Noncirrhotic portal hypertension (NCPH) represents a relatively infrequent group of conditions that causes portal hypertension in the absence of cirrhosis. An association between NCPH and patients infected with human immunodeficiency virus (HIV) has been reported. Six consecutive patients with HIV infection and NCPH were the subject of this series. Case histories, including medication lists, liver biopsy and laboratory data were reviewed. Age at diagnosis was 43 ± 3 years (range, 37-47). Liver disease was diagnosed 12 ± 4 years (range, 8-18) after initiation of antiretroviral therapy (ART). All patients developed esophageal varices, S patients presented at least one bleeding episode and 2 required TIPS. Serum liver tests showed a mean total bilirubin of 1.4 ± .7 mg/dL (range, .S-2.S) and INR was 1.2 ± .14 (range, 1.0-1.4). CD4 count was 326 ± 124 cells/mL (range, 198-467) and all patients presented HIV viral load < 7S copies/mL. Didanosine (ddl) was the most common ART drug being used by 4 patients. Portal vein thrombosis was diagnosed in 2 patients. Hepatic portal sclerosis (HPS) alone was observed in 1 patient, nodular regenerative hyperplasia (NRH) alone in 2 patients and combined HPS/NRH in 3 patients. In conclusion, NCPH should be included in the differential diagnosis of HIV-individuals presenting with clinical manifestations of portal hypertension and well preserved liver synthetic function. Prolonged exposure to ART, specially ddI, can play a pathogenic role. Rarely, liver synthetic function is sufficiently severe to warrant liver transplantation.
Noncirrhotic portal hypertension (NCPH) represents a relatively infrequent group of conditions that causes portal hypertension in the absence of cirrhosis. Among the entities that cause NCPH are nodular regenerative hyperplasia (NRH), hepatoportal sclerosis (HPS), congenital hepatic fibrosis, schistosomiasis and various hypercoagulable states.1 They usually have an asymptomatic course but can manifest consequences of portal hypertension that include splenomegaly and hypersplenism, variceal bleeding, and ascites, and rarely liver failure that may necessitate liver transplantation as well.2,3 NRH is characterized by diffuse transformation of normal hepatic parenchyma into a repetitive nodular pattern due to relative hepatocellular atrophy in the centrovenular region; fibrosis is not increased. The prevalence is estimated to be 2.6% in autopsy series and has been reported to occur in association with certain drugs and other systemic diseases as vascular, rheumatologic and myeloproliferative disorders.4,5 HPS is also an uncommon disease characterized by portal and septal fibrosis associated with portal hypertension occurring predominantly in adults, mostly in men, living in tropical zones.1It can be associated with partial or complete extrahepatic portal vein obstruction.6
With the advent of antiretroviral therapy (ART), the prognosis of HIV infection has dramatically improved, and with a reduction in morbidity and mortality secondary to classical opportunistic infections.7 However, other illnesses such as liver disorders have become an important cause of death among these patients. Hepatitis C (HCV) or B (HBV) viruses, alcoholic and non-alcoholic steatohepatitis and drug-induced toxicity account for most cases of liver disease in the HIV infected individuals; yet there remains a small group of patients where the etiology of liver disease remains unknown.8 It is believed that 0.5% of the HIV infected patients will develop cryptogenic liver disease.9 Recently, an association between HIV infected patients and NCPH has been reported, especially with NRH and HPS.10-16
We describe six HIV infected patients who presented to the Hospital of the University of Pennsylvania and Austral University Hospital with features of portal hypertension and after a thorough work up these patients were diagnosed with NCPH.
MethodsSeven patients with diagnosis of HIV infection and clinical or endoscopic evidence of portal hypertension presented at the outpatient clinic of the Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania and the Austral University Hospital, Pilar, Argentina. All patients underwent routine laboratory screening tests for the known etiologies of liver disease including HBsAg (Hepatitis B surface antigen), anti-HCV (antibody to hepatitis C antibody), serum hepatitis B DNA, hepatitis C RNA, anti-smooth muscle antibody, antinuclear antibody, a-1 antitrypsin level, ceruloplasmin, ferritin and transferring saturation. Past or recent history of heavy alcohol consumption (> 20 g/day) and previous exposure to hepatotoxic drugs were ruled out. Testing for hyper-coagulable disorders including protein C and protein S activity, anticardiolipin antibody, antithrombin III, antiphospholipid antibody, and factor V Leiden mutation were performed in those patients with thrombosic episodes.
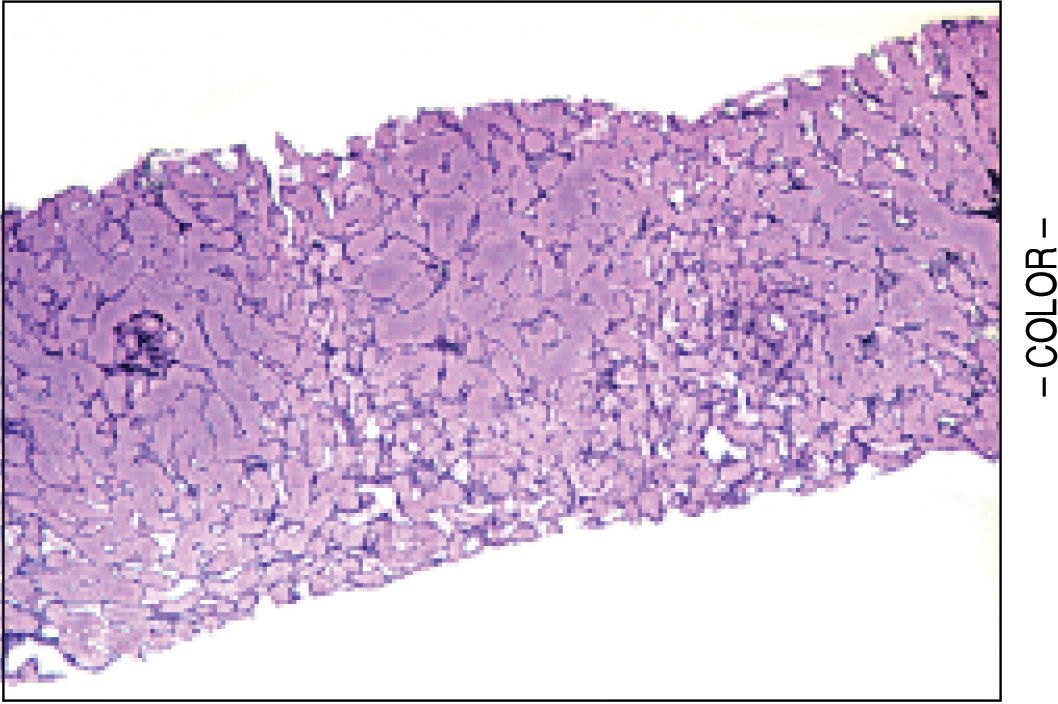
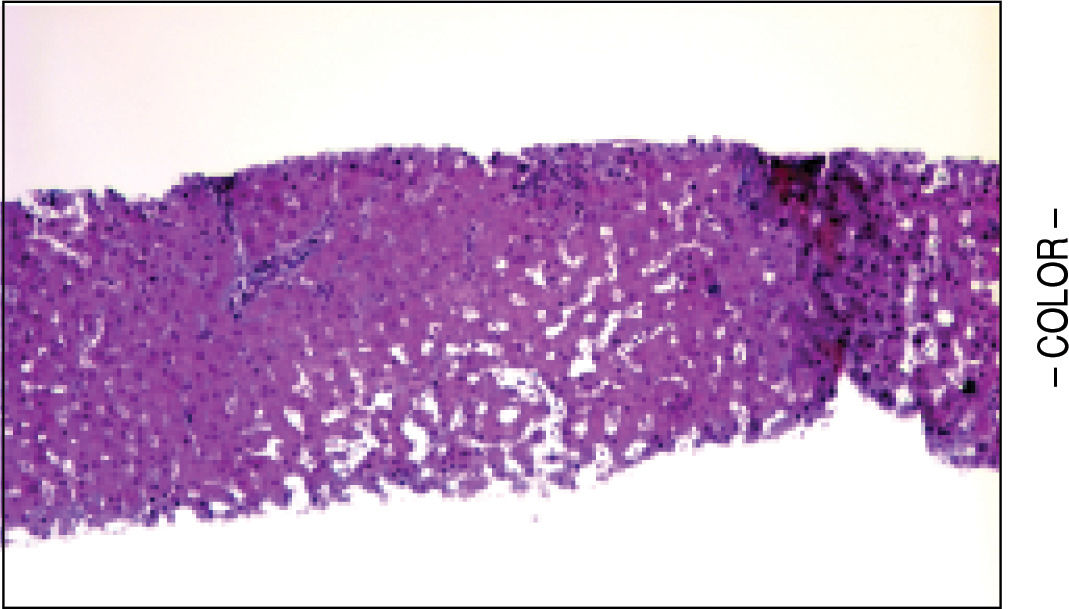
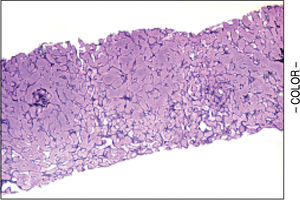
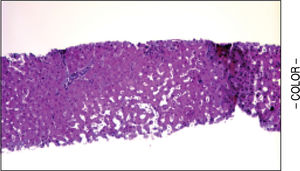
A liver biopsy was done in 6 patients through a transjugular approach (n = 4), percutaneously (n = 1) or laparoscopically (n = 1); one patient declined having a liver biopsy. Histologic criteria for the diagnosis of HPS included varying degrees of fibrosis and sclerosis of the portal vein branches with or without marked dilatation of sinusoids.1 NRH histology includes the presence of hepatocellular nodules < 3 mm in diameter that are not surrounded by fibrosis.4
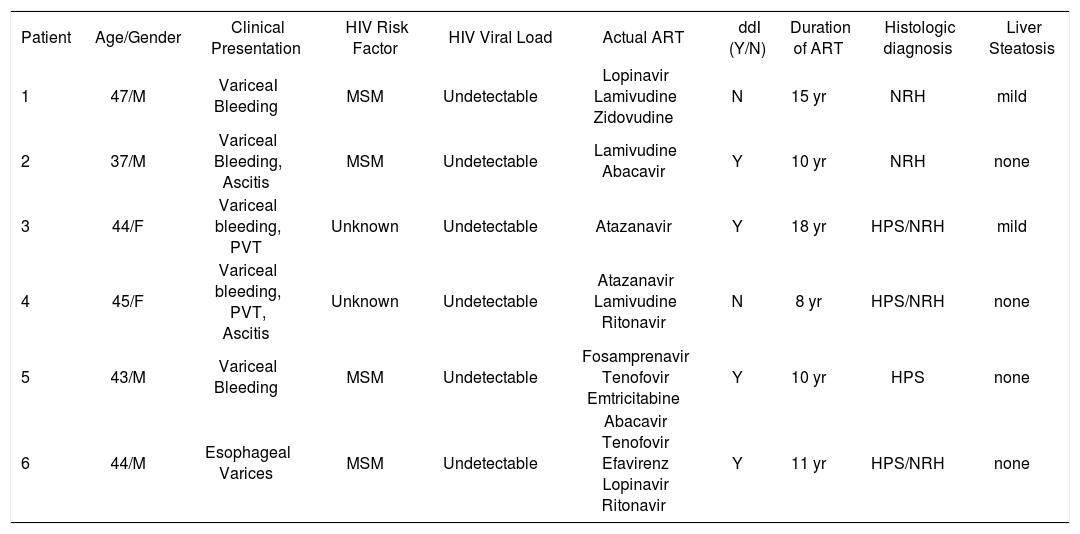
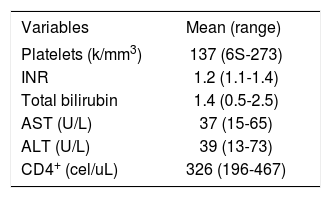
ResultsFour patients were Caucasian and two were Hispanics. Two were female and mean the age at diagnosis of NCPH was 43 ± 3 years (range, 37-47). Liver disease was diagnosed 12 ± 4 years (range, 8-18) after initiation of ART. With the exception of HIV infection and the associated liver disease, none of these patients had significant past medical history, especially a history of opportunistic infections, cancer, renal pathology or cardiac disease. The initial mode of presentation was variceal bleeding (n = S) or an isolated finding of esophageal varices (n = 1). Ascites was present in 2 patients. All but one patient had thrombocytopenia with a median platelet count of 137 ± 77 K/mm3 (range, 6S-273 k/mm3). Serum liver tests showed a mean total bilirubin of 1.4 ± .7 mg/dL (range, 0.S-2.S), alanine aminotransferase 39 ± 21 U/L (range, 13-73), aspartate aminotransferase 37 ± 18 U/L (range, 1S-6S) and INR was 1.2 ± .14 (range, 1.0-1.4). Previous HBV exposure was detected in one patient with positive hepatitis B core antibody. All patients with variceal bleeding received endoscopic treatment and two patients with refractory variceal hemorrhage underwent transjugular intrahepatic portosystemic shunt (TIPS) placement. Surgical splenorenal shunt was performed in one patient after TIPS occluded twice. Hepatic vessels were evaluated with Doppler ultrasound and computed axial tomography or magnetic resonance imaging with intravenous contrast in all patients. Portal vein thrombosis was diagnosed in 2 patients, and only one tested positive for an underlying hypercoagulable state (anticardiolipin antibody).
None of the patients were diagnosed with an opportunistic infection. The median CD4 cell count was 326 ± 124 cells/mL (range, 198-467). At the time of diagnosis, all patients were receiving ART with an efficient immune restoration. HIV viral load was undetectable (< 7S copies/mL) in all patients. Among the several antiretroviral drugs the patients received before the diagnosis of NCPH, didanosine (ddl) was the most common and was used by 4 patients. None of the patients were identified as having had previous exposure to azathioprine, arsenic, copper sulfate, vitamin A or vinyl chloride as these are known to cause HPS. The clinical data and laboratory findings of these patients are summarized in Table 1 and Table 2.
Clinical Characteristics of HIV patients with noncirrhotic portal hypertension.
| Patient | Age/Gender | Clinical Presentation | HIV Risk Factor | HIV Viral Load | Actual ART | ddI (Y/N) | Duration of ART | Histologic diagnosis | Liver Steatosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 47/M | VariceaI Bleeding | MSM | Undetectable | Lopinavir Lamivudine Zidovudine | N | 15 yr | NRH | mild |
| 2 | 37/M | Variceal Bleeding, Ascitis | MSM | Undetectable | Lamivudine Abacavir | Y | 10 yr | NRH | none |
| 3 | 44/F | Variceal bleeding, PVT | Unknown | Undetectable | Atazanavir | Y | 18 yr | HPS/NRH | mild |
| 4 | 45/F | Variceal bleeding, PVT, Ascitis | Unknown | Undetectable | Atazanavir Lamivudine Ritonavir | N | 8 yr | HPS/NRH | none |
| 5 | 43/M | Variceal Bleeding | MSM | Undetectable | Fosamprenavir Tenofovir Emtricitabine | Y | 10 yr | HPS | none |
| 6 | 44/M | Esophageal Varices | MSM | Undetectable | Abacavir Tenofovir Efavirenz Lopinavir Ritonavir | Y | 11 yr | HPS/NRH | none |
M: Male. F: Female. PVT: Portal vein thrombosis. MSM: Men having sex with men. TB: Total bilirubin, AST: Aspartate aminotransferase. ALT: Alanine aminotransferase. Y: Yes. N: No. NRH: Nodular regenerative hyperplasia. HPS: Hepatoportal sclerosis.
Laboratory findings of HIV patients with noncirrhotic portal hypertension.
| Variables | Mean (range) |
|---|---|
| Platelets (k/mm3) | 137 (6S-273) |
| INR | 1.2 (1.1-1.4) |
| Total bilirubin | 1.4 (0.5-2.5) |
| AST (U/L) | 37 (15-65) |
| ALT (U/L) | 39 (13-73) |
| CD4+ (cel/uL) | 326 (196-467) |
INR: International normalized ratio. AST: Aspartate aminotransferase. ALT: Alanine aminotransferase.
HPS alone was observed in 1 patient, nodular regenerative hyperplasia alone in 2 patients and combined HPS/NRH in 3 patients. Two patients have mild steatosis without steatohepatitis. Figures 1 and 2 show representative microscopic findings. To date, all patients are alive and not in consideration of liver transplantation.
NCPH encompasses a heterogeneous group of diseases that are secondary to intrahepatic or extrahepatic etiologies. Recent reports have found an association between HIV and intrahepatic causes of NCPH, especially HPS and NRH.9,13,15,16 Clinically they usually present with generally well-tolerated episodes of portal hypertension related hemorrhage and splenomegaly with its consequent hypersplenism. Development of ascites, jaundice and hepatic encephalopathy is uncommon and may be seen only after a bleeding episode.
HPS, also known as noncirrhotic portal fibrosis and idiopathic portal hypertension, is characterized by varying degrees of portal fibrosis and phleboesclerotic changes of the portal vein system. It has been associated with many potential causes such as chemical exposure (arsenic, vinyl chloride, copper sulfate), herbal and fungal toxins, parasitic infection, thrombophlebitis secondary to abdominal infections and an immunologic/immunogenetic origin has also been suggested. Histologic evaluation shows obliterative venopathy of the liver and suben-dothelial thickening of the large and medium-sized intrahepatic branches of the portal vein with a patchy, segmental distribution; marked dilatation of the sinusoids may also be present.17,18
In NRH, hepatocellular nodules are distributed throughout the liver in the absence of fibrous septae between the nodules. Histologically, hypertrophied and hyperplastic hepatocytes are identified in the periportal areas while the centrovenular zone features sinusoidal dilatation with relative hepatocellular atrophy; fibrosis is usually little or absent. NRH is usually associated with a variety of hepatic and systemic diseases as an adaptation to heterogeneous distribution of blood flow rather than due to a distinct disease entity. Conditions most frequently associated with NRH are myeloproliferative disorders, collagen vascular diseases (lupus erythematous, rheumatoid arthritis), infections, drug toxicity (azathioprine, thioguanine) and malignancies.1,18
Various mechanisms have been postulated to explain the development of NCPH in HIV infected patients. Hepatotoxicity induced by ART has been recently reported with an incidence of 2-18% in treated patients.19 Among all the antiretroviral drugs usually used, nevirapine (NVP) has been associated with short and long term consequences of iatrogenic liver damage. NVP has been implicated in causing severe hepatitis soon after the initiation of therapy, especially in women with low BMI.20 However, in other reports, NVP hepatotoxicity had a later onset, with an increase cumulative incidence over time.21 In patients with chronic HCV infection, NVP was associated with a more rapid progression of fibrosis,22 but a large series showed no association between NVP use and chronic liver disease.9 On the other hand, prolonged exposure to ddl has described to correlate strongly with the presence of cryptogenic liver disease.9,12,15 Recently, Maida, et al. examined the clinical outcome following ddl removal on HIV-infected patients who exhibited persistently elevated aminotransferases and/or liver fibrosis of unknown cause, resulting in significant normalization of the transaminases and fewer episodes of hepatic clinical decompensation.23 Interestingly, liver fibrosis regression assessed by percutaneous elastography was not significant; this may be explained by the low amount of baseline fibrosis found in patients with HPS and NRH. Fulminant hepatitis with severe lactic acidosis secondary to ddI has been described as well.24 Some drugs like 6-thioguanine and azathioprine may affect the endothelial cells in the portal system resulting in veno-occlusive disease,25 and a similar mechanism should perhaps explored for ddI. HIV itself has been suggested to cause NRH, but a recent report failed to find any association between cryptogenic liver disease and the duration of the HIV infection, high plasma viremia levels and/or low CD4 count.9
Portal vein thrombosis (PVT) has also been described in HIV patients who developed NCPH. In our series, we identified two patients with PVT. Maida, et al.23 suggested that this may be due to repeated episodes of pylephlebitis caused by gastrointestinal pathogens, particularly in men who have sex with men. These episodes of septic microthrombophlebitis could possibly occur as a consequence of an enhanced expression of tissue factors,26 disruption of the intestinal immunological barrier27 and lower levels of anticoagulation factors such as antithrombin III, protein C and S27 seen in HIV infection. Finally, a “two-hit” model to explain the association between ART and liver disease has been proposed,23 in which NRH could be secondary to the portal endothelial damage caused by long-term exposure to ddI, in conjunction with repeated episodes of pylephlebitis. Blood stasis secondary to increase liver resistance to portal venous flow may also potentiate the risk of thrombosis. Other possible explanations should be explored because other causes of NCPH, like HPS, are not known to cause endothelial vascular damage.
Management of patients with HIV associated liver disease secondary to NCPH is primarily directed at the complications. The immediate approach to variceal bleeding and ascites does not differ from that of any other patient with the same clinical manifestations due to any other etiology of chronic liver disease. In case of variceal hemorrhage, effective decrease in portal pressure can be addressed by non-selective beta-blockers or portosystemic shunts, with the latter being pursued usually in those who have failed beta blocker prophylaxis and/or endoscopic therapy. TIPS or surgical shunts may serve as a bridge to liver transplant. In this scenario, where the synthetic liver function is preserved and where liver transplantation might not become a consideration, a surgical shunt might be preferable to TIPS given its long-term patency. However, surgical shunts are difficult operations requiring significant expertise. TIPS technology is advancing with newer coated stents with lower rates of restenosis,29 so this might turn to be the more generalizable and accepted treatment. Infrequently, hepatic synthetic function can be compromised or the complications of portal hypertension are sufficiently severe to warrant liver transplantation. A few cases of liver transplantation for NCPH have been reported with good long-term graft function outcome.2 Recently, Tateo, et al.3 reported 3 patients with NRH and HIV who developed liver failure and were successfully treated with liver transplantation. However, NRH ocurrence in the posttransplant setting has also been described.30
In summary, we have described six HIV infected patients who developed NCPH while receiving ART. It remains unclear if HIV infected patients who present with abnormal hepatic biochemical tests should routinely undergo upper endoscopy surveillance for varices. On the other hand, clinicians should definitely include HPS and NRH in their differential diagnosis in this clinical scenario, particularly if there are features of hypersplenism. If ART regimen includes ddI, substitution of this antiretroviral drug with an alternative agent should be considered. Further studies are required to clarify whether antire-troviral drugs are responsible for endothelial damage of the portal system or if septic microthrom-bophlebitis plays a role in modifying the hepatic histological architecture.
Abbreviations- •
NCPH: Noncirrhotic portal hypertension
- •
NRH: Nodular regenerative hyperplasia
- •
HPS: Hepatoportal sclerosis
- •
ART: Antiretroviral therapy
- •
HCV: Hepatitis C virus
- •
HBV: Hepatitis B virus
- •
TIPS: Transjugular intrahepatic portosystemic shunt
- •
ddl. Didanosine
- •
NVP: Nevirapine
- •
PVT: Portal vein thrombosis