activation of ERK1/2 [45].
Additionally, in a mouse model of PSC (multidrug resistance 2, Mdr2−/−) with BDL, the increase of the SCT/SR axis induced the activation of stellar cells and liver fibrosis. In the same model, the use of SR antagonist (Section 5-27) decreases liver fibrosis and hyperplasia [44]. This shows that the SCT/SR axis plays a fundamental role in the proliferative regulation of large cholangiocytes in the obstructive cholestatic process.
6.2Bile acids and loss of the bile acid-activated receptorAs we already know, bile acids are essential for the processes of digestion, absorption, lipid metabolism, cell proliferation, among others [47]. Due to their signaling and detergent properties, the increase in their levels can translate into cell death processes, either due to their accumulation within hepatocytes or cholangiocytes due to their filtration caused by lesions in the bile ducts [48]. El grado de daño que los acidos Biliares pueden causar depende de su tipo. The degree of damage that bile acids can cause depends on their type and is characterized by the degree of hydrophobicity of bile acids, having an order from the most hydrophobic to the most hydrophilic to lithocholic > deoxycholic > chenodeoxycholic > cholic > ursodeoxycholic acids. Therefore, the more hydrophobic it is, the greater the toxicity generated [47].
For example, in in vitro studies, taurocholic and taurolithocholic acid induce the proliferation of large cholangiocytes, the expression of SR, increased cAMP levels, and the activity of AE2. Therefore, increasing these can result in hyperplasia and increased biliary secretion of cholangiocytes, this being dangerous in scenarios with already established cholangiocellular damage such as BDL [49].
However, an imbalance of them damages the hepatic epithelia, as well as the dysregulation of molecules that depend on them, such as the case of G protein-coupled bile acid receptor 1 (GPBAR1) or better known as TGR5, a specific bile acids receptor, which after being activated raises intracellular levels of cAMP [50], controlling bile Cl− secretion, gallbladder filling and cholangiocellular proliferation [51].
Hepatocytes and cholangiocytes express this receptor to a greater degree in bile cells. The cholangiocyte TGR5 localizes in the primary biliary cilium [52] in both types of cholangiocytes [53, 54].
The importance of this receptor is more evident in TGR5 Knockout (KO) mice, where BDL and a bile acids-enriched diet aggravate the cholestatic damage [55] since they induce the appearance of bile infarcts [56], the damage being greater in both types of ducts (large and small) when the diet is supplemented with hydrophobic bile acids such as lithocholic [55] as bile acids stimulate hyperplasia in cholangiocytes [50]. However, other studies indicate that the loss of TGR5 reduces the hyperplasia of the bile ducts, ensuring that the receptor is necessary for the proliferation of cholangiocytes mediated bile acids or for cholestasis [57]. Cholangiocellular proliferation induced by TGR5 and lithocholic acid has been reported to occur due to increased ROS and activation of ERK 1/2 [58].
TGR5 protects the biliary epithelium in a BDL model by regulating paracellular permeability through tight junctions (TJ). Therefore, the activation of TGR5 leads to the stabilization of junctional adhesion molecule A (JAM-A) through protein kinase C-ζ (PKCζ), this decreases the permeability of TJs, protecting the liver from the loss of bile acids; thus, TGR5 is essential for bile acid-mediated liver protection [51].
However, it has been observed that the presence of this receptor in macrophages enhances inflammation previously induced by the accumulation of bile salts or by the presence of LPS [59].
6.3MelatoninMelatonin is a hormone synthesized by arylalkylamine N-acetyltransferase (AANAT) [60] in the pineal gland, secreted mainly at night. Melatonin regulates various antioxidant defense processes, functioning as a reactive oxygen species (ROS) scavenger, and preventing lipid oxidation and the immune response, among others [61]. Administration of melatonin decreases serum cytokine levels and fibrosis in rats with BDL [62].
Melatonin has protective effects in cholestatic models such as BDL and ANIT administration [17]. Studies showed that melatonin binding to the melatonin receptor (MT1) reduced cAMP levels and proliferation of large cholangiocytes in BDL rats [63]. Another study demonstrated that the absence of AANAT increases bile duct hyperplasia in BDL, and in in vitro studies, its overexpression in cholangiocytes decreases proliferation [64]. Therefore, the presence of AANAT produces melatonin to induce proliferation.
6.4GastrinGastrin is a hormone that regulates gastric acid secretion through the CCK2 receptor [65]. Administration of gastrin to BDL-purified rat cholangiocytes decreases bicarbonate-rich choleresis and secretin-stimulated cAMP levels. Gastrin induces the expression of PKCα, β1, and β2 by inhibiting cAMP, which is related to proliferation, secretion, and apoptosis effects [66]. Proliferating cholangiocytes may adopt a neuroendocrine phenotype in extrahepatic cholestasis [12] since cholangiocytes express dopamine receptors and GABA that increase the levels of IP3 and Ca2+, which decreases the secretion induced by secretin by the inhibition of cAMP and Cl− release [35]. Besides, the decrease in proliferation and ductular secretion due to the effect of gastrin was associated with an increase in apoptosis [66]
6.5HistamineHistamine is an amine synthesized by mast cells that accumulate within cells in vesicles and is released under-stimulation. Histamine carries out its effects by binding to G protein-coupled histamine receptors 1 to 4 (H1R-H4R) [67, 68]. It affects the immune response, muscle contraction, vasodilation, mucus secretion, tachycardia, arrhythmias, etc. [69].
During the chronic cholestatic process, as in PBC, the number of mast cells in the liver increases, and with this, the histamine production [70] regulating cholangiocyte hyperplasia and fibrosis [17].
Considering the stimulatory and inhibitory effects of H1R-H2R and H3R-H4R, respectively, Dr. Francis Heather demonstrated in the BDL model how H3R prevents the proliferation of large cholangiocytes by decreasing the levels of cAMP already turn off the activation of PKA/ERK1/2/Elk-1 [71]. However, H2R stimulated the growth of large cholangiocytes through cAMP and PKA [26]. On the other hand, H1R could induce the proliferation of small cholangiocytes by increasing IP3 and CA2+ levels, which led to phosphorylation of CaMKI and increased cAMP-response element binding protein (CREB) activity [33], suggesting that histamine plays a vital role in biliary repopulation during the obstructive cholestatic process.
6.6Substance PSubstance P (SP) is a neuropeptide proteolytic product of tachykinin (Tac1). SP is expressed in endothelial, inflammatory, immune, and neuronal cells [72]. SP binds to the neurokinin-1 receptor (NK-1R) that regulates the connections between the nervous system and peripheral organs [73].
SP increases in experimental animals with chronic cholestatic diseases and in patients with liver diseases and cholangiopathies. Therefore the SP/NK-1R junction has been linked to chronic liver diseases [74].
In the extrahepatic obstructive cholestasis outstanding model (BDL) in mice, the expression of NK-1R increased in the bile ducts and large cholangiocytes, increasing their proliferation through cAMP-dependent PKA [75]; also, the administration of SP in sham mice induced fibrosis and hyperplasia [76]. In NK-1R−/− mice, the opposite was observed, a decrease in ductular hyperplasia and cell proliferation [75]. Similarly, in the Mdr2−/− model with BDL, the administration of an NK-1R antagonist (L-733,060) decreased the presence of fibrosis [76]. Clarifying that the increase in SP aggravates the cholestatic disease.
6.7Vascular endothelial growth factorVascular endothelial growth factor (VEGF) regulates vasodilation, migration, permeability, proliferation, and survival in endothelial cells; however, cholangiocytes secrete this factor, and at the same time, they express type 2 and 3 receptors (VEGFR-2 and VEGFR- 3), increased in BDL-induced obstructive cholestasis. This increment favors cholangiocyte proliferation through the IP3/ Ca2+/PKCα/Src/ERK1/2 [77]. Another study in BDL showed how biliary obstruction directly affects PBVP (due to its proximity to large bile ducts), leading to a decrease in VEGF secretion, which increases apoptosis with a reduction in bile growth and ductular secretion. Otherwise, BDL animals treated with VEGF-A (which binds to VEGFR-2) preserve the integrity of the PBVP [78]. The increase in the expression of VEGF-A and C and VEGFR-2 and -3 was related with the protection of the bile ducts in a caffeic acid model under taurocholic administration bile acid [79]. With this, we can realize that VEGF is an essential factor in the balance of proliferation and apoptosis in diseases of the biliary epithelium.
6.8SerotoninSerotonin is a neuroendocrine hormone that is also secreted by cholangiocytes. This molecule is associated with the regulation of the bile ducts' growth, precisely due to the expression of serotonin receptors 1A and 1B, which, when activated, decrease the bile duct's growth and functionality during cholestasis.
Lack of serotonin in proliferating large cholangiocytes associates with the inhibition of cAMP/PKA/Src/ERK1/2 pathway, favoring the activation of the IP3/Ca2+/PKC pathway and with it the reduction of proliferation, where there is a kind of feedback, in which the excessive growth of bile ducts is controlled through the release of serotonin [80]. Besides, serotonin is the cause of pruritus in patients with PBC [12].
6.9Glucagon-like peptide-1Glucagon-like peptide-1 (GLP-1) is a hormone secreted by enteroendocrine L cells; this molecule binds to the G-protein coupled receptor (GLP1R) and regulates glucose homeostasis. GPL-1 regulates the cholangiocellular response in BDL-induced obstructive cholestasis [81]. The expression of this hormone increases after BDL. In rats with BDL, an increase in cholangiocytes' proliferation was observed after the administration of exedin-4, a GLP-1 agonist. These rats expressed preproglucagon, suggesting the importance of GLP-1 in bile growth in cholestasis. GLP-1 carries out its effects by activating PI3, cAMP/PKA, and Ca2+ CaMKII [81]. Exedin-4 also showed protective effects against apoptosis after administration with CCl4.
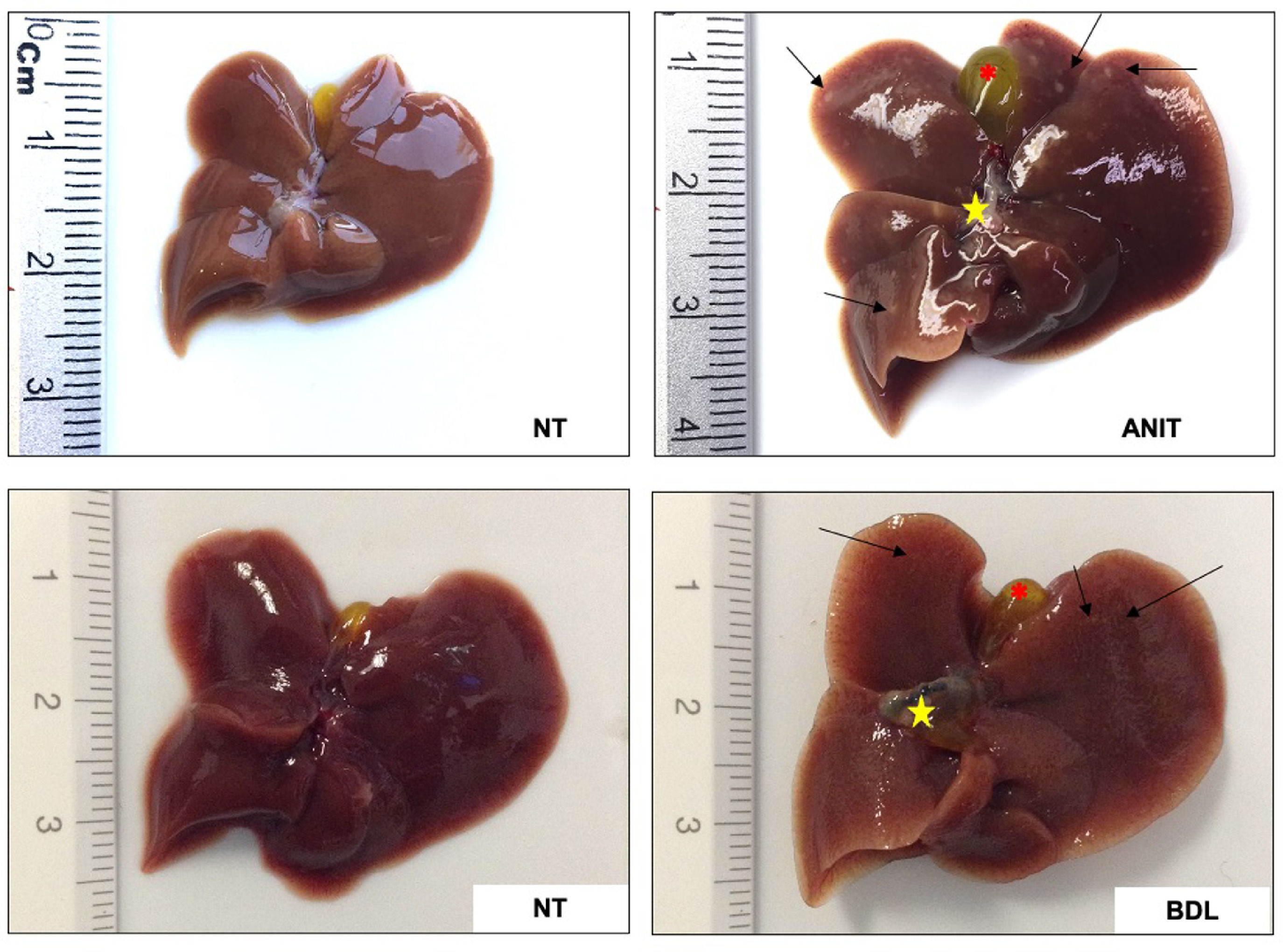
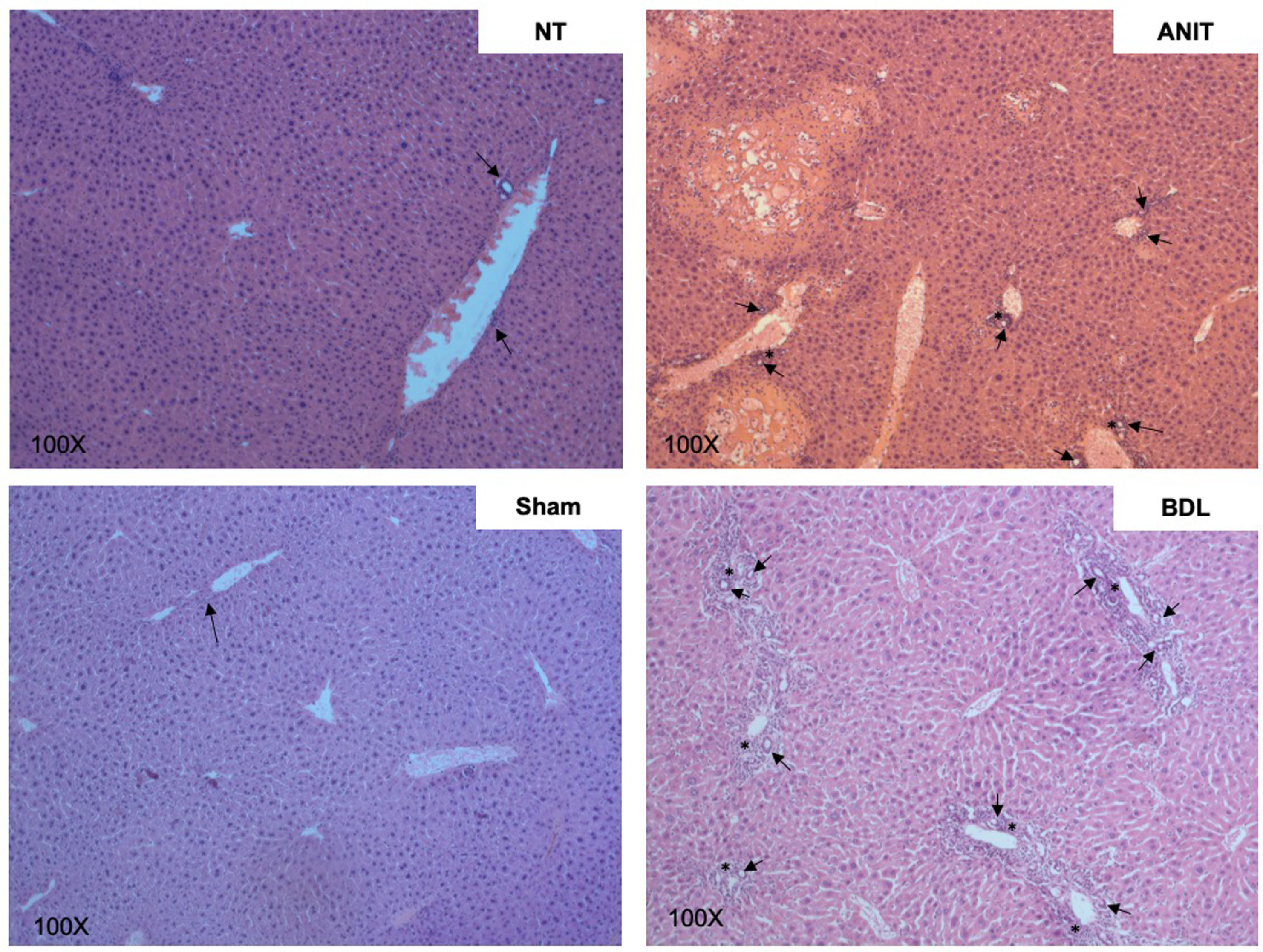
7Functional cholestasis models7.1α-naphthylisothiocyanateANIT is one of the main drug-induced cholestasis models. This is a versatile model because it allows studying the mechanisms of both obstructive and functional cholestasis, depending on the dose and time of exposure, where the cholestatic damage is established 36 h after ANIT administration and inducing cholestatic processes such as delocalization of ATP binding cassette (ABC) transporters and at late times >72 h biliary lesions, even obstructive phenomena, are presented with liver lesions (arrows), hepatomegaly, gallbladder, and common bile duct swelling and dark coloring (stars) [4, 82], similar to what happens during bile duct ligation (Fig. 3).
The administration of this hepatotoxic compound induces apoptosis on large and small cholangiocytes, and as a consequence, the proliferation of healthy cholangiocytes.
ANIT also induces profound hepatocellular damage, being the oxidative injury due to the generation of ROS (Fig. 4) [36], the main harmful mechanism. As we can see in Fig. 5, where ANIT treatment generates severe liver tissue damage, characterized by numerous and extensive necrotic areas, ductular hyperproliferation (arrows), and a significant increase in the inflammatory infiltrate that surrounds the bile ducts (asterisk). Although the amount of inflammatory infiltrate is less than that observed in BDL (a very aggressive obstructive model), it is still highly relevant in the inflammatory process and the generation of ROS, fibrosis, and other bile flow functions.
Cholangiocellular damage induced by chemical (ANIT) and obstructive (BDL) cholestatic models. Ductular hyperproliferation (arrows) and inflammatory infiltrate (asterisk) around bile ducts. Bile duct ligation (BDL) model generates a more remarkable ductular proliferation surrounded by inflammation than α-naphthylisothiocyanate (ANIT) model. 100x original magnification.
The proliferation of small cholangiocytes induced by ANIT (in animals with partial hepatectomy), as in other models, associates with the de novo synthesis of SR and cAMP activation [83].
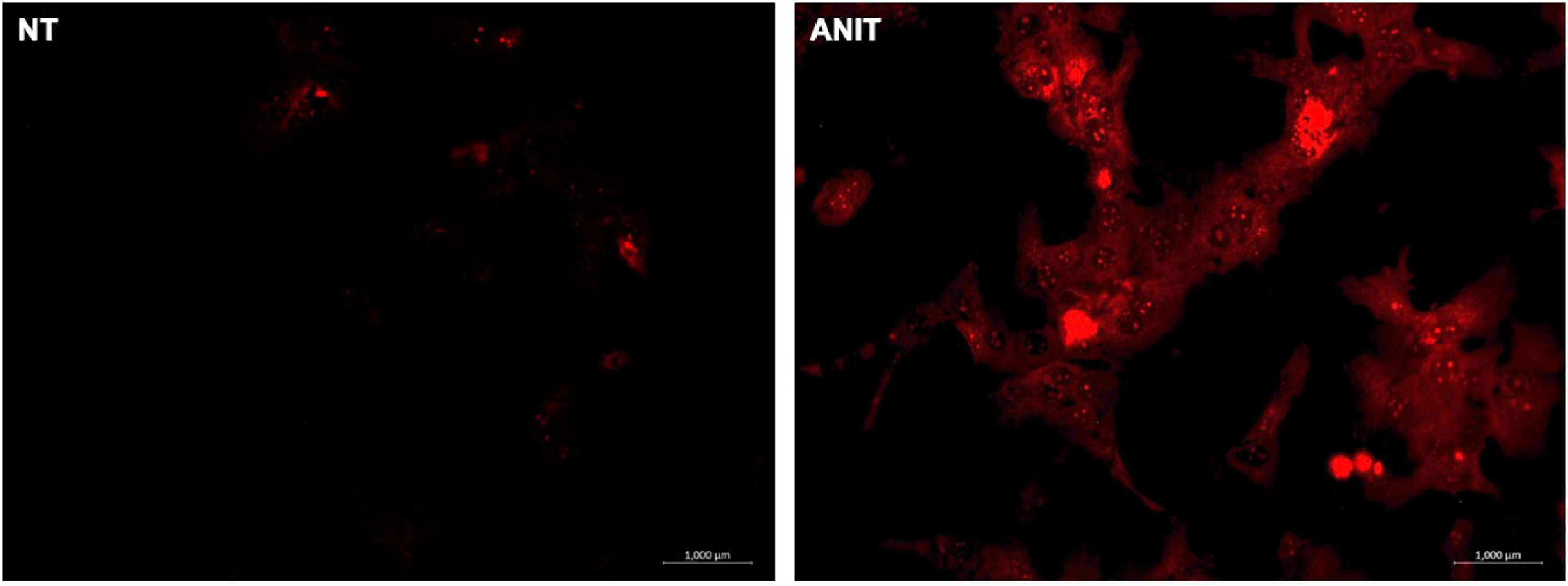
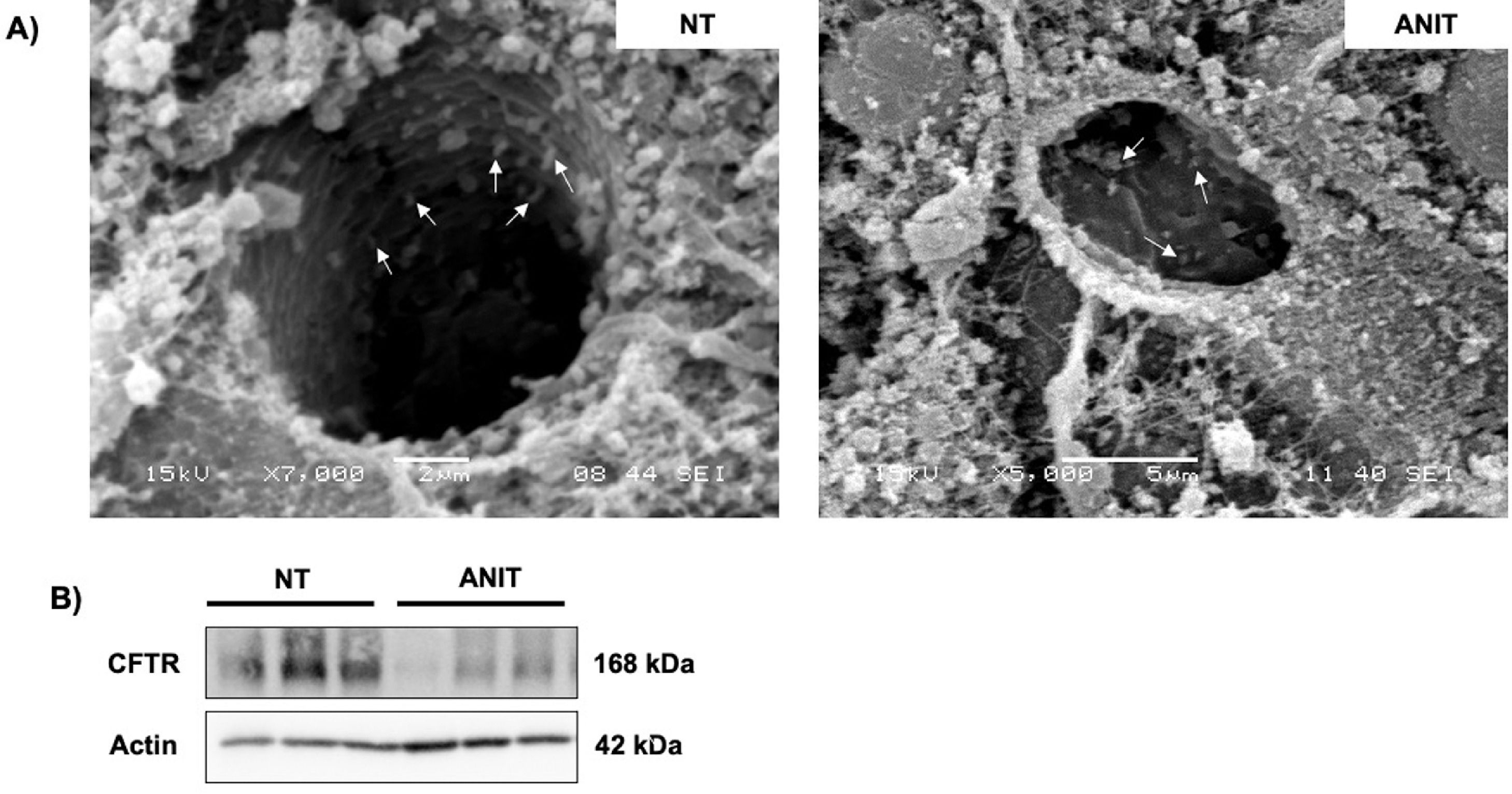
Recently we reported that ANIT decreased the content of CFTR in cholangiocytes, in addition to the effect of shortening or absence of primary cilia (Fig. 6A-B) [4]. These findings explain the strong inflammatory response generated by ANIT and the impact of CFTR in the presence of pro-inflammatory cytokines [40, 84] due to increased ROS production. The effect of ANIT on CFTR go beyond its decrease since this contributes to the loss of the Cl−/HCO3− exchange, favoring the obstructive process and the formation of the "bicarbonate umbrella" [85].
Cholangiocyte changes induced by α-naphthylisothiocyanate (ANIT)
A) shortening or absence of primary cilia in liver tissue from animals treated with ANIT, scanning electron micrograph from livers showing cilia from bile canaliculi (arrows). B) Cystic fibrosis transmembrane conductance regulator (CFTR) is downregulated in cholestasis damage.
As we previously stated, the nature of bile acids could be an advantage in the treatment of some diseases; such is the case of ursodeoxycholic acid (UDCA, the only one approved for use in patients with PBC), a hydrophilic bile acid that displaces the pool of toxic bile acids (responsible for cholangiocellular damage) [86]. UDCA has been shown to have an effect too on immunosuppressive effects, maintenance in the expression and localization of ABC transporters, especially canalicular with the subsequent effect choleretic [87].
In Abcb4−/− mice, UDCA improved damage to cholangiocytes due to decreased ductular proliferation, portal, and ductular inflammation by Ca2+ and PKCα-dependent mechanisms [88].
In recent years, UDCA's effect has been observed in the mouse ezrin-knockout (Vil2kd/kd) model. Ezrin is a cytoskeletal protein expressed in intrahepatic bile ducts, which interacts with transporters, scaffold proteins, and the actin cytoskeleton. Ezrin regulates the location and activity of CFTR. However, in the ezrin-KO model, CFTR, AE2, and AQP1 were deregulated since when they are dysfunctional, the formation of the "bicarbonate umbrella" is impossible [86, 89]. UDCA administration (intraperitoneally) reduces periductular fibrosis, the proliferation of cholangiocytes, and the decrease of inflammatory and profibrotic genes in this model. However, prolonged administration of UDCA or administered in the diet generated harmful effects associated with liver dysfunction and hepatocellular carcinoma since it inhibits DNA repair mechanisms [86]; Which would suggest its use only in the early stages of obstructive cholangiopathies. In this sense, we can realize that UDCA's effects are differential depending on the dose, time, administration via, and pathology and why its use remains controversial.
7.3Cystic fibrosis transmembrane conductance regulatorCFTR is a cAMP-dependent Cl− channel found in secretory ductular epithelial membrane. Mutations in the gene of this protein lead to the development of CF [84]. As we have seen during this work, cholestatic alterations affect the activity of this protein, just as the absence of CFTR promotes the cholestatic process.
One of the proposed mechanisms of this protein was determined in CFTR-KO mice and exposed to lipopolysaccharide (LPS), where there was an increase in nuclear factor κB (NF-κB) through the activation of Toll-like receptor 4 (TLR4) and the secretion of more TLR4-dependent pro-inflammatory cytokines [90] as monocyte chemoattractant protein 1 (MCP-1) and interleukin-8 (IL-8) compared to control animals. This explains because the lack of CFTR induces the actin cytoskeleton rupture, causing Src activation, which activates TLR4 and this, in turn, NF-κB [84].
The use of induced pluripotent stem cells (iPSCs) derived from healthy or patients with mutated CFTR (ΔF508) by Fiorotto et al. demonstrated that differentiate human iPSCs into monolayer polarized cholangiocytes acquired the secretory functions of the biliary epithelium with high efficiency, without losing these characteristics with the culture passages. It is worth highlighting the importance of this model to study differentiation mechanisms and damage and repair in various cholangiopathies. PP2 (an inhibitor of Src) treatment on these polarized cholangiocytes induced the reversion of inflammation and aberrant cytoskeleton structures; besides that, the administration of CFTR potentiators in combination with PP2 reestablished fluid secretion at normal levels in cholangiocytes derived from ΔF508 iPSCs [40]. These works clarify the role in the regulation of innate immunity of cholangiocytes through CFTR [90]. With this, the importance of CFTR in developing cholestatic processes in cholangiopathies and some pathologies typical of hepatocytes is straightforward (Fig. 7).
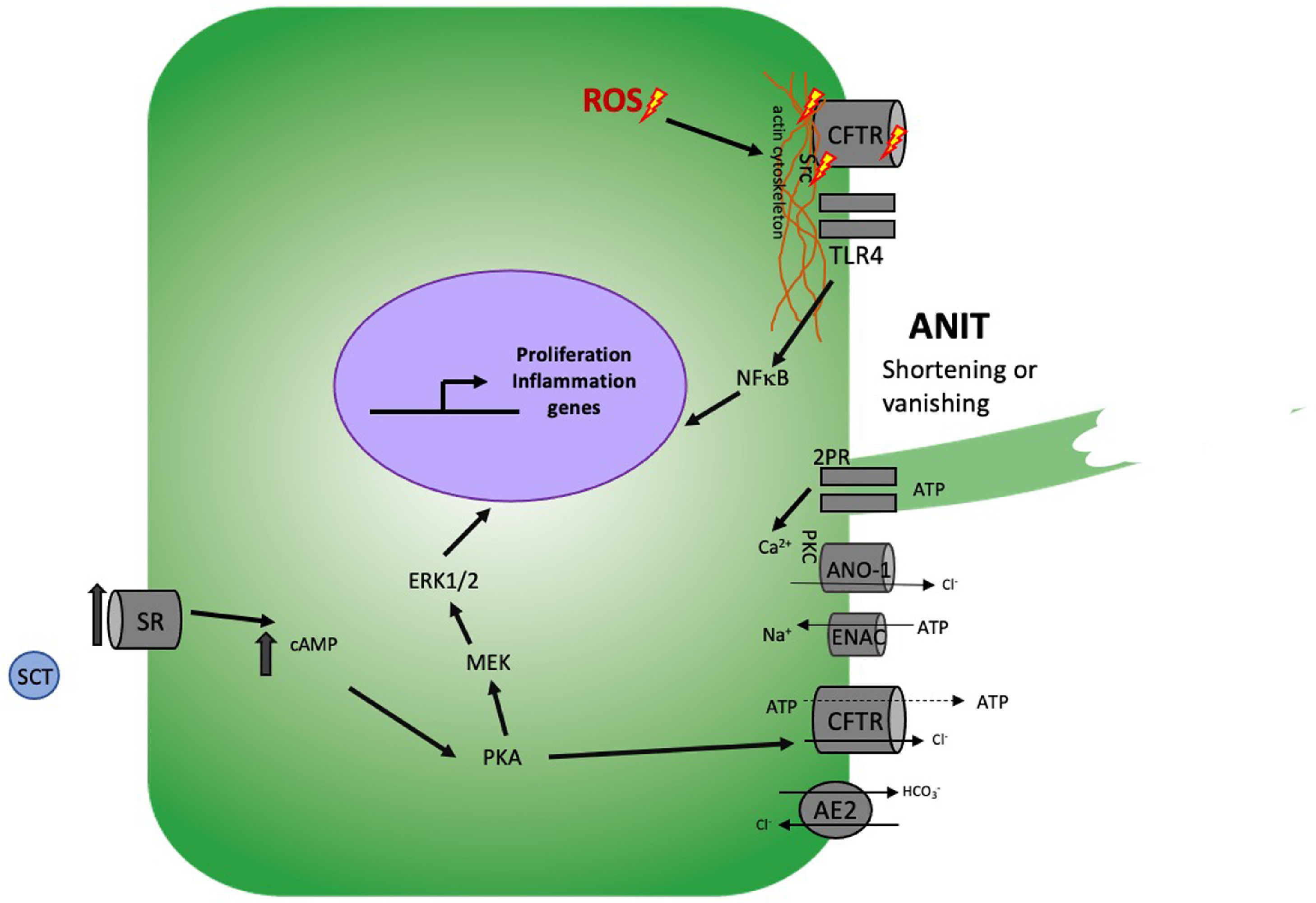
Cholangiocellular damage mechanisms induced by functional cholestasis
Under conditions of damage induced by ANIT, CFTR levels decrease, as well as the ROS produced induce rearrangement of the actin cytoskeleton, inducing the imbalance between the processes of secretion and absorption of Cl−, HCO3−, Na+ and release of ATP aggravating this damage. This induces the release of Src and at the same time the activation of TLR4 and this in turn to NF-κB causing the release of pro-inflammatory cytokines. On the other hand, treatment with ANIT induces SR overexpression, leading to sustained proliferation and exhausting the secretory function of CFTR.
Finally, we will review some cholangiocellular damage mechanisms that can induce the development of cholangiocarcinoma (CCA). CCA results from the malignant transformation of biliary epithelial cells, that is, cholangiocytes. This type of cancer can develop in intra- or extrahepatic bile ducts [91, 92]. It comprises a series of alterations that generate an activation and inhibition imbalance between oncogenes and tumor suppressor genes, respectively [61]. Given its aggressiveness, the deficiency in early diagnosis protocols, and the limited therapeutic options, this type of cancer occurs more frequently. The increasing mortality levels position it as one of the most critical clinical emergencies [91, 92].
As previously mentioned, persistent aggressions on the biliary epithelium and sustained inflammation, whether in the small or large ducts intra or extrahepatic, can culminate in developing cancer. For this reason, we will review some of the mechanisms that lead to this disease.
8.1Bile acids and their receptorsAs we have already seen, the exhausted increase in bile acids during cholestasis can generate carcinogenic processes due to cell proliferation. For example, deoxycholic acid (DA) induces cell survival by increasing Mcl-1 (an anti-apoptotic protein) [93].
Bile acid overload activates epidermal growth factor receptor (EGFR) [94], which increases the release of cyclooxygenase-2 (COX2), which regulates the production of inflammatory molecules such as prostaglandins [95]. The increase in COX2 induces the activation of the p38 and ERK1/2 pathways, resulting in cholangiocellular proliferation. EGFR, COX2, and MAPKs increase in samples from patients with CCA and even with PSC [94, 96].
Another proposed pathway for the development of CCA is through Sphingosine-1-Phosphate Receptor 2 (S1PR2), through which the ERK1/2, NFκB and Akt pathways are activated, which again leads to an increase in COX2, favoring cell survival and migration [93]. Activation of ERK1/2 explains through a study that verified that TGR5 through the administration of cholic acid (CA) induced cell growth through the generation of ROS and the subsequent activation of Src/EGFR/ERK1/2. Increased survival is directly associated with cancer progression [97].
8.2Inflammation and Interleukin-6The SOCS3 protein negatively regulates the IL-6 signaling pathway, which eventually activates STAT3/Mcl1, preventing cell death. However, in CCA, epigenetic changes are repressed, causing apoptosis to be interrupted in a sustained way [93]. Furthermore, p21 decrement favors mitosis due to the activation of p38 by IL-6 [98]. As we can see, inflammatory processes characterized by IL-6, such as cholangiopathies, present the risk of developing CCA through the IL-6/STAT3 pathway.
8.3Primary cilium defectsHistone deacetylase 6 (HDAC6) overexpression in cholangiocytes associates with the loss of the primary cilium. In CCA, the primary cilia are reduced and HDAC6 is overexpressed. Treatment of CCA cell line with HDAC6 pharmacologic inhibitor tubastatin-A decreased cell proliferation and anchor-independent cell growth.
Therefore, as we stated above, cholestasis could cause the disappearance of the primary cilia of the cholangiocytes; this induces proliferation regardless of the anchorage, which favors cell migration and generates the activation of the Hedgehog (Hh) and MAPK pathways [99, 100].
8.4HistamineBy the last, histamine, through its H4HR receptor, has been shown to affect the progression of CCA, since by inhibiting it, the epithelium-mesenchyme transition (EMT) and the degradation of the extracellular matrix (ECM) are interrupted. During this process, the stem cell factor receptor (SCF)/c-Kit is related as observed in tumors of mice treated with cromolyn sodium, where mesenchymal markers such as vimentin or paxillin decreased, as well as the expression of epithelial markers such as E-cadherin increased significantly compared to tumors of control animals. The inhibition of the expression of c-Kit (that binds with SCF) also decreased the expression of ECM degradation proteins such as MMP-9 and MMP-2. [101].
9ConclusionNowadays, numerous studies address the mechanisms of biliary epithelium damage during the cholestatic process and chronic cholestatic diseases, including the mechanisms that lead to cholangiocarcinoma development and progression. There are still many unsolved questions; however, with this review, we can realize the critical role of cAMP, and the activation of PKA, which, as we figure out, are determining factors in the damage and repair processes, mainly during obstructive cholestasis. In contrast, during functional cholestasis, they are orchestrated by the presence of both cAMP and Ca2+-dependent PKC, probably due to the ability of small cholangiocytes to adopt characteristics of large cholangiocytes, which suggests giving particular attention to the regulation of these two key molecules for the development of cholangiocellular damage, thus, with support for what already studied, it will be possible to offer better therapeutic options for these diseases as well as their early diagnosis.
Author contributionsAll authors made substantial contributions to all of the following: SSS, ASN and LEGQ conceptualization, data curation, formal analysis and investigation, SSS, ASN, LCR, MCGR, LBO and LEGQ writing-review and editing, SSS and LEGQ supervision and validation.
This work was partially funded by a grant from the Consejo Nacional de Ciencia y Tecnología (CONACYT), Mexico (CB-A1-S-38154, CB-252942, Infra-2017 280788, Infra-2013 205941), “Estímulo Antonio Ariza Cañadilla 2017” from FUNDHEPA, Mexico. Universidad Autónoma Metropolitana Iztapalapa, Mexico.