Background. Anti-tuberculosis drug-induced hepatotoxicity (ATDH) is one of the most prevalent and serious adverse drug reactions in the course of anti-tuberculosis (TB) treatment. Some researchers suggested that determination of N-acetyltransferase 2 (NAT2) genotype may be clinically useful to identify patients at high risk of developing ATDH. Aim. To evaluate whether the NAT2 genotype could be as a predictor for ATDH in Chinese community TB population.
Material and methods. A total of 4304 community-based TB patients were followed up six to nine months prospectively. A nested case-control study was designed. Each ATDH case was 1:4 matched with controls by age (within 5 years old), gender, treatment history, disease severity and drug dosage. The polymorphisms of NAT2 were determined using polymerase chain reaction with restriction fragment length polymorphism. Conditional Logistic regression model was used to calculate odds ratio (OR) and 95% confidence interval (CI), as well as corresponding P-values.
Results. A total of 89 ATDH cases and 356 controls were included in this study. Allele frequency of NAT2*5, NAT2*6 and NAT2*7 in cases and controls were 4.5 and 3.2%, 25.3 and 26.5%, and 13.5 and 13.5%, respectively. Frequencies of genotypes and alleles of NAT2*5, NAT2*6 and NAT2*7 did not differ significantly between cases and controls. The OR of intermediate acetylator and slow acetylator compared with rapid acetylator was 1.040 (95%CI 0.616-1.758) and 0.990 (95%CI 0.509-1.925), respectively. The NAT2 haplotype distribution in cases was similar to controls. Conclusions. In conclusion, we did not find significant association between NAT2 genotype and ATDH in community-based Chinese population. It may be deficient to take NAT2 genotype as a predictor for ATDH in Chinese community TB patients.
Adverse drug reactions (ADRs) in the course of anti-tuberculosis treatment are great challenges for tuberculosis (TB) control. ADRs would increase the patient suffering and socioeconomic burden associated with TB. Meanwhile, ADRs may cause incompliance and eventually contributing to the treatment duration extending, drug resistance and treatment failure.1,2 Anti-tuberculosis drug-induced hepatotoxicity (ATDH) is one of the most prevalent and serious ADRs in the course of anti-tuberculosis treatment in China and many other regions.3-5
Among the first line anti-tuberculosis drugs, isoniazid (INH) is mainly responsible for the occurrence of ATDH. The predominant metabolic pathway of INH is acetylation by the hepatic enzyme N-ace tyltransferase 2 (NAT2).4 Hydrazine is the major hepatotoxic metabolite of INH and its quantity is related to the acetylation rate of N-acetyltransfe-rase 2.6 The polymorphisms of NAT2 gene can cause changes in the structure of NAT2 and so are associated with rapid, intermediate, and slow acetylation rate.7 The presence of any two mutant alleles of NAT2 defines the slow acetylator (SA) genotype, whereas intermediate (IA) and rapid acetylator (RA) have one and two wild alleles (NAT2*4) respectively.8 The association between the polymorphisms of NAT2 and ATDH had been studied in different Asian populations.9-21 Except three studies in Indian,9-11 most of recent Asian studies including those in Chinese found that slow acetylators were more susceptible to developing ATDH and some researchers suggested that determination of NAT2 genotype may be clinically useful to identify patients at high risk of developing ATDH.12-21
Pharmacogenetic testing has been more and more applied to identify and exclude individuals with a certain genetic makeup for individual treatment in clinical practice.22-24 It will be quite valuable if the clinicians could apply NAT2 genotype testing as useful new biomarkers for predicting ATDH prior to their administration. However, most of the related previous Asian studies were hospital-based12-19,21 and it was reported that only about 8.6% ATDH patients required a hospital stay in China.25 A community-based cohort of 4,304 Chinese sputum smear positive TB patients who received short-course chemotherapy had been set up by our research group.26 Before the generalization of NAT2 genotype testing prior to TB treatment in Chinese TB patients, it is necessary and meaningful to confirm the relationship between NAT2 genotype and ATDH in our cohort.
In view of NAT2*5 (C481T, rs1799929), NAT2*6 (G590A, rs1799930) and NAT2*7 (G857A, rs1799931) mutations accounted for virtually all of the slow acetylator alleles in Asian,27 the goal of the present study was to evaluate the roles of NAT2*5, NAT2*6 and NAT2*7 in the developing of ATDH basing on our community-based 4304 TB patients cohort.26
Material and MethodsSubject sourceFrom October 2007 to June 2008, a total of 4,488 sputum smear positive pulmonary TB patients who received standard short-course chemotherapy recommended by WHO were recruited from 52 counties within four regions of China by a national-level cohort study entitled Anti-tuberculosis Drugs Induced Adverse Reactions in China National Tuberculosis Prevention and Control Scheme Study (ADACS).26 All primary/retreatment patients took INH (600 mg), rifampicin (RIF) (600 mg, or 450 mg if body weight was < 50 kg), pyrazinamide (PZA) (2,000 mg), and ethambutol (EMB) (1,250 mg) every other day in the first two months and then INH and RIF were continued for another four/six months. The retreatment patients meanwhile received streptomycin (SM) (750 mg) every other day in the first two months and continued receiving EMB for another six months. When patients developed some ADRs (such as ATDH, gastrointestinal reaction, allergic reaction, nervous system disorder, arthralgia), their treatment would be adjusted according to the severity of ADRs. A total of 4,304 patients ultimately finished six to nine months follow-up. The ADACS was approved by the Ethics Committee of Peking University Health Science Center. Written informed consent was obtained from every participant before enrolment.
Patient monitoringBefore anti-tuberculosis therapy, serum hepatitis B virus surface antigen, serum alanine transaminase (ALT), aspartate amino transaminase (AST), direct and total bilirubin levels, renal function, blood and urine routine test were measured. Serum ALT, AST, and total bilirubin levels would be measured again within two months after anti-tuberculosis treatment beginning or whenever patients had symptoms of suspected hepatitis (such as anorexia, nausea, vomiting, malaise, tea-colored urine). Patients would be required to fill in their ADACS calendars to record their drug usages and discomfort symptoms. The information, including gender, age, weight, height, TB treatment history, disease history, co-mediation, anti-tuberculosis treatment induced ADRs, was interviewed and recorded with a set of questionnaires. The causality between ADRs and anti-tuberculosis drugs was assessed basing on the WHO Uppsala Monitoring Center system.26,28 The causality between ATDH and anti-tuberculosis drugs in this study was reevaluated basing on the Council for the International Organization of Medical Sciences (CIOMS) scale,30 which was a liver specific, structured, and quantitative causality assessment method. The patients, nutritional status were expressed using body mass index.
Cases and controls selectionATDH was designated as:
- •
An increase of over two times the upper limit of normal value (ULN) in ALT or a combined increase in AST and total bilirubin provided one of them is above two times ULN.29 In this study, the ULN of ALT, AST and total bilirubin were 40 U/L, 40 U/L and 19 umol/L, respectively.
- •
Causality assessment result was highly probable, probable or possible basing on the CIOMS scale.30
All suspected ATDH patients were then strictly reviewed and assessed by experts. Once diagnosed with ATDH, the patient would be assigned to the case group. Incidence density sampling method was adopted to select controls from patients free of ADRs up to the date when the paired cases were diagnosed with ATDH. For each ATDH case, four controls were randomly selected and matched with age (within five years old), sex, treatment history, disease severity and drug dosage.
Patients with any of the following conditions were excluded from the present study:
- •
Positive serum hepatitis B virus surface antigen or other liver disease.
- •
Potentially hepatotoxic medications that would confound the picture.
- •
Abnormal serum ALT, AST or total bilirubin levels before anti-tuberculosis treatment.
One drop of each patient's intravenous blood was saved on Whatman FTA® card (WB120210, Whatman International Ltd, Maidstone, UK) at the ADACS study beginning.26 According to Whatman FTA Protocol BD01, one 1.2 mm disk was punched out from each FTA blood card sample, and then washed with FTA purification reagent (WB120204, Whatman International Ltd, Maidstone, UK) three times, following Tris-Ethylene Diamine Tetraacetic Acid buffer two times. The washed disks were air dried and subjected directly to polymerase chain reaction (PCR) amplification.
PCR-restriction fragment length polymorphism (PCR-RFLP) was used to genotype NAT2. The primers were 5,-GCCTCAGGTGCC TTGCATTT-3,and 5,-CGTGAGGGTAGAGAGGATAT-3, as previously reported.31 PCR was performed in a volume of 40 uL according to the following protocol: initial denaturation at 95 oC for 5 min; 40 cycles of denaturation at 95 oC for 30 s, annealing at 55 oC for 30 s, and extension at 72 oC for 45 s; and a final extension at 72 oC for 10 min. Subsequently, the PCR product of 535 bps was cut with 3 different restriction enzymes: KpnI, TaqI, and BamHI respectively. A loss of a KpnI restriction site denotes NAT2*5, a TaqI res-triction site denotes NAT2*6, and a BamHI restriction site denotes NAT2*7. To ensure the quality, genotyping was performed by blinding the case or control status and with a positive control of a DNA sample with a known heterozygous genotype in each test. The genotyping results were judged by two researchers independently. At last, 5% of the samples were randomly selected for sequencing to confirm the validation of PCR-RFLP results.
Statistical analysisValues were expressed as median and interquartile range (IQR) or as numbers and percentages. Continuous variables were compared between cases and controls using two-factor analysis of variance test or non-parametric test. Chi-square test was used to compare the categorical variables and test the Hardy-Weinberg equilibrium. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated using conditional logistic regression. A two-tailed P-value < 0.05 was considered statistically significant. The statistical analyses were performed using SPSS for Windows (version 13.0, SPSS Inc., Chicago, Illinois, USA). Haplotype reconstruction was performed using the program PHASE v2.1.1.
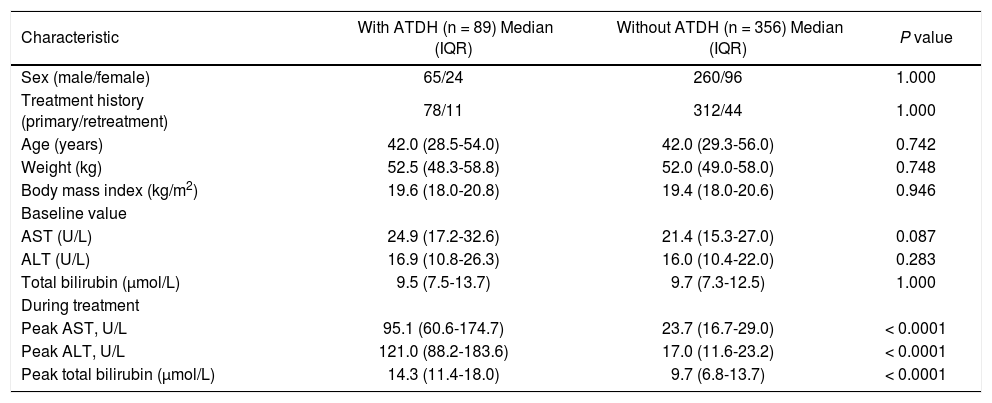
ResultsCharacteristics of patientsA total of 445 TB patients consisting of 89 cases and 356 controls were selected from the 4304 community-based cohort. Among 89 cases, 79 patients had over two times increased ALT level and 10 patients had a combined increase in AST and total bilirubin with at least one of them above two times of ULN. A total of 37 (41.6%) had two to three times of ULN (34 in ALT and 3 in AST/total bilirubin), 29 (32.6%) had three to five times of ULN (25 in ALT and 4 AST/total bilirubin) and 23 (25.8%) had more than five times of ULN (20 in ALT and 3 AST/total bilirubin). Basing on the CIOMS scale, 4 of 89 cases get 4 score (4.5%), 67 get 5 score (75.3%) and 18 get 6 score (20.2%). That is to say, 79.8% cases were judged possible and 20.2% probable. Sixty-nine (77.5%) cases had clinical symptoms such as nausea, vomiting, anorexia and so on. The median time between the initial TB treatment and the detection of ATDH was 35 days. Baseline characteristics, including age, weight, and body mass index, in the control group were similar to the case group. Before treatment, all cases and controls had normal AST, ALT, and total bilirubin level, and there were no significant differences between the groups (P > 0.05). Compared with the controls, the peak value of AST, ALT, and total bilirubin in cases were significantly higher (P < 0.0001) (Table 1).
Characteristics of patients with and without ATDH.
| Characteristic | With ATDH (n = 89) Median (IQR) | Without ATDH (n = 356) Median (IQR) | P value |
|---|---|---|---|
| Sex (male/female) | 65/24 | 260/96 | 1.000 |
| Treatment history (primary/retreatment) | 78/11 | 312/44 | 1.000 |
| Age (years) | 42.0 (28.5-54.0) | 42.0 (29.3-56.0) | 0.742 |
| Weight (kg) | 52.5 (48.3-58.8) | 52.0 (49.0-58.0) | 0.748 |
| Body mass index (kg/m2) | 19.6 (18.0-20.8) | 19.4 (18.0-20.6) | 0.946 |
| Baseline value | |||
| AST (U/L) | 24.9 (17.2-32.6) | 21.4 (15.3-27.0) | 0.087 |
| ALT (U/L) | 16.9 (10.8-26.3) | 16.0 (10.4-22.0) | 0.283 |
| Total bilirubin (μmol/L) | 9.5 (7.5-13.7) | 9.7 (7.3-12.5) | 1.000 |
| During treatment | |||
| Peak AST, U/L | 95.1 (60.6-174.7) | 23.7 (16.7-29.0) | < 0.0001 |
| Peak ALT, U/L | 121.0 (88.2-183.6) | 17.0 (11.6-23.2) | < 0.0001 |
| Peak total bilirubin (μmol/L) | 14.3 (11.4-18.0) | 9.7 (6.8-13.7) | < 0.0001 |
ATDH: anti-tuberculosis drug-induced hepatotoxicity. IQR: interquartile range. AST: aspartate amino transferase. ALT: alanine aminotransferase.
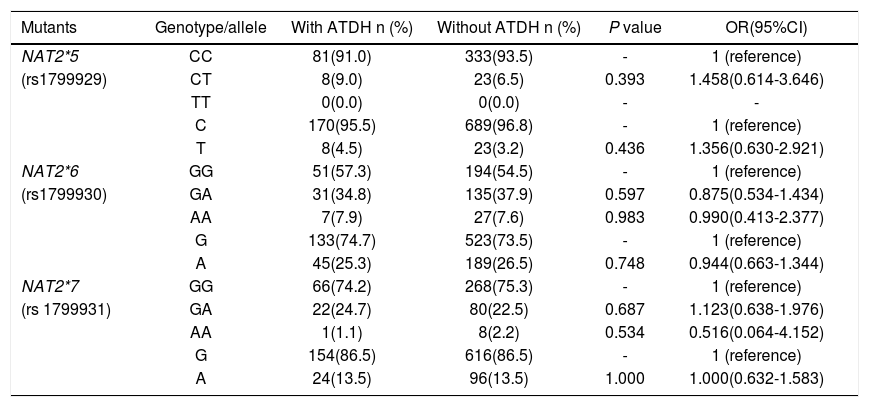
NAT2*5, NAT2*6 and NAT2*7 were successfully genotyped in the 445 subjects. Distribution of NAT2*5, NAT2*6 and NAT2*7 among controls coincided well with the Hardy-Weinberg equilibrium (P > 0.05, respectively). NAT2*5 mutant homogenous genotype was not found in all subjects. The wild homogenous genotype of NAT2*5, NAT2*6 and NAT2*7 had the highest distribution in both the study groups. Taken the wild genotype and allele as the reference group respectively, there were no statistical differences in NAT2*5, NAT2*6 and NAT2*7 genotype and allele frequencies between cases and controls (Table 2).
Genotypes and alleles frequencies of NAT2*5, NAT2*6 and NAT2*7 in patients with and without ATDH.
| Mutants | Genotype/allele | With ATDH n (%) | Without ATDH n (%) | P value | OR(95%CI) |
|---|---|---|---|---|---|
| NAT2*5 | CC | 81(91.0) | 333(93.5) | - | 1 (reference) |
| (rs1799929) | CT | 8(9.0) | 23(6.5) | 0.393 | 1.458(0.614-3.646) |
| TT | 0(0.0) | 0(0.0) | - | - | |
| C | 170(95.5) | 689(96.8) | - | 1 (reference) | |
| T | 8(4.5) | 23(3.2) | 0.436 | 1.356(0.630-2.921) | |
| NAT2*6 | GG | 51(57.3) | 194(54.5) | - | 1 (reference) |
| (rs1799930) | GA | 31(34.8) | 135(37.9) | 0.597 | 0.875(0.534-1.434) |
| AA | 7(7.9) | 27(7.6) | 0.983 | 0.990(0.413-2.377) | |
| G | 133(74.7) | 523(73.5) | - | 1 (reference) | |
| A | 45(25.3) | 189(26.5) | 0.748 | 0.944(0.663-1.344) | |
| NAT2*7 | GG | 66(74.2) | 268(75.3) | - | 1 (reference) |
| (rs 1799931) | GA | 22(24.7) | 80(22.5) | 0.687 | 1.123(0.638-1.976) |
| AA | 1(1.1) | 8(2.2) | 0.534 | 0.516(0.064-4.152) | |
| G | 154(86.5) | 616(86.5) | - | 1 (reference) | |
| A | 24(13.5) | 96(13.5) | 1.000 | 1.000(0.632-1.583) |
NAT2: N-acetyltransferase 2 gene. ATDH: anti-tuberculosis drug-induced hepatotoxicity. OR: odds ratio. 95%CI: 95% confidence intervals.
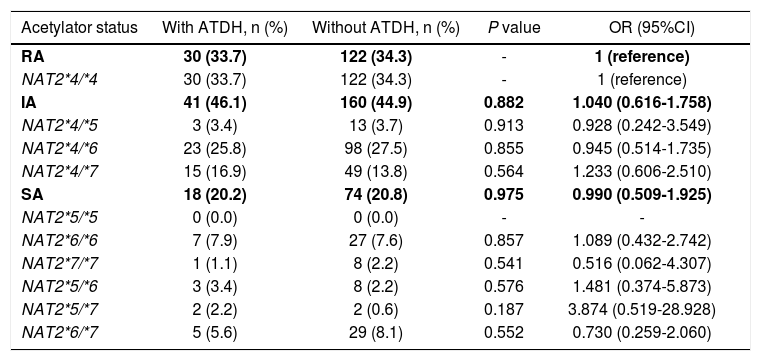
A total of 30 (33.7%) and 122 (34.3%) RAs, 41 (46.1%) and 160 (44.9%) IAs, 18 (20.2%) and 74 (20.8%) SAs were defined in cases and controls, respectively. There was no statistical difference in the distribution of NAT2 acetylator status between cases and controls (x2 = 0.037, P = 0.981). The OR of IA and SA compared with RA was 1.040 (95%CI 0.616-1.758) and 0.990 (95%CI 0.509-1.925), respectively (Table 3). The association was also explored by stratified analysis basing on sex, age, primary/retreatment situation, the serious of ATDH, and the CIOMS score grade, respectively. In the subgroups of female, older than 35 years old, primary treatment TB patients, more than three times of ULN and the causality "possible" group, the frequencies of RA were lower in cases than controls, but there were no significant differences between cases and controls in all of the subgroups (data not shown).
NAT2 acetylator status and susceptibility of ATDH.
| Acetylator status | With ATDH, n (%) | Without ATDH, n (%) | P value | OR (95%CI) |
|---|---|---|---|---|
| RA | 30 (33.7) | 122 (34.3) | - | 1 (reference) |
| NAT2*4/*4 | 30 (33.7) | 122 (34.3) | - | 1 (reference) |
| IA | 41 (46.1) | 160 (44.9) | 0.882 | 1.040 (0.616-1.758) |
| NAT2*4/*5 | 3 (3.4) | 13 (3.7) | 0.913 | 0.928 (0.242-3.549) |
| NAT2*4/*6 | 23 (25.8) | 98 (27.5) | 0.855 | 0.945 (0.514-1.735) |
| NAT2*4/*7 | 15 (16.9) | 49 (13.8) | 0.564 | 1.233 (0.606-2.510) |
| SA | 18 (20.2) | 74 (20.8) | 0.975 | 0.990 (0.509-1.925) |
| NAT2*5/*5 | 0 (0.0) | 0 (0.0) | - | - |
| NAT2*6/*6 | 7 (7.9) | 27 (7.6) | 0.857 | 1.089 (0.432-2.742) |
| NAT2*7/*7 | 1 (1.1) | 8 (2.2) | 0.541 | 0.516 (0.062-4.307) |
| NAT2*5/*6 | 3 (3.4) | 8 (2.2) | 0.576 | 1.481 (0.374-5.873) |
| NAT2*5/*7 | 2 (2.2) | 2 (0.6) | 0.187 | 3.874 (0.519-28.928) |
| NAT2*6/*7 | 5 (5.6) | 29 (8.1) | 0.552 | 0.730 (0.259-2.060) |
NAT2: N-acetyltransferase 2 gene. ATDH: anti-tuberculosis drug-induced hepatotoxicity. OR: odds ratio. 95%CI: 95% confidence intervals. RA: rapid acetylator. IA: intermediate acetylator. SA: slow acetylator.
In the 445 TB patients examined, we identified four haplotypes composed of three mutations (rs1799929C > T-rs1799930G > A-rs1799931G > A). The wild type (C-G-G) was the major type in both groups (56.7 and 56.7% respectively). The haplotype distribution in cases was similar to controls (P > 0.05).
DiscussionThe present study did not find NAT2*5, NAT2*6 and NAT2*7 were risk for developing ATDH in community TB patients. In addition, our result showed that NAT2 acetylator status and haplotypes had no significant association with ATDH. In the subgroups of female, older than 35 years old, primary treatment TB patients, more than three times of ULN and the causality "possible" group, the frequencies of RA were lower in cases than controls, but there were no significant differences between ca-ses and controls in all of the subgroups. A meta-analysis reported that the frequency of RA, IA and SA in healthy Chinese population was 29.9% (132/ 441), 44.7% (197/441) and 25.4% (112/441), respectively.32 The frequency of RA, IA and SA in our controls was respectively 34.3% (122/356), 44.9% (160/ 356) and 20.8% (74/356) and the distribution of acetylator status was similar to the meta-analysis result (x2 = 2.960, P = 0.228).
A recent nested case-control study basing on a 1,200 cohort whose research design was similar to ours also did not demonstrate an increased risk of ATDH related to the presence of NAT2 slow poly-morphisms.33 These results showed that the NAT2 genetic testing for ATDH prevention in community TB patients is currently a matter of debate and the target population should be clearly determined before the application of NAT2 genetic testing. Compared with the previous Asian studies that found the significant association between NAT2 genotype and ATDH,12-21 several explanations of this contradictory observation can be proposed. Firstly, our subjects were from a 4,488 community-based cohort while others were hospital-based. In hospital-based studies, the participants were likely to have more complex and serious ATDH. For example, the median peak value of AST and ALT in cases studied by Higuchi, et al.,20 was 294.5 and 316.2 U/L, respectively, compared with 95.1 and 121.0 U/L, respectively in our study. It was reported that slow acetylators are prone to develop more severe hepatotoxicity than rapid acetylators.12 Therefore, it is possible that NAT2 genotype is not an independent risk factor for mild ATDH cases. Although we did not find significant association between NAT2 polymorphisms and relative mild hepatotoxicity, our results would probably be valuable for validating the application of NAT2 polymorphisms in community-based TB patients. Secondly, the primary TB patients in our study were treated with INH + RFP + PZA + EMB and the retreatment TB patients also took SM meanwhile. The pa-tients took the combination chemotherapy every other day other than daily. It was shown that daily TB treatment in comparison with thrice-weekly treatment increased the risk of ATDH.34 These treatment differences may lead to some change in pharma-cokinetic and pharmacodynamic interactions, which are very likely to represent a major confounding factor impeding proper interpretation of the results obtained in these studies.12-21 What's more, differences in the study design, ATDH definition, sample size, follow-up period, molecular detection method and the presence of other relevant polymorphisms may have given rise to different results among studies.
According to the model of the pathogenesis of drug-induced hepatotoxicity proposed by Russmann, et al., the biochemical mechanism of drug-induced hepatotoxicity may involve a complex interplay between the chemical properties of the drug, environmental factors (such as age, sex, diet, alcohol consumption, compliance of drug intake, existing liver disease, concomitant use of other drugs and comorbid illness) and genetic factors that control the handling of the drug (metabolism, detoxification, and transport), as well as those that influence cell injury and repair.35,36 Some studies proposed that CYP2E1 genotype, GSTM1 homozygous null genotype, a mutant C allele (T/C or C/C genotype) of manganese superoxide dismutase, the absence of HLA-DQA1*0102 and the presence of HLA-DQB1*0201 alleles were significantly associated with ATDH.37-39 In addition, patients who were slow acetylators and carried CYP2E1 c1/c1 genotype had more severe hepatotoxicity.37 In a word, the biochemical mechanism and pathogenesis of ATDH is complicated and the development of ATDH is influenced by many factors. It may be valuable to combine several risk factors together to identify the susceptible population of ATDH.
The major strength of this study is that the controls were from the same cohort as cases and all the subjects were followed up prospectively, thereby the selection bias and recall bias were reduced efficiently. Because of the large sample size of the cohort, we could perform 1:4 matching to increase efficiency and minimize the impact of potential confounders. Each case was reviewed and assessed by experts strictly and the causality was evaluated basing on the CIOMS scale. Therefore, misclassification was less likely in our study than in most others. Potential limitation of the present study should be considered. The patients enrolled in the present study all had taken combination chemotherapy including INH, RFP, EMB, PZA and some also got SM. Rechallenge with the suspected drug were not done for most of cases considering safety and practical necessary, so it is difficult to confirm which one or some of the multiple drugs should be criminated. Some of the cases might not be directly related with INH and NAT2. However, combination chemotherapy is the standard treatment for TB and it is meaningful to evaluate NAT2 genotype testing in TB patients taking multiple drugs in clinical practice. Another potential limitation is the still small sample size of this present study.
In summary, our results did not suggest a significant association between the NAT2 genotypes, alleles, acetylator status, or haplotypes and hepatotoxicity due to anti-tuberculosis drugs in Chinese community TB patients. It may be deficient to take NAT2 genotype as a predictor of ATDH for Chinese community TB patients.
AcknowledgementsWe are grateful to all cooperating organizations (Center for Disease and Control Prevention in Zhe-jiang, Chongqing, Guangxi and Jilin Provinces) and their staffs whose hard work made this study possible. Special thanks to TB patients participating in this study.
GrantsThis study was funded by National Natural Science Foundation of China (N0.81072387) and Beijing Natural Science Foundation (N0.7111006).
Abbreviations- •
ATDH: anti-tuberculosis drug-induced hepatotoxicity.
- •
TB: tuberculosis.
- •
NAT2: N-acetyltransferase 2 gene.
- •
INH: isoniazid.
- •
RIF: rifampicin.
- •
PZA: pyrazinamide.
- •
EMB: ethambutol.
- •
SM: streptomycin.
- •
ADRs: adverse drug reactions.
- •
SA: slow acetylator.
- •
IA: intermediate.
- •
RA: rapid acetylator.
- •
ADACS: Antituberculosis Drugs Induced Adverse Reactions in China National Tuberculosis Prevention and Control Scheme Study.
- •
ALT: alanine transaminase.
- •
AST: aspartate amino transaminase.
- •
ULN: upper limit of normal.
- •
PCR: polymerase chain reaction.
- •
RFLP: restriction fragment length polymorphism.
- •
IQR: interquartile range.
- •
OR: odds ratios.
- •
95% CI: 95% confidence intervals.