Although unlimited sessions of conventional transarterial chemoembolization (cTACE) may be performed for liver metastases, there is no data indicating when treatment becomes ineffective. This study aimed to determine the optimal number of repeat cTACE sessions for nonresponding patients before abandoning cTACE in patients with liver metastases.
Materials and MethodsIn this retrospective, single-institutional analysis, patients with liver metastases from neuroendocrine tumors (NET), colorectal carcinoma (CRC), and lung cancer who underwent consecutive cTACE sessions from 2001 to 2015 were studied. Quantitative European Association for Study of the Liver (qEASL) criteria were utilized for response assessment. The association between the number of cTACE and 2-year, 5-year, and overall survival was evaluated to estimate the optimal number of cTACE for each survival outcome.
ResultsEighty-five patients underwent a total of 186 cTACE sessions for 117 liver metastases, of which 30.7 % responded to the first cTACE. For the target lesions that did not respond to the first, second, and third cTACE sessions, response rates after the second, third, and fourth cTACE sessions were 33.3 %, 23 %, and 25 %, respectively. The fourth cTACE session was the optimal number for 2-year survival (HR 0.40; 95 %CI: 0.16–0.97; p = 0.04), 5-year survival (HR 0.31; 95 %CI: 0.11–0.87; p = 0.02), and overall survival (HR 0.35; 95 %CI: 0.13–0.89; p = 0.02).
ConclusionsRepeat cTACE in the management of liver metastases from NET, CRC, and lung cancer was associated with improved patient survival. We recommend at least four cTACE sessions before switching to another treatment for nonresponding metastatic liver lesions.
Liver metastases originating from organs such as colorectal carcinoma (CRC), neuroendocrine tumors (NETs), and lung cancer are a growing healthcare challenge worldwide and are among the most commonly reported causes of cancer-related deaths [1]. Although surgical resection is the curative treatment option for liver metastasis, most patients with liver metastasis are not candidates due to the advanced stage of the disease at diagnosis [2,3]. Therefore, intraarterial therapies (IAT), such as transarterial chemoembolization (TACE), which includes conventional transarterial chemoembolization (cTACE) and drug-eluting beads transarterial chemoembolization (DEB-TACE), represent a pivotal locoregional therapeutic option in interventional oncology approaches to hepatic metastases [4]. These locoregional tumor therapies have demonstrated excellent local tumor control rates and improved overall survival when compared with the best supportive care [2,5].
While cTACE has become part of the treatment algorithm for most patients with primary and metastatic liver cancer [6], there is no standardized TACE treatment protocol to date. Treating physicians generally tailor the number of TACE sessions based on inadequate evidence in the literature [2,7]. In terms of hepatocellular carcinoma (HCC) as a primary liver tumor, the limited available data favors repeat TACE in managing patients who fail to respond to the first TACE or develop progressive disease after response to TACE [8-12]. However, the utility of repeat TACE in patients with hepatic metastasis remains unsettled, with only a limited number of studies on patients with NETs [13,14]. Therefore, we evaluated the radiologic responses and the 2-year, 5-year, and overall survival outcomes associated with different numbers of cTACE sessions among patients with liver metastases from CRC, NETs, and lung cancer. We also determined the optimal number of repeat cTACE for each survival interval accordingly.
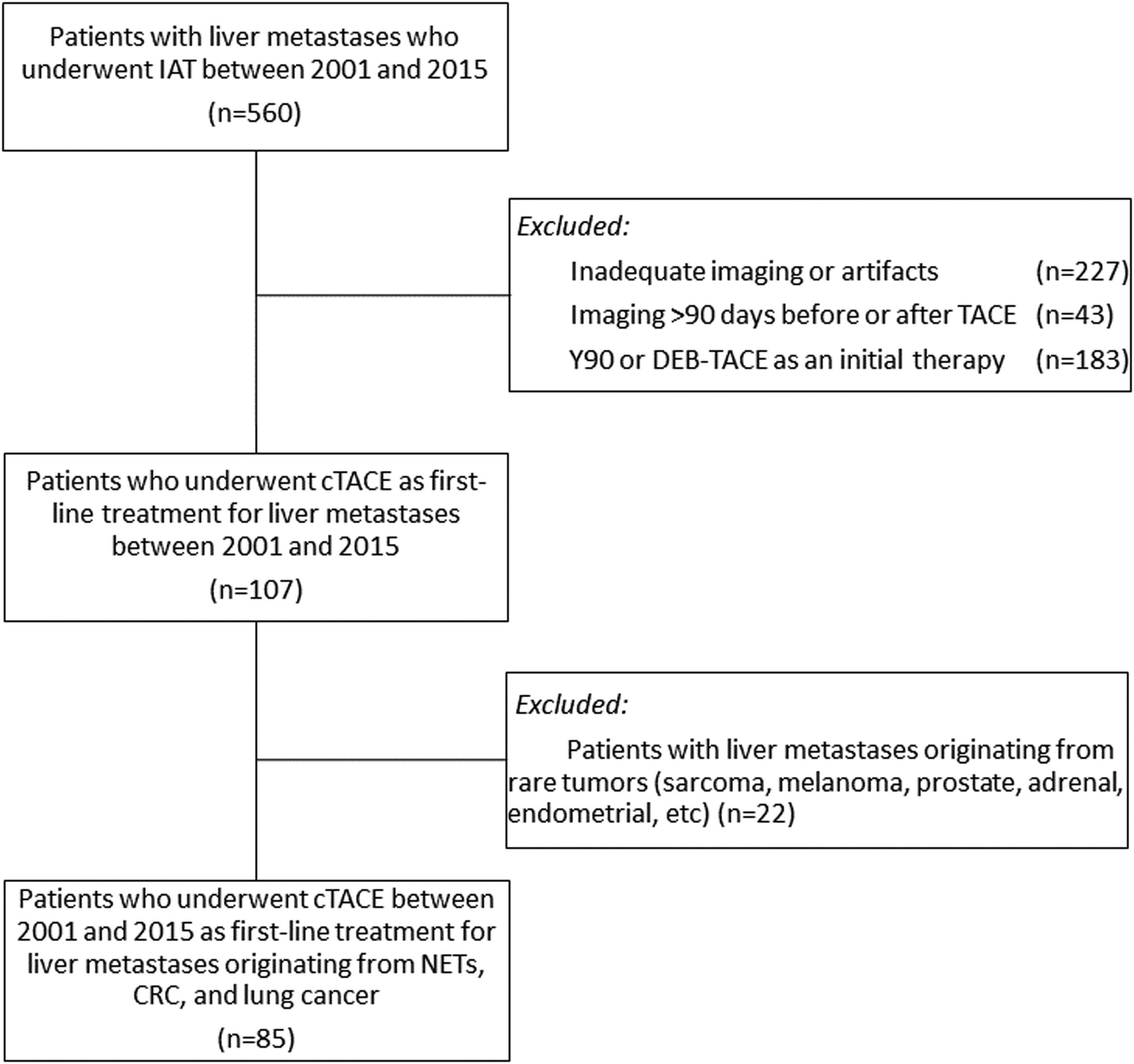
2Materials and Methods2.1Study design and patient populationWe performed a retrospective analysis of 560 consecutive patients with biopsy-proven liver metastasis from other organ malignancies, who underwent a total of 1320 IAT sessions from 2001 to 2015. Patients were included if they received initial Lipiodol-based cTACE, followed by consecutive cTACE treatments if they did not respond to the initial therapy, and had baseline and follow-up contrast-enhanced magnetic resonance imaging (MRI) within 90 days before and after TACE. Patients were excluded if their imaging was inadequate or affected by artifacts such as respiratory motion artifact, or if they had received Yttrium-90 radioembolization (Y90) or DEB-TACE as initial therapy. Additionally, patients with liver metastases from sources other than CRC, NETs, or lung cancer were excluded due to the limited number of such patients, which could have affected the reliability of the subsequent analyses. The study flow is demonstrated in Fig. 1.
Flowchart of the study's eligibility criteria illustrates the selection of patients based on the inclusion and exclusion criteria. CRC = colorectal carcinoma; cTACE = conventional transarterial chemoembolization; DEB-TACE = drug-eluting bead transarterial chemoembolization; IAT = intraarterial therapy; NETs = neuroendocrine tumors; Y90 = Yttrium-90 radioembolization.
The cTACE procedure was performed according to standard institutional protocol by one of two interventional radiologists, each with over 15 years of experience in hepatic interventions [15]. First, hepatic arteriography was conducted, followed by dual-phase cone-beam computed tomography (CBCT) with intraarterial injection of contrast medium to evaluate the hepatic arterial anatomy and tumor vascularity. Then, selective and super-selective cTACE was performed using a combination of 50 mg doxorubicin (Adriamycin; Pharmacia & Upjohn, Peapack, NJ) and 10 mg mitomycin-C in an emulsion with an ethiodized oil (Lipiodol; Guerbet, France), followed by the infusion of bland microspheres (diameter: 100–300 μm; Embospheres, Merit Medical, South Jordan, UT) [16].
2.3Imaging protocolsAll patients received baseline contrast-enhanced MRI and follow-up contrast-enhanced MRI between two weeks and three months post-procedure. If more than three months had elapsed from a previous cTACE session, patients underwent further follow-up/re-staging imaging before an additional cTACE session. All patients had intraprocedural angiography or CBCT, or postprocedural non-contrast CT imaging recorded within one month to determine embolization targets.
2.4Image analysisImage-based tumor response was reviewed by three readers for each cTACE session using RadiAnt DICOM viewer (Medixant, Poznan, Poland). Then, 3D image segmentation was implemented using GeoBlend, a prototype software (Medisys, Philips Research, Suresnes, France), to create 3D volumes of pre-selected hepatic lesions. These segmentation masks were generated on contrast-enhanced T1-weighted MRIs of the arterial phase acquired before and after cTACE treatment. This method provided the basis for target tumor volumetric changes (Fig. 2. A and B).
To produce qEASL values, a 3D segmentation of the tumor boundaries was acquired using semi-automated segmentation software to generate volumetric maps of the tumors (IntelliSpace Portal 8.0, Philips Healthcare, Haifa, Israel). Finally, a manual selection of a 3D region of interest from the non-tumoral liver parenchyma allowed quantification of enhancing tumor volume. Within the 3D segmentation mask, viable enhancing tumor tissue (red) and necrotic non-enhancing tissue (blue) are illustrated in Fig. 2. C and D. The reproducibility and inter-reader reliability of the segmentation software has been previously demonstrated [17-19].
Patients were categorized into responders (complete response and partial response) and nonresponders (stable and progressive disease) based on the radiologic tumor response criteria using the qEASL criterion [20].
2.5Statistical analysisStatistical analysis of the data was performed using R 4.0.2 (http://www.R-project.or). For continuous variables, descriptive statistics are presented as mean and standard deviation, or median and interquartile range (IQR). For categorical variables, descriptive statistics are presented as absolute numbers and percentages. The rates of radiologic response in nonresponders to previous cTACE sessions were calculated for each tumor type subgroup. Additionally, the median 2-year, 5-year, and overall survival were determined for the entire cohort and each tumor type subgroup. At the lesion level, multivariable logistic regression analysis was performed to analyze the associations between the number of cTACE sessions, tumor type (CRC, NET, or lung cancer), and enhancing tumor volume with the responder status of the tumor. At the patient level, survival curves (at 2 years, 5 years, and overall) among patients undergoing one to six cTACE sessions were estimated with Kaplan-Meier curves and analyzed with the log-rank test. The Cox proportional hazards model was used to determine significant prognostic factors, including the number of cTACE sessions, on 2-year, 5-year, and overall survival. Based on these analyses, we estimated the optimal number of cTACE sessions. Overall survival was calculated as the interval between the date of the first cTACE and death or the last known observation. We used median values of 60 cm3 and 100 cm3 as thresholds for enhancing tumor volume in the multivariable logistic regression and Cox proportional hazards model analyses, respectively. A p value <0.05 was considered statistically significant.
2.6Ethical statementThis single-institution, retrospective study was conducted in compliance with the Health Insurance Portability and Accountability Act. The institutional review board approved the study and waived the requirement for informed consent.
3Results3.1Patient characteristicsA total of 85 patients with liver metastases originating from CRC (n = 31, 36.5 %), NET (n = 43, 50.6 %), and lung cancer (n = 11, 12.9 %) were studied. Table 1 summarizes the study population's characteristics. The median age of the patients at the time of initial cTACE was 59.4 years (IQR: 51.6–65.4). Among the 85 patients, 117 unique metastatic targets were identified with a median number of 1.38 (IQR: 1–2) per patient. A total of 186 cTACE sessions were performed, with a mean of 1.59 (SD: 0.95) per target lesion. The maximum number of cTACE sessions for any specific metastatic target was six. The 30-day survival rate after the initial cTACE session was 97.7 %. No patient died before receiving at least one follow-up imaging assessment. At the last follow-up, 68 of the 85 patients had died. The median overall survival was 12.0 months (IQR: 5.6–27.4).
Baseline characteristics of the study cohort.
| Variable | Study Cohort (n = 85) |
|---|---|
| Sex, n (%) | |
| Male | 49 (57.6 %) |
| Female | 36 (42.4 %) |
| Age (years), median (IQR) | 59.4 (51.6–65.4) |
| Ethnicity, n (%) | |
| White | 64 (75.3) |
| African-American | 14 (16.5) |
| Other | 7 (8.2) |
| cTACE treatments, median (range) | 2 (1–6) |
| Tumor type, n (%) | |
| NET | 43 (50.6) |
| CRC | 31 (36.5) |
| Lung cancer | 11 (12.9) |
| Serum total bilirubin (mg/dL), median (IQR) | 0.5 (0.3–0.7) |
| Tumor size (cm), median (IQR) | 12 (8.7–15.3) |
| Serum albumin (g/dL), median (IQR) | 3.9 (3.7–4.2) |
| Serum INR, median (IQR) | 1 (0.9–1) |
| Ascites, n (%) | |
| Absent | 69 (81.2) |
| Slight | 13 (15.3) |
| Moderate | 3 (3.5) |
| Encephalopathy, n (%) | |
| Absent | 85 (100) |
| Present | 0 (0) |
| Cirrhosis, n (%) | 0 (0) |
| ALBI score, median (IQR) | −2.7 (−2.9 to −2.5) |
| ALBI grade, n (%) | |
| 1 | 54 (63.5) |
| 2 | 25 (29.4) |
| 3 | 1 (1.2) |
| Missing data | 5 (5.9) |
| Child-Pugh Class, n (%) | |
| A | 63 (74.1) |
| B | 8 (9.4) |
| Unclassified | 14 (16.5) |
ALBI, Albumin-Bilirubin; CRC, colorectal carcinoma; cTACE, conventional transarterial chemoembolization; INR, international normalized ratio; NET, neuroendocrine tumors.
After the first cTACE of 117 target lesions, 36 (30.7 %) lesions responded. Following the second cTACE of 45 nonresponding target lesions, 15 (33.3 %) lesions responded. Only three (23 %) lesions responded to the third cTACE of 13 nonresponding target lesions. Among the eight nonresponding target lesions that underwent the fourth cTACE session, only two (25 %) lesions responded. The rates of radiologic response in nonresponders to previous cTACE session for each tumor type subgroup are presented in Table 2.
Rates of radiologic response in nonresponders to previous cTACE session for the entire cohort and each tumor type subgroup.
| Total | Nonresponder | Responder | P-value | ||
|---|---|---|---|---|---|
| CRC | Number of lesions | 37 | 25 | 12 | – |
| Enhancing tumor volume (cm3), median (IQR) | 60.5 (13.3–133.4) | 40.7 (11.4–108.0) | 123.4 (16.8–196.9) | 0.25 | |
| Responder after | – | – | – | ||
| 1st cTACE | 9/37 (24.3 %) | ||||
| 2nd cTACE | 4/11 (36.3 %) | ||||
| 3rd cTACE | 0/3 (0 %) | ||||
| 4th cTACE | 0/3 (0 %) | ||||
| 5th cTACE | 0/1 (0 %) | ||||
| NET | Number of lesions | 68 | 42 | 26 | – |
| Enhancing tumor volume (cm3), median (IQR) | 56.4 (12.3–210.6) | 44.0 (7.7–164.5) | 97.6 (21.2–401.6) | 0.07 | |
| Responder after | – | – | – | ||
| 1st cTACE | 22/68 (32.3 %) | ||||
| 2nd cTACE | 10/32 (31.2 %) | ||||
| 3rd cTACE | 3/10 (30 %) | ||||
| 4th cTACE | 2/5 (40 %) | ||||
| 5th cTACE | 0/2 (0 %) | ||||
| Lung cancer | Number of lesions | 12 | 7 | 5 | – |
| Enhancing tumor volume (cm3), median (IQR) | 60.7 (5.5–279.2) | 61.9 (4.0–441.1) | 39.5 (5.9–89.0) | 0.53 | |
| Responder after | – | – | – | ||
| 1st cTACE | 5/12 (41.6 %) | ||||
| 2nd cTACE | 1/2 (50 %) | ||||
| 3rd cTACE | – | ||||
| 4th cTACE | – | ||||
| 5th cTACE | – | ||||
| Entire cohort | Number of lesions | 117 | 74 | 43 | – |
| Enhancing tumor volume (cm3), median (IQR) | 59.5 (12.0–188.7) | 46.7 (10.1–138.9) | 80.5 (17.9–197.5) | 0.12 | |
| Responder after | – | – | – | ||
| 1st cTACE | 36/117 (30.7 %) | ||||
| 2nd cTACE | 15/45 (33.3 %) | ||||
| 3rd cTACE | 3/13 (23 %) | ||||
| 4th cTACE | 2/8 (25 %) | ||||
| 5th cTACE | 0/3 (0 %) |
CRC, colorectal carcinoma; cTACE, conventional transarterial chemoembolization; NET, neuroendocrine tumors.
At the lesion-level, a multivariable logistic regression model including tumor type (CRC, NET, or lung cancer), enhancing tumor volume ≥60 cm3, and number of cTACE sessions showed no significant association with the radiologic response of the lesion to cTACE (p > 0.05; Supplementary Table 1). Figs. 3-5 illustrate the Kaplan-Meier curves for 2-year, 5-year, and overall survival. The median 2-year, 5-year, and overall survival are presented in Supplementary Table 2 for the entire cohort and each tumor type subgroup. At the patient-level, based on univariate Cox regression analyses, compared to one cTACE session, the optimal number of cTACE sessions was four for 2-year survival (HR 0.29; 95 %CI: 0.10–0.83; p = 0.02), 5-year survival (HR 0.23; 95 %CI: 0.08–0.63; p = 0.004), and overall survival (HR 0.27; 95 %CI: 0.11–0.65; p = 0.004). This finding persisted after adjusting for tumor type and enhancing tumor volume ≥100 cm3 in multivariate Cox regression analyses (Table 3). Four vs. one cTACE sessions was associated with better 2-year survival (HR 0.40; 95 %CI: 0.16–0.97; p = 0.04), 5-year survival (HR 0.31; 95 %CI: 0.11–0.87; p = 0.02), and overall survival (HR 0.35; 95 %CI: 0.13–0.89; p = 0.02). In addition, tumor type (NEC vs. CRC) (HR 0.35 for 2-year survival; HR 0.30 for 5-year and overall survival; p < 0.001 for all) and enhancing tumor volume ≥100 cm3 (HR 1.87 for 5-year survival; HR 1.91 for overall survival; p = 0.03 for both) were identified as independent predictors of patient survival (Table 3).
Multivariable Cox regression analysis investigating impact of tumor type, enhancing tumor volume, and different number of cTACE sessions on 2-year, 5-year, and overall survival in patients with liver metastases.
| 2-year survival | 5-year survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| HR (95 % CI) | P value | HR (95 % CI) | P value | HR (95 % CI) | P value | |
| Tumor type | ||||||
| CRC | 1.00 (Ref) | – | 1.00 (Ref) | – | 1.00 (Ref) | – |
| NEC | 0.35 (0.19–0.65) | <0.001 | 0.30 (0.16–0.55) | <0.001 | 0.30 (0.16–0.55) | <0.001 |
| Lung | 0.56 (0.25–1.28) | 0.17 | 0.81 (0.35–1.88) | 0.62 | 0.81 (0.35–1.88) | 0.67 |
| Enhancing tumor volume ≥ 100 cm3 | 1.10 (0.62–1.95) | 0.73 | 1.87 (1.04–3.37) | 0.03 | 1.91 (1.08–3.39) | 0.03 |
| Number of cTACE session | ||||||
| 1 | 1.00 (Ref) | – | 1.00 (Ref) | – | 1.00 (Ref) | – |
| 2 | 1.04 (0.55–1.94) | 0.89 | 1.03 (0.54–1.94) | 0.92 | 0.99 (0.53–1.87) | 0.99 |
| 3 | 0.40 (0.15–1.08) | 0.07 | 0.44 (0.18–1.09) | 0.07 | 0.37 (0.15–0.94) | 0.03 |
| 4 | 0.40 (0.16–0.97) | 0.04 | 0.31 (0.11–0.87) | 0.02 | 0.35 (0.13–0.89) | 0.02 |
| 5 | 0.41 (0.10–1.43) | 0.16 | 0.42 (0.14–1.26) | 0.12 | 0.50 (0.18–1.37) | 0.17 |
CI, confidence interval; cTACE, conventional transarterial chemoembolization; HR, hazard ratio.
Our findings showed that a significant proportion of liver metastases (69.3 %) did not respond to the first cTACE session. Among the nonresponders to the first cTACE who underwent repeat sessions, 33.3 %, 23 %, and 25 % responded to the second, third, and fourth cTACE sessions, respectively. Importantly, our findings revealed that the fourth cTACE session was associated with optimal outcomes in the management of liver metastases, showing improved 2-year, 5-year, and overall survival.
The current literature on the utility of repeat TACE in patients with liver metastases is limited. A retrospective study of 27 patients with liver metastases from NET, who showed radiologic or clinical progression after initial cTACE, found that repeat cTACE was well tolerated and associated with comparable survival outcomes to the first cTACE [14]. In contrast, a recent retrospective study of 202 patients with NET liver metastases reported a decreased time to progression with repeat TACE cycles, although stable response rates were observed [13]. This contrasts with our findings, which showed better survival outcomes with repeat cTACE sessions. This discrepancy between our study and that of Touloupas et al. [13] may stem from methodological differences. In our study, we used qEASL for response assessment of liver metastases from NET, CRC, and lung cancer, to evaluate the association between repeat cTACE and overall survival. Notably, our study exclusively included patients who underwent cTACE and excluded those treated with DEB-TACE. In contrast, Touloupas et al. [13] used Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST (mRECIST) criteria to assess response in patients with NET liver metastases, exploring the association between repeat TACE and time to progression as a survival outcome measure.
Our study of nonresponding liver metastases suggests that four cTACE sessions may be optimal before considering switching to other treatments. To our knowledge, no previously published data have established the optimal number of cTACE sessions before abandoning this therapy for liver metastases. In contrast, guidelines for HCC from the European Association for the Study of the Liver discourage repeat TACE after two unsuccessful TACE treatments without significant necrosis induction [21]. However, a recent retrospective study of 4154 patients with HCC recommended up to three cTACE sessions before considering alternative treatments for nonresponding intermediate-stage HCC [8]. This approach suggests that a third cTACE session could potentially induce tumor vulnerability to embolization and local chemotherapy, leading to a favorable tumor response [8].
Our study identified that an enhancing tumor volume of less than 100 cm³ was associated with better patient survival in liver metastases. This finding highlights the prognostic significance of tumor burden in determining outcomes following cTACE. Similar observations have been reported in studies assessing the impact of tumor size on treatment response and survival in liver metastases originating from CRC and NET [22,23].
The qEASL metric has proven to be superior in predicting tumor response to IAT across various cancers, including NET, CRC, sarcoma, and uveal melanoma [24-27]. Recently, volumetric enhancement-based assessment using qEASL has been suggested as a valuable diagnostic marker for evaluating tumor response following cTACE in liver metastases originating from rare tumors [16]. The enhanced diagnostic performance of qEASL is attributed to the distinct effects of embolotherapy on the local tumor environment compared to systemic chemotherapy. While systemic agents typically cause tumor shrinkage, IAT induce tumor necrosis, often without an immediate change in tumor diameter [28]. This makes purely anatomical response criteria such as RECIST less effective and highlights the importance of biological criteria for assessing response to IAT [29]. The growing adoption of enhancement-based response metrics such as qEASL and mRECIST in clinical practice is expected to improve the accuracy of response assessment and guide more effective management decisions [30].
Our study has several limitations. First, it was conducted at a single institution with a retrospective design. Second, the study population was heterogenous, including hypervascular metastases originating from NET, and hypovascular metastases from lung cancer or CRC. The difference in vascularity could potentially influence the response to therapy [31]. Nevertheless, our finding of an association between repeat cTACE in the management of liver metastases and improved patient survival persisted after including the tumor type in the multivariable Cox regression model. Third, there were some surviving nonresponders to cTACE sessions who did not receive repeat cTACE, introducing potential selection bias. Fourth, during the study period (2001 to 2015), the treatment regimens were modified multiple times, and new systemic therapies were adopted. However, data on the regimens of systemic therapy for the primary cancer, as well as therapies subsequently provided to patients who progressed after cTACE, were not recorded. These factors may have affetcted the survival outcomes observed in the present study. Moreover, data on treatment-related toxicity or post-procedure complications, such as postembolization syndrome, were not included in the present study. Finally, our focus was on liver metastases response to cTACE, and it is possible that patients may have died due to the primary tumor or metastases to other organs. Therefore, evaluating additional endpoints, such as progression-free survival, could have provided more comprehensive insights. However, we chose to focus on overall survival as the primary endpoint, which is often considered ideal in liver cancer research [32]. Despite these limitations, our study includes a large cohort of relatively rare tumor types. The patients were treated and imaged using consistent protocols and were followed up for an extended duration.
5ConclusionsRepeat cTACE in the management of liver metastases from NET, CRC, and lung cancer was associated with improved overall survival. Four cTACE sessions was associated with a better 2-year, 5-year, and overall survival in patients with metastatic liver disease from CRC, NET, and lung cancer. We recommend at least four cTACE sessions for nonresponding lesions in patients with liver metastases before switching to another treatment option.
FundingThis study was funded by NIH/NCIR01 CA206180 grant.
Authors contributionsStudy concept and design (K.G., A.W.H., J.C., N.N.), acquisition of data (K.G., A.W.H., N.M., T.B., L.C.A., F.L.G., M.L., N.N.), analysis and interpretation of data (K.G., A.W.H., M.K., N.M., T.B., L.C.A., F.L.G, M.L., J.C., C.G, N.N.), drafting of the manuscript (K.G., A.W.H., M.K., N.N.), critical revision of the manuscript for important intellectual content (K.G., M.K., C.G., N.N.).
Data availability statementThe raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
None