Objective. To determine the clinical characteristics of NAFLD in asymptomatic obese women.
Methods. A total of 457 asymptomatic obese women were enrolled in a cross-sectional study and allocated into groups with and without NAFLD. Irrespective of ALT levels, diagnosis of NAFLD was established by ultrasonographic findings; irrespective of fibrosis, NASH was defined by hepatic histological changes.
Results. One hundred ninety five (42.7%) women had elevated ALT levels. Diagnosis of NAFLD was established in 228 (49.9%) women; among women with NAFLD, 34 (14.9%) have ALT levels within the normal range. On the other hand, based on the healthy range for ALT levels (19 UI/L), 336 (73.5%) women had elevated ALT, but only 2 (0.9%) women with NAFLD exhibited ALT levels within normal healthy values. Furthermore, 93 (41%) women who had AST/ALT levels 3 1 underwent liver biopsy; of these, 90 (96.8%) had diagnosis of NASH and 3 (3.2%) of hepatic cirrhosis. Women with NAFLD were more obese and have higher fasting plasma glucose, triglycerides, ALT, and AST levels than obese women without NAFLD. Seventy six (16.6%) women had diagnosis of diabetes; of these 47 (61.8) in the NAFLD group.
Conclusions. Results of this study support the statement that women with NAFLD have an adverse metabolic profile. Furthermore, our results show that hyperglycemia, hypertriglyceridemia and markers of liver injury such as AST/ALT ≥ 1 may be useful for early recognition of NAFLD.
The Nonalcoholic fatty liver disease (NAFLD), a clinical condition characterized by histological features that resemble those of alcohol-induced liver injury, occurs in individuals who do not consume alcohol and is involved in the development of chronic liver disease.1
Individuals with NAFLD commonly are asymptomatic or have minimal clinical symptoms; thus, diagnosis of NAFLD is suggested by elevation of aminotransferase levels or changes in the hepatic echogenicity induced by liver fatty infiltration.2 Obesity and their commonly associated metabolic disorders such as hyperglycemia and hypertriglyceridemia are well-known risk factors for NAFLD.3,4 The prevalence of NAFLD reach 14–21%,5 but it is as high as 90%-95% in obese persons and up to 70% in diabetic patients.6 Irrespective of age, gender, and body mass index (BMI), it has been noted that liver fat content also is significantly increased in the subjects with metabolic syndrome.7
Hepatic ultrasonographic changes of NAFLD appear when steatosis involve 15% to 20% of the liver; however ultrasound is unable to distinguish nonalcoholic steatohepatitis (NASH) from other forms of NAFLD, distinction that has important prognostic implications.8,9 Clinically, the main features that dis-tinguish cirrhosis caused by NAFLD from cirrhosis caused by alcohol ingestion is that patients, who have NAFLD-related cirrhosis, frequently exhibited serious end organ complications of metabolic syndrome, particularly those related with type 2 diabetes.10
The NAFLD comprises a wide spectrum of histological categories; steatosis alone (type I), steatosis plus inflammation (type 2), steatosis plus hepatocy-te injury (balonization) (type 3), and steatosis plus sinusoidal fibrosis and polymorphonuclear cell infiltrates with or without Mallory-Denk bodies (type 4). Types 3 and 4 are considered as NASH.11 Furthermore, the NAFLD activity score (NAS), which includes features of active injury such as steatosis, lobular inflammation, and ballooning, is a useful tool for assessing severity of disease.12
In this study, we determine the clinical characteristics of NAFLD in asymptomatic obese women.
MethodsWith the approval by the Mexican Social Security Institute Research Committee, and after obtaining the subject informed consent, a cross-sectional study was carried out from December 2007 to May 2009.
Obese women (defined by BMI ≥ 30 kg/m2) aged 20 to 65 years were enrolled and allocated into the following groups: a) asymptomatic obese women without NAFLD, and b) asymptomatic obese women with diagnosis of NAFLD.
The sampling strategy was based on advertising to general population of Durango, city in northern Mexico, to invite obese women to participate in the study. Durango city has approximately 800,000 inhabitants and the prevalence of obesity is 27.27.13
Alcohol consumption ≥ 30 g per week, viral hepatitis, medical treatment with drugs that promote cholestasis or liver injury, and previous diagnosis of chronic liver disease were exclusion criteria.
Data about family history as well as diagnosis of diabetes, hypertension and hypertriglyceridemia were recorded.
Liver biopsy using tru-cut needle ultrasound-guided, was offered to women with aspartate aminotrans-ferase (AST)/alanine aminotransferase/(ALT) levels ≥ I. Biopsy specimens were stained using hematoxilin and eosin, Masson’s trichrome, and Perls stains.5
DefinitionsIrrespective of ALT levels, diagnosis of NAFLD was established by the presence of hepatic ultraso-nographic findings such as hepatorenal echo contrast, bright liver, deep attenuation, and blurred vessels.14,15 In general, ultrasonography has a sensitivity of 897 and specificity of 937 for detecting steatosis, and sensitivity and specificity of 777 and 897 for detecting fibrosis.4
Irrespective of fibrosis, NASH was defined by hepatic histological changes characterized by macro-vesicular steatosis; ballooning, inflammation, and Mallory-Denk bodies.16 The NAS was estimated by the sum of scores for steatosis (0–3), lobular inflammation (0–3) and ballooning (0–2). The NAS value = 5 suggests diagnosis of NASH.12
Family history of diabetes (FHD), obesity, and hypertriglyceridemia was defined by the presence of type 2 diabetes, obesity, and hypertension in at least one first degree relative.
Diabetes was defined according criteria of the American Diabetes Association.17
Hypertriglyceridemia was defined by serum trigly-cerides levels ≥ 150 mg/dL.18
MeasurementsIn the standing position, weight and height were measured with the women in light clothing using a fixed scale with stadimeter (Tanita TBF-215, Tokyo, Japan). The precision of weight and height measurements was 0.1 kg and 0.01 m. BMI was calculated as weight (kilograms) divided by height (meters) squared. Total body fat was measured by bioelectric impedance using a body composition analyzer (Tanita TBF-215, Tokyo, Japan) with 0.1 percent increment.
Abdominal ultrasonography was performed using ultrasound scanner (General Electric, USA). Ultra-sonographic evaluations were performed by two in-dependent experts, who were blinded regard results of laboratory.
AssaysA venous whole blood sample was collected after 8–10 hours of fasting. Plasma glucose was assessed by glucose-oxidase method; the inter and intrassay variations were 2.1, and 1.57. Total-cholesterol (inter-and intrassay variations of 3.0 and 2.57) and serum triglycerides (inter-and intrassay variations of 3.5, and 3.07) were determined by enzymatic methods. AST and ALT levels were determined by UV kinetic methods (Erlic, Tlalnepantla, Estado de Mexico, Mex.). Normal reference values of AST and ALT levels were of 38 and 40 UI/L.19 In addition, data were re-addressed using the healthy range for ALT levels (19 UI/L for women).20
All measurements were performed in an Express 500 clinical chemistry autoanalyzer (Ciba Corning, Diagnostic Corp., Overling, Ohio).
Statistical analysisNumerical values are reported as mean ± standard deviation, and categorical variables are expressed as proportions.
For bivariate analysis, Student’s t test (or alternately Mann-Whitney U test for skewed data) and chi-squared test were used for numerical and categorical data, respectively.
Sensitivity, specificity, positive predictive value, and negative predictive value of ALT for diagnosis of NAFLD were estimated.21
A p value < 0.05 defined statistical significance. Data were analyzed by using the statistical package SPSS for Windows 15.0.
ResultsA total of 457 obese women with average age and BMI of 45.0 ± 10.7 years and 35.5 ± 5.0 kg/ m2 were enrolled.
One hundred ninety five (42.7%) women had elevated ALT levels. Diagnosis of NAFLD was established in 228 (49.9%) women; among women with NAFLD, 34 (14.9%) have ALT levels within the normal range (sensitivity 85%, specificity 99.6%, positive predictive value 99.5% and negative predictive value 87%).
On the other hand, based on the healthy range for ALT levels, a total of 336 (73.5%) women had elevated ALT, but only 2 (0.9%) women with NA-FLD exhibited ALT levels within normal healthy values (sensitivity 99.1%, specificity 52%, positive predictive value 67.3%, and negative predictive value 98.3%).
Ninety three (40.8%) women had AST/ALT levels ≥ 1; all of them accepted underwent liver biopsy.
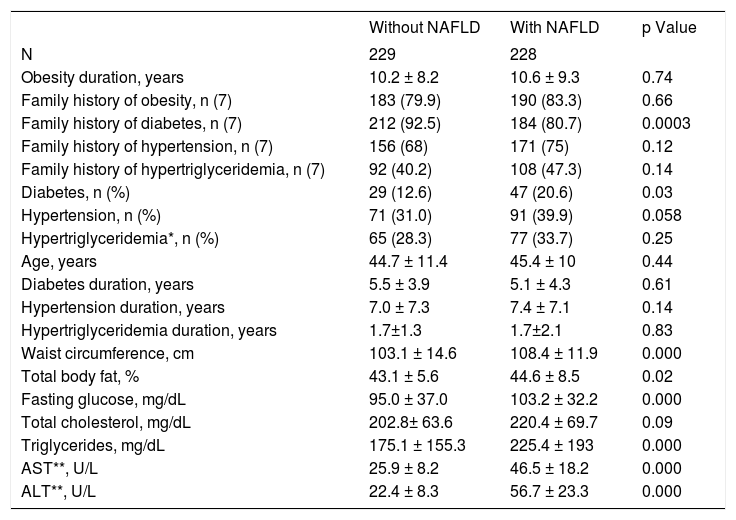
The frequency of diabetes was higher in the women with NAFLD than in women without NAFLD. In addition, women with NAFLD were more obese and had higher fasting plasma glucose, triglycerides, ALT, and AST levels than obese women without NAFLD (Table 1).
Characteristics of obese women with and without nonalcoholic steatohepatitis
| Without NAFLD | With NAFLD | p Value | |
|---|---|---|---|
| N | 229 | 228 | |
| Obesity duration, years | 10.2 ± 8.2 | 10.6 ± 9.3 | 0.74 |
| Family history of obesity, n (7) | 183 (79.9) | 190 (83.3) | 0.66 |
| Family history of diabetes, n (7) | 212 (92.5) | 184 (80.7) | 0.0003 |
| Family history of hypertension, n (7) | 156 (68) | 171 (75) | 0.12 |
| Family history of hypertriglyceridemia, n (7) | 92 (40.2) | 108 (47.3) | 0.14 |
| Diabetes, n (%) | 29 (12.6) | 47 (20.6) | 0.03 |
| Hypertension, n (%) | 71 (31.0) | 91 (39.9) | 0.058 |
| Hypertriglyceridemia*, n (%) | 65 (28.3) | 77 (33.7) | 0.25 |
| Age, years | 44.7 ± 11.4 | 45.4 ± 10 | 0.44 |
| Diabetes duration, years | 5.5 ± 3.9 | 5.1 ± 4.3 | 0.61 |
| Hypertension duration, years | 7.0 ± 7.3 | 7.4 ± 7.1 | 0.14 |
| Hypertriglyceridemia duration, years | 1.7±1.3 | 1.7±2.1 | 0.83 |
| Waist circumference, cm | 103.1 ± 14.6 | 108.4 ± 11.9 | 0.000 |
| Total body fat, % | 43.1 ± 5.6 | 44.6 ± 8.5 | 0.02 |
| Fasting glucose, mg/dL | 95.0 ± 37.0 | 103.2 ± 32.2 | 0.000 |
| Total cholesterol, mg/dL | 202.8± 63.6 | 220.4 ± 69.7 | 0.09 |
| Triglycerides, mg/dL | 175.1 ± 155.3 | 225.4 ± 193 | 0.000 |
| AST**, U/L | 25.9 ± 8.2 | 46.5 ± 18.2 | 0.000 |
| ALT**, U/L | 22.4 ± 8.3 | 56.7 ± 23.3 | 0.000 |
* Serum triglycerides levels ≥ 150 mg/dL. Reference value of 40 and 38 U/L for AST and ALT levels.
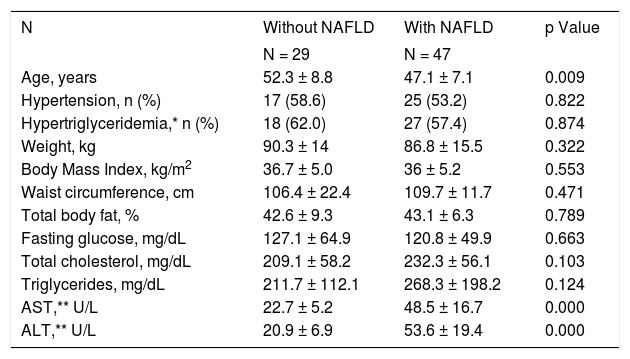
Women with NAFLD and diabetes were younger more obese, and have higher AST and ALT levels than women without NAFLD (Table 2).
Characteristics of diabetic obese women with and without nonalcoholic steatohepatitis, n = 76.
| N | Without NAFLD | With NAFLD | p Value |
|---|---|---|---|
| N = 29 | N = 47 | ||
| Age, years | 52.3 ± 8.8 | 47.1 ± 7.1 | 0.009 |
| Hypertension, n (%) | 17 (58.6) | 25 (53.2) | 0.822 |
| Hypertriglyceridemia,* n (%) | 18 (62.0) | 27 (57.4) | 0.874 |
| Weight, kg | 90.3 ± 14 | 86.8 ± 15.5 | 0.322 |
| Body Mass Index, kg/m2 | 36.7 ± 5.0 | 36 ± 5.2 | 0.553 |
| Waist circumference, cm | 106.4 ± 22.4 | 109.7 ± 11.7 | 0.471 |
| Total body fat, % | 42.6 ± 9.3 | 43.1 ± 6.3 | 0.789 |
| Fasting glucose, mg/dL | 127.1 ± 64.9 | 120.8 ± 49.9 | 0.663 |
| Total cholesterol, mg/dL | 209.1 ± 58.2 | 232.3 ± 56.1 | 0.103 |
| Triglycerides, mg/dL | 211.7 ± 112.1 | 268.3 ± 198.2 | 0.124 |
| AST,** U/L | 22.7 ± 5.2 | 48.5 ± 16.7 | 0.000 |
| ALT,** U/L | 20.9 ± 6.9 | 53.6 ± 19.4 | 0.000 |
* Serum triglycerides levels = 150 mg/dL. Reference value of 40 and 38 U/L for AST and ALT levels.
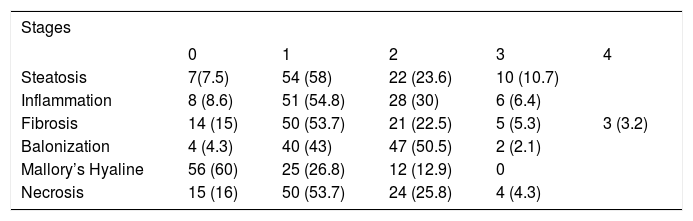
Among women who underwent hepatic biopsy, 90 (96.8%) had diagnosis of NASH and 3 (3.2%) of hepatic cirrhosis. Among women with NASH, 85 (91.4%) had severe NASH, or the most severe and irreversible form of NAFLD, exhibiting steatosis, balonization, inflammation, fibrosis, and necrosis. Only 40% of the women had Mallory-Denk bodies (Table 3). There were not significant differences by age between different stages of fibrosis.
Hepatic histological changes in women with nonalcoholic steatohepatitis who underwent hepatic biopsy n = 93.
| Stages | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Steatosis | 7(7.5) | 54 (58) | 22 (23.6) | 10 (10.7) | |
| Inflammation | 8 (8.6) | 51 (54.8) | 28 (30) | 6 (6.4) | |
| Fibrosis | 14 (15) | 50 (53.7) | 21 (22.5) | 5 (5.3) | 3 (3.2) |
| Balonization | 4 (4.3) | 40 (43) | 47 (50.5) | 2 (2.1) | |
| Mallory’s Hyaline | 56 (60) | 25 (26.8) | 12 (12.9) | 0 | |
| Necrosis | 15 (16) | 50 (53.7) | 24 (25.8) | 4 (4.3) | |
Values are n (%).
According to NAS 36% of biopsies had a score ≥ 5 and 53% a score > 3 and < 5; however, all of these biopsies showed histological activity; only 8% of biopsies had score ≤ 3 of NAS.
DiscussionOur results show that obese women with NAFLD have higher frequency of diabetes, fasting plasma glucose, and triglycerides levels than obese women without NAFLD; this finding supports the statement that women with NAFLD have an adverse metabolic profile.
Among obese individuals, the prevalence of NA-FLD range from 50% to 90%4,22,23 and in diabetic patients from 34 to 74%. In the subjects with obesity and type 2 diabetes, NAFLD is a common finding.24 In our population, the prevalence of NAFLD reach 49.9%, higher than that in previous reports;25 on the other hand, the frequency of risk factors such as type 2 diabetes; hypertension, and hypertriglyceride-mia was similar to the observed in US population.26
In this study, 14.9% of the women with normal ALT levels had NAFLD; on this regard, in the Dallas Heart Study27 and Dyonisios study,25 79% and 55% of the subjects with normal ALT exhibited NAFLD.
These findings suggest that liver enzymes have an elevated rate of false negative results in the diagnosis of NAFLD. Using the healthy range for ALT levels increase the sensitivity and negative predictive value but decrease specificity and predictive positive value; these finding suggest that healthy range for ALT le-vels are more appropriate as a screening tool for NA-FLD. However, using the appropriate methodological design study, the best cutoff point of ALT for recognizing NAFLD should be established in a Receiver Operating Characteristic scatter plot.
In agree with other studies,27 the hepatic histolo-gical changes of obese women with NAFLD were compatible with a severe and progressive form of NASH. Furthermore, in agree with the report by Marchesini,28 the hepatic disease was more severe in the presence of type 2 diabetes. However, the presence of moderate or severe fibrosis was lower than the reported in other populations, in which 30 to 40% of the obese patients with NAFLD have advanced fibrosis and 10–15% cirrhosis.4
According NAS, 89% of the women in this study have NASH; taking into account that NAS is a tool for assessing disease severity12 we hypothesized
that the target population had a severe hepatic disease.
Some clinical parameters such as duration of obesity and diabetes, the presence of AST/ALT ≥ 1, and hyperglycemia may be useful for predicting NA-FLD.29,30 In addition, we have previously showed that triglycerides levels ≥ 300 mg/dL are associated to NAFLD (unpublished data). However, because NAFLD and NASH represent advanced stages of hepatic steatosis that are associated with metabolic diseases; and a high proportion of individuals with fatty liver do not show laboratory abnormalities,31 based on the results of this study, we develop an algorithm to facilitate recognition of NAFLD, Figure 1. The algorithm suggests that in diabetic obese women aged < 40 years, triglycerides and ALT levels should be assessed; if triglycerides are ≥ 300 mg/dL and healthy ALT levels < 20 U/L, hepatic ultraso-nography is mandatory; in the presence of hepatic steatosis and AST/ALT levels ≥ 1, hepatic biopsy should be offered. Although our results suggest that the algorithm facilitates recognition of NASH, further research is required to validate it.
Algorithm for the early clinical recognition of NAFLD. In the presence of obesity and diabetes, women aged <40 years would have a determination of triglycerides levels; if triglycerides are ≥ 300 mg/dL and healthy ALT levels ≥ 20 UIL, hepatic ultrasonography is mandatory; in the presence of steatosis and AST/ALT levels ≥ I, hepatic biopsy should be proposed.
Some limitations of the present study deserve to be mentioned: first, only obese women were studied; in consequence, our results cannot be applied to men; second, only 41% of obese women underwent hepatic biopsy; so, is probable that the frequency of NASH and cirrhosis have been underestimated; third, Mallory-Denk bodies were estimated using he-matoxilin and eosin stain but not more sensitive techniques such as ubiquitin; thus, it is probable that we underestimated the frequency of Mallory-Denk bodies. However, is necessary to keep in mind that Mallory-Denk body is a frequent feature of alcoholic steatohepatitis rather than a feature of NASH. Nonetheless, these limitations do not influence our conclusions.
In conclusion, the present study shows that women with NAFLD are more obese and have higher hyperglycemia, hypertriglyceridemia, and elevated rates of diabetes than obese women without NAFLD. Furthermore, our results show that hyperglycemia, hypertriglyceridemia and markers of liver injury as such AST/ALT ≥ 1 may be useful for early recognition of NAFLD and severe form of NASH.
AcknowledgementsThis work was partially supported by grants from the Mexican Social Security Institute Foundation, Civil Association.
Authors state that they have not any financial or other potential conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Serum triglycerides levels ≥ 150 mg/dL.
Reference value of 40 and 38 U/L for AST and ALT levels.