Abstracts of the 2023 Annual Meeting of the ALEH
More infoAtezolizumab and bevacizumab (Atez/Bev) are the new standard of care for first-line systemic therapy of hepatocellular carcinoma (HCC). Real-world data on safety and efficacy of Atez/Bev are scarce in Latin America. We aimed to describe safety and efficacy of Atez/Bev in patients with HCC Barcelona Clinic Liver Cancer (BCLC) B and C stages.
Materials and MethodsProspective cohort study at two tertiary healthcare centers in Porto Alegre, Southern Brazil, included consecutive HCC patients within BCLC B or C stages started with Atez/Bev as first line therapy between 2020-2023. Demographics, tumor response, overall survival (OS), and adverse events were assessed.
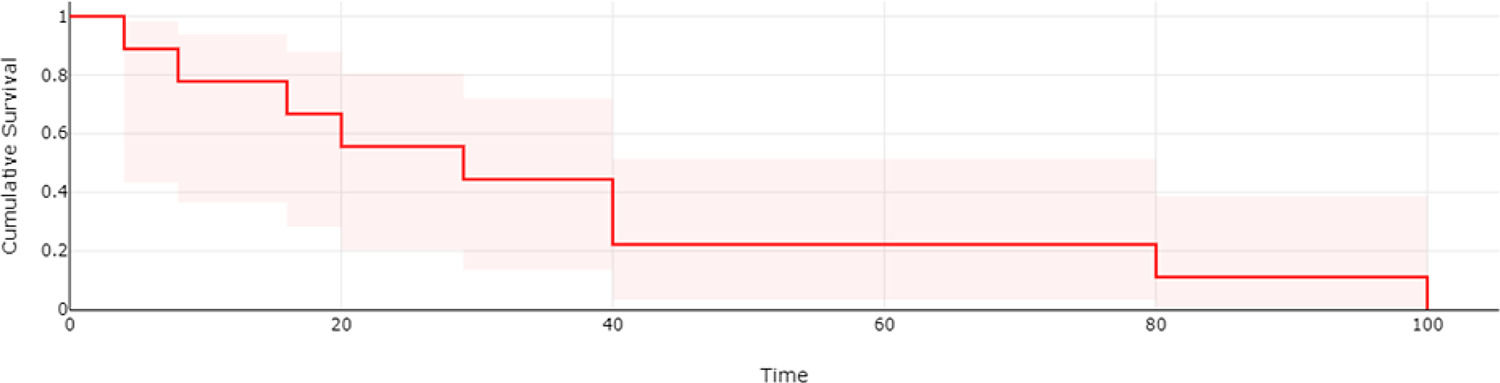
ResultsA total of 20 patients, 16 males (80%), all with cirrhosis (HCV 13, HBV 3, NASH 3, alcohol 1). Child-Pugh were A and B (17 and 3, respectively). Median MELD was 8 (IQR 7-10.5) and median age 70.5 years-old (IQR 61-72.8). Median baseline alfa-fetoprotein was 36.8 (IQR 6.6-2.696). Esophageal varices in 11 individuals (65%). Majority (19/20) was BCLC stage C and ECOG 0/1. Previous HCC treatment was surgery (n=2, 10%), radiofrequency ablation (n=1, 5%) or transarterial chemoembolization (n=10, 50%). Macrovascular invasion and extra-hepatic metastasis were detected in 9 (45%) and 5 (25%) patients, respectively. Median Atez/Bev cycles were 5.5 (IQR 3-8.8) and dose reduction occurred in 5 patients (25%). Tumor response was evaluated in 13 patients: partial response in 3 (23.1%), stable disease in 1 (7.6%), and progressive disease in 9 (69.3%). Median follow-up (last visit or death) was 31.5 weeks (IQR 16-47.5). Median OS was 55% (Fig 1). Cirrhosis decompensation occurred in 11/20 individuals (55%) with variceal bleeding in 5/20 (25%), which was the only significant variable associated with mortality (p=0.04).
ConclusionsAtez-Bev in a real-world cohort of intermediary and advanced HCC patients showed efficacy and safety comparable to published studies with similar inclusion criteria.