In 2020, a total of approximately 900,000 individuals worldwide were diagnosed with hepatocellular carcinoma (HCC), with 830,000 fatalities attributed to the disease. HCC ranks among the leading causes of cancer-related mortality globally [1].
Liver transplantation (LT) is considered the optimal treatment for patients who have early HCC in conjunction with decompensated cirrhosis and/or clinically significant portal hypertension, as it addresses both the tumor and the underlying liver disease [2].
Despite the stringent selection criteria applied to patients with HCC for transplantation, recurrence still occurs in approximately 16 % of individuals [3]. While the Milan Criteria (MC) [4] have represented a significant breakthrough in the management of HCC patients, they rely solely on morphometric criteria, and the size and number of nodules alone cannot consistently predict tumor biology, as discrepancies exist between radiological and pathological assessments [5]. Consequently, there is a critical need to develop a reliable method that can effectively discern the risk of tumor recurrence in eligible patients.
Over the past few decades, numerous prognostic models have been developed to accurately assess the risk of HCC recurrence following LT. These models incorporate various pre- and post-transplant variables, including tumor biological markers and inflammatory indicators [6]. The selection of an appropriate prognostic model plays a pivotal role in guiding treatment decisions and organ allocation, thereby enhancing the likelihood of successful LT and improving patient survival.
The objective of this study was to evaluate the performance of different predictive models for HCC recurrence after LT in a cohort of patients who were not included in the development of these models.
2Materials and Methods2.1Study design and populationThis was a retrospective cohort study. Patients ≥ 18 years of age, of both sexes, with diagnosis of liver cirrhosis and imaging findings suggestive of HCC who underwent deceased donor LT between June 2007 and December 2019 were considered for inclusion. The Brazilian Milan Criteria (BMC), based on the MC, was used for listing patients with HCC and compensated cirrhosis [6–10]. According to the BMC, eligible patients must meet one of the following criteria: (1) a single nodule measuring 2 to 5 cm, or (2) up to three nodules measuring between 2 and 3 cm each, plus any number of nodules smaller than 2 cm.
HCC was demonstrated on the explant or, in the case of non-viable tumors in liver explant, diagnosis of HCC was based on pre-LT imaging findings. HCC diagnosis before LT was based on the American Association for the Study of Liver disease diagnostic criteria [2]. After the LT, all patients were followed up on in the same transplant referral center. Standard immunosuppression was performed with tacrolimus and prednisone, after induction with methylprednisolone. Prednisone was gradually reduced, usually being discontinued around six months after transplantation. Since May 2013, everolimus has been used routinely for immunosuppression in these patients.
Demographic information, including age and sex, along with the etiology of the underlying liver disease, MELD score within 24 h before LT, and pretransplant tumor characteristics (such as the number of nodules and the sum of nodule diameters based on imaging), were collected for the study cohort. Additionally, details regarding HCC treatments received prior to LT, such as ablative therapies, chemoembolization, or resection, as well as alpha-fetoprotein (AFP) levels, were obtained. Posttransplant tumor characteristics, including the number of nodules and the sum of nodule diameters, tumor grade/differentiation, complete tumor necrosis, and the presence of microvascular invasion, were assessed through histopathological examination of the explanted liver. Based on imaging studies at HCC diagnosis and the data from the explanted liver, tumor staging was performed according to the BMC [10] or the MC [4].
The diagnosis of post-LT recurrence of HCC was determined using the following criteria: 1) imaging examinations revealing lesions suggestive of HCC recurrence, along with elevations in α-fetoprotein levels; 2) biopsy or examination of surgical specimens confirming the presence of HCC that developed after LT. Subsequently, patients were categorized into two groups: with or without HCC recurrence after LT. The rates of recurrence and overall survival were analyzed on June 30, 2022.
2.2Predictive models assessed in the studyThe study assessed predictive models by utilizing pre- and post-LT variables. The following models were calculated: AFP model [11], University of California, Los Angeles (UCLA) nomogram [12], Model of Recurrence after Liver Transplantation (MORAL): Pre-MORAL, Post-MORAL, and Combo MORAL [13], Risk Estimation of Tumor Recurrence (RETREAT) [14], Platelet to Lymphocyte Ratio (PLR) model [15], and R3-AFP model [16]. For reference, the formulae used in these prognostic models are provided in Table S1.
To compute the scores, we employed the following methods for handling missing data: for continuous variables, we utilized the median value, while for categorical variables, we used the most frequent values. However, when calculating the R3-AFP score, we made a modification by considering the maximum AFP value instead of the last pre-LT AFP value. In instances where complete tumor necrosis was detected, histopathological variables including the number of nodules, tumor size, and microvascular necrosis were recorded as absent for each prognostic model.
2.3Statistical analysisThe primary objective of the study was HCC recurrence after LT. Baseline patient characteristics were described using standard statistical methods. Continuous variables were compared using either Student's t-test or the Mann-Whitney test in cases where distributional assumptions were uncertain. Categorical variables were evaluated using the chi-square test or Fisher's exact test, as applicable. The accuracy of the prognostic models was assessed by calculating the area under the receiver operating characteristics curve (AUROC), accompanied by their respective 95 % confidence interval (CI). Comparisons between the areas were conducted using the Delong Test, without adjustments for multiple comparisons. The 5-year overall survival rate was estimated using the Kaplan-Meier method. The analyses were performed using IBM-SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) and R program version 4.0. Statistical significance was defined as p-values < 0.05.
2.4Ethical aspectsThe study adhered to the ethical guidelines outlined in the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of our institution. Informed consent was waived as the study had a non-interventional design and involved retrospective data collection. To ensure the safe and ethical use of data, all investigators signed a data use document. The study followed the guidelines for publishing observational studies [17].
Approval of the research protocol: Approved by the Institutional Review Board of Santa Casa de Misericórdia de Porto Alegre (study protocol No. 4.250.889).
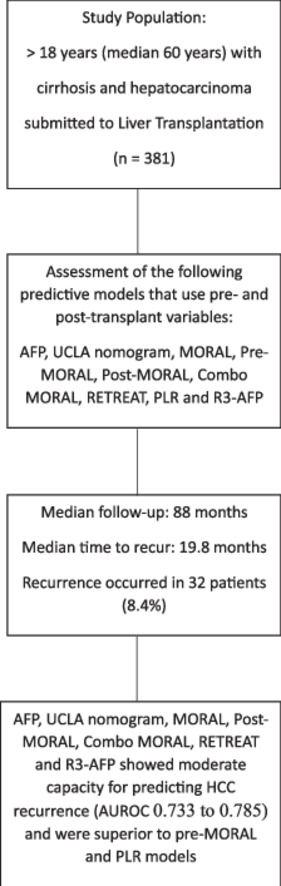
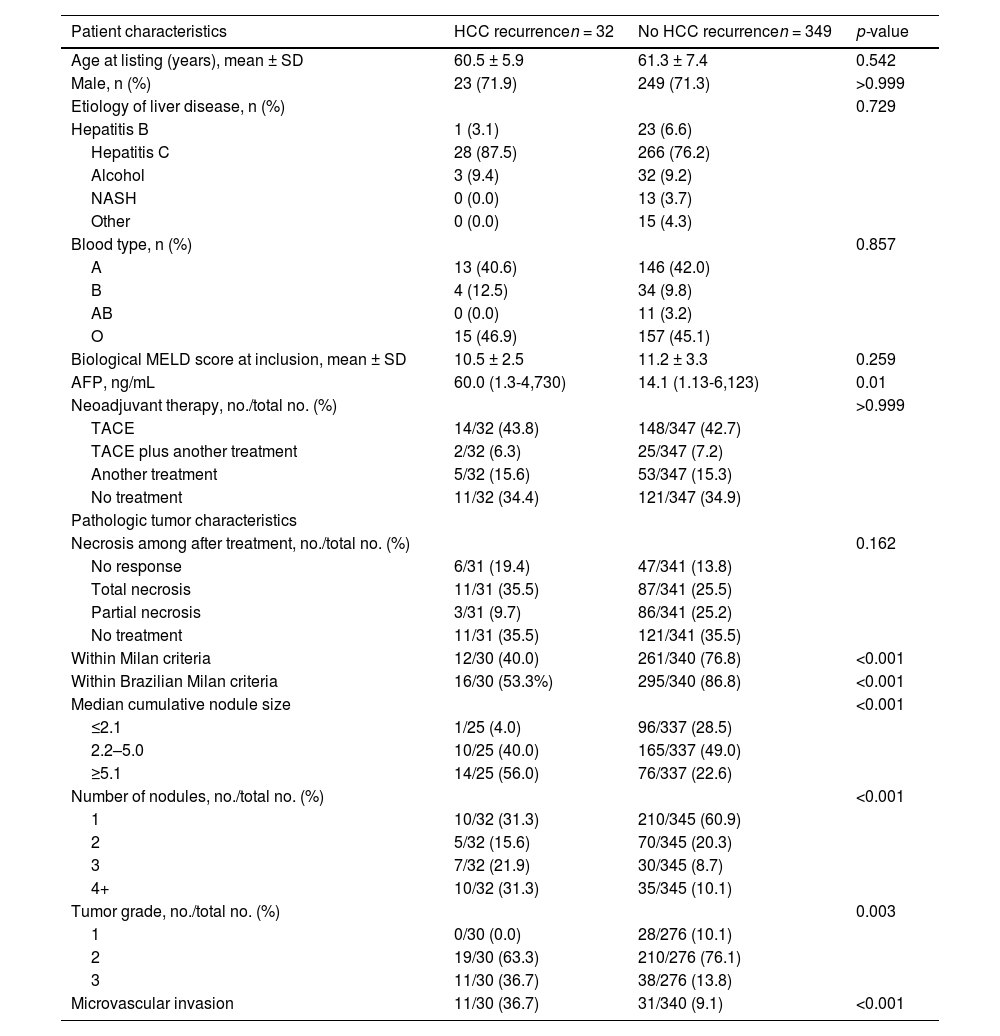
3Results3.1Study populationFollowing the application of the inclusion criteria, a total of 381 HCC patients who underwent LT during the specified period were deemed eligible for the final analysis. Table 1 provides a summary of the demographic data, clinical information, radiological findings, and explant data of the included patients, categorized based on the occurrence of HCC recurrence after LT. Most of the patients were male, and their average age at listing was approximately 60 years. The primary cause of cirrhosis was attributed to the hepatitis C virus. Bridging therapy was administered to 65 % of patients while on the waiting list, with transarterial chemoembolization being the most frequently performed procedure (49.6 %). None of the pre-transplant characteristics showed statistical significance in differentiating between patients who experienced HCC recurrence and those who did not, except AFP levels, which were significantly higher in the recurrence group (60 vs. 14.4 ng/mL; p = 0.01).
Comparison of demographical and clinical parameters in patients with or without HCC recurrence
| Patient characteristics | HCC recurrencen = 32 | No HCC recurrencen = 349 | p-value |
|---|---|---|---|
| Age at listing (years), mean ± SD | 60.5 ± 5.9 | 61.3 ± 7.4 | 0.542 |
| Male, n (%) | 23 (71.9) | 249 (71.3) | >0.999 |
| Etiology of liver disease, n (%) | 0.729 | ||
| Hepatitis B | 1 (3.1) | 23 (6.6) | |
| Hepatitis C | 28 (87.5) | 266 (76.2) | |
| Alcohol | 3 (9.4) | 32 (9.2) | |
| NASH | 0 (0.0) | 13 (3.7) | |
| Other | 0 (0.0) | 15 (4.3) | |
| Blood type, n (%) | 0.857 | ||
| A | 13 (40.6) | 146 (42.0) | |
| B | 4 (12.5) | 34 (9.8) | |
| AB | 0 (0.0) | 11 (3.2) | |
| O | 15 (46.9) | 157 (45.1) | |
| Biological MELD score at inclusion, mean ± SD | 10.5 ± 2.5 | 11.2 ± 3.3 | 0.259 |
| AFP, ng/mL | 60.0 (1.3-4,730) | 14.1 (1.13-6,123) | 0.01 |
| Neoadjuvant therapy, no./total no. (%) | >0.999 | ||
| TACE | 14/32 (43.8) | 148/347 (42.7) | |
| TACE plus another treatment | 2/32 (6.3) | 25/347 (7.2) | |
| Another treatment | 5/32 (15.6) | 53/347 (15.3) | |
| No treatment | 11/32 (34.4) | 121/347 (34.9) | |
| Pathologic tumor characteristics | |||
| Necrosis among after treatment, no./total no. (%) | 0.162 | ||
| No response | 6/31 (19.4) | 47/341 (13.8) | |
| Total necrosis | 11/31 (35.5) | 87/341 (25.5) | |
| Partial necrosis | 3/31 (9.7) | 86/341 (25.2) | |
| No treatment | 11/31 (35.5) | 121/341 (35.5) | |
| Within Milan criteria | 12/30 (40.0) | 261/340 (76.8) | <0.001 |
| Within Brazilian Milan criteria | 16/30 (53.3%) | 295/340 (86.8) | <0.001 |
| Median cumulative nodule size | <0.001 | ||
| ≤2.1 | 1/25 (4.0) | 96/337 (28.5) | |
| 2.2–5.0 | 10/25 (40.0) | 165/337 (49.0) | |
| ≥5.1 | 14/25 (56.0) | 76/337 (22.6) | |
| Number of nodules, no./total no. (%) | <0.001 | ||
| 1 | 10/32 (31.3) | 210/345 (60.9) | |
| 2 | 5/32 (15.6) | 70/345 (20.3) | |
| 3 | 7/32 (21.9) | 30/345 (8.7) | |
| 4+ | 10/32 (31.3) | 35/345 (10.1) | |
| Tumor grade, no./total no. (%) | 0.003 | ||
| 1 | 0/30 (0.0) | 28/276 (10.1) | |
| 2 | 19/30 (63.3) | 210/276 (76.1) | |
| 3 | 11/30 (36.7) | 38/276 (13.8) | |
| Microvascular invasion | 11/30 (36.7) | 31/340 (9.1) | <0.001 |
HCC, hepatocellular carcinoma; SD, standard deviation; AFP, alpha-fetoprotein; NASH, nonalcoholic steatohepatitis; MELD, Model for End-stage Liver Disease; TACE, transarterial chemoembolization.
Data expressed as mean ± SD or median (interquartile range).
The anatomopathological examination of the explanted livers revealed that 11 patients exhibited complete tumor necrosis, which was attributed to one of the neoadjuvant treatments. Among the remaining 370 patients, there were statistically significant differences observed between those who experienced HCC recurrence and those who did not. Patients who experienced recurrence more frequently exceeded the BMC or MC, showing larger and a greater number of nodules. Additionally, they had less differentiated tumors and a higher incidence of microvascular invasion, in comparison to patients who did not experience recurrence (Table 1).
3.2Recurrence rate and overall survivalThe median follow-up period for the study was 88 months. Within this time frame, 32 patients (8.4 %) experienced HCC recurrence, which was confirmed through either imaging or histopathological analysis. The median time from liver transplantation to HCC recurrence was 19.8 months. Post-transplant HCC recurrence rates were observed in 5.1 % of patients within the BMC and 4.4 % within the MC.
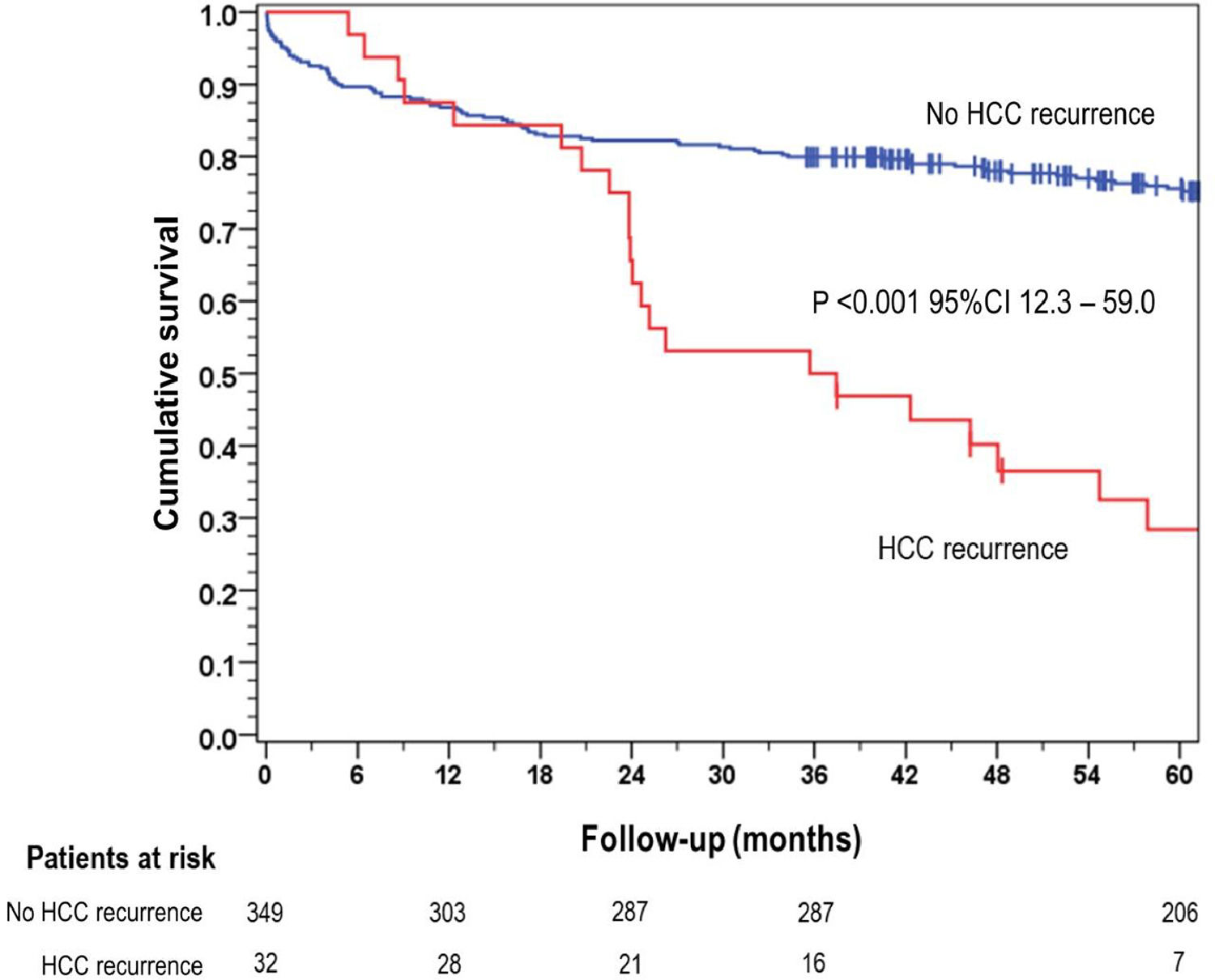
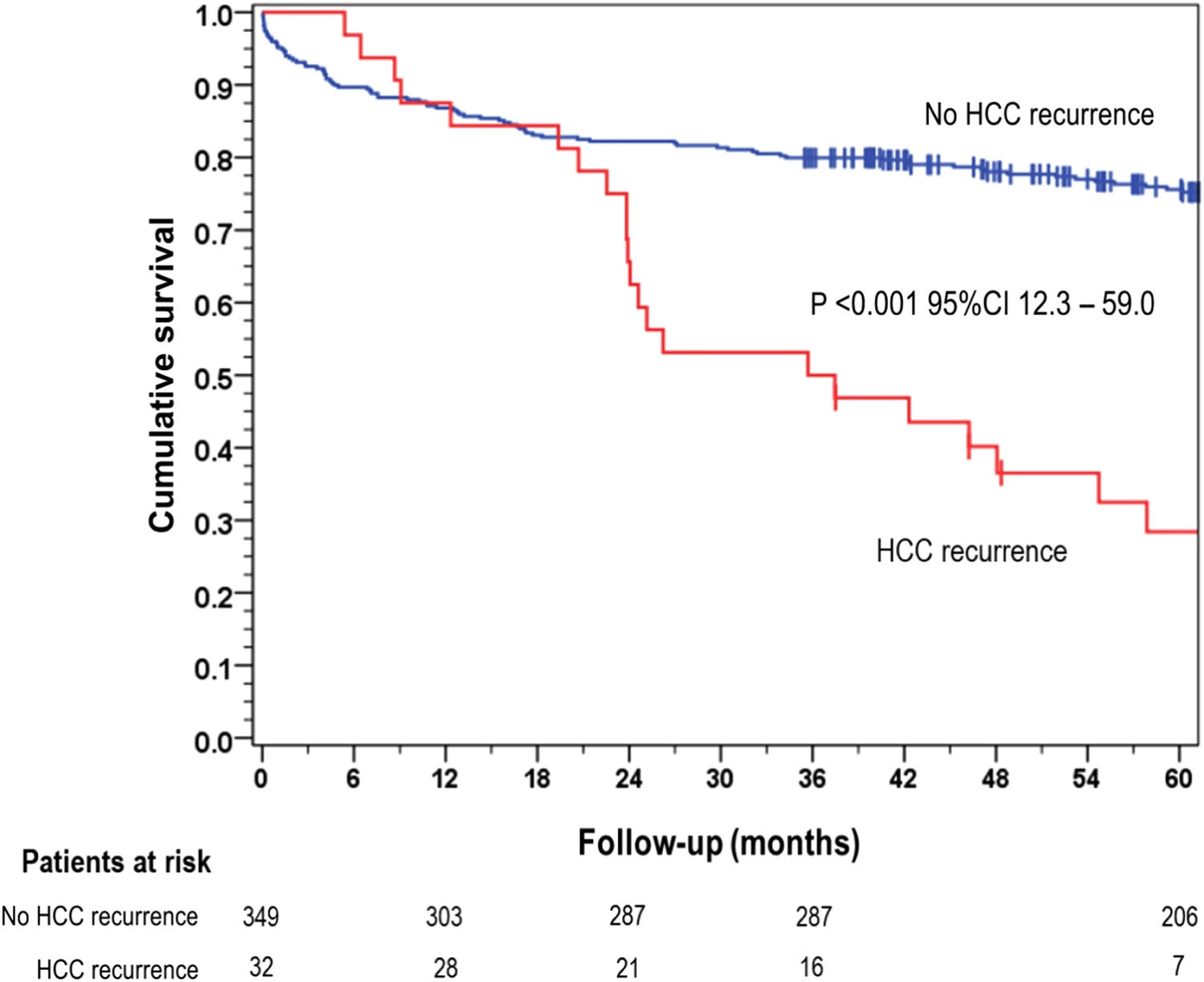
The overall 5-year survival rate was 28.4 % for patients who experienced HCC recurrence, significantly lower than the 75.6 % survival rate observed in the group of patients without recurrence (p < 0.001) (Fig. 1).
3.3Accuracy of prediction as assessed by area under the receiver operating characteristics curve (AUROC) and comparison of the modelsTo determine which was the recurrence-risk assessment model with greater discriminatory power, we calculated the AUROC for each model studied. Subsequently, AUROCs were compared with each other with the Delong test (Fig. 2). According to this analysis, the AUROCs for R3-AFP model (0.785 [95 % CI 0.687-0.883]) and AFP model (0.764 [95 % CI 0.670-0.858]) were superior to pre-MORAL and PLR models (superscript letter a). UCLA nomogram (0.765 [95 % CI 0.671-0.858]), RETREAT score (0.743 [95 % CI 0.634-0.851)], Combo MORAL model (0.749 [95 % CI 0.643-0.854] and Post- MORAL model (0.733 [95 % CI 0.592-0.792]) performed similarly to the first two models but were only superior to PLR (superscript letter b). Pre- MORAL model and PLR presented low AUROCs: 0.692 (95 % CI 0.592-0.792) and 0.566 (95 % CI 0.457-0.675), respectively (superscript letter c). However, pre-MORAL model shares characteristics with models with intermediate values of AUROCs (superscript letter b).
Comparison of AUROCs for different predictive models of HCC recurrence after liver transplantation.
Abbreviations: AUROC, areas under the ROC curve, HCC, hepatocellular carcinoma; AFP, alpha fetoprotein; CI, confidence interval; MORAL, Model of Recurrence After Liver Transplant; PLR, platelet to lymphocyte ratio; RETREAT, Risk Estimation of Tumor Recurrence After Transplant; UCLA, University of California.
*Estimates followed by a common letter do not represent statistically significant differences (p < 0.05) at the pairwise DeLong test (least significant difference approach without multiple comparisons adjustment)
To our knowledge, this is the first study to validate and compare in a single center eight models developed to estimate the risk of HCC recurrence after LT: AFP model [11], UCLA nomogram [12], Pre-MORAL, Post MORAL and Combo MORAL [13], RETREAT [14], PLR model [15], and R3-AFP model [16].
The use of Milan criteria [4] for the selection of candidates with HCC for LT has long been the subject of debate. Studies based mainly on North American and European cohorts investigated predictive models based on additional risk factors [6,18]. In our cohort, the R3-AFP score [16], UCLA nomogram [12], AFP model [11], RETREAT [14], Combo MORAL, and Post-MORAL models [13] exhibited similar AUROCs ranging from 0.785 to 0.733, indicating a moderate predictive capacity with clinical utility. Conversely, the pre-MORAL score [13] (AUROC 0.692) and RPL score [15] (AUROC 0.566) performed poorly in our cohort. This study emphasizes the importance of validating models developed for specific populations by applying them to external cohorts from different geographical areas before their widespread adoption. For example, the Pre-MORAL score demonstrated excellent performance with an AUROC of 0.82 in the North American cohort where it was developed [13]. However, in our cohort, it did not perform as well, with an AUROC of 0.692. On the other hand, the AFP model achieved comparable accuracy (AUROC 0.764) in our center to that obtained in the original study (AUROC 0.701) [11].
Based on our results, we cannot definitively conclude that one recurrence risk assessment model is superior to another. The small nominal differences observed in the AUROC curves can be attributed to the limited sample size utilized in the study. Therefore, it is challenging to make conclusive statements regarding the superiority of one model over another based solely on these small differences. Indeed, caution should be exercised when interpreting the results of the R3-AFP model [16] compared to the Pre-MORAL model [13] and RPL [15]. While a statistically significant difference was observed, it is important to note that no prognostic model has been definitively established as superior in the current context. When evaluating these models, it is crucial to consider not only the pre- or post-transplant factors but also the practicality and feasibility of implementing them in clinical practice. Factors such as ease of use, accessibility of required data, and applicability to different patient populations should be contemplated when considering the adoption and implementation of these models. In our pairwise comparisons, we did not employ any multiple adjustment strategies. Conducting multiple tests without p-value adjustment increases the probability of obtaining spurious results. However, given the exploratory nature of our analyses and the small nominal differences observed in the AUROC curves, it is important to interpret the results with caution and consider them as preliminary findings that warrant further investigation and validation in larger studies.
Constetin et al. [16] introduced the R3 AFP score, developed in a cohort with a significant proportion of transplanted patients with HCC beyond the MC. The variables included in the score are the last AFP value before LT and explant findings (number and diameter of the largest nodule, presence of microvascular invasion and degree of tumor differentiation). Given that our study's database only includes the highest recorded AFP value for each patient, the median value of the highest AFP value (14.5 ng/mL) was used. For comparison, in the cohort of Constetin et al, 1.3 % of patients had an AFP value > 1,000 ng/mL and, in our cohort, this was found in 7 % of patients (in both cohorts, most patients had AFP values < 100 ng/mL). In the study where the R3-AFP score was developed, the discriminatory power of the model was 0.78 (95 % CI 0.73-0.83) using Wolber's c-index. In our cohort, using c-statistics, it was 0.785 (95 % CI 0.687-0.883).
The UCLA nomogram [12] is a predictive model that incorporates seven variables, including five pre-transplantation data, for predicting HCC recurrence. The original study reported an AUROC of 0.86 (95 % CI 0.82-0.89) for the UCLA nomogram. In our cohort, the AUROC was 0.765 (95 % CI 0.671-0.858). To date, it appears that the UCLA nomogram has not been validated in other centers, possibly due to its complexity. The inclusion of multiple variables and the calculations involved in the nomogram may pose challenges for implementation and validation in different clinical settings. However, our study provides initial evidence of the performance of the UCLA nomogram in our specific cohort, highlighting its potential usefulness in predicting HCC recurrence.
The AFP model [11] is a prognostic model that utilizes three pre-transplant variables for its calculation: the number and size of nodules and the AFP value, variables considered important predictors of HCC recurrence. By incorporating these factors, the AFP model aims to provide a quantitative assessment of the risk of HCC recurrence in transplant patients. In our study, the AFP model demonstrated an AUROC of 0.764 (95 % CI 0.670-0.858), which is comparable to the performance reported in the original study. The AFP model's simplicity and reliance on readily available pre-transplant variables make it a practical tool for risk assessment in clinical practice.
The RETREAT [14] score was developed retrospectively in three North American cohorts, where most patients were within the MC while on the waiting list for LT. This score incorporates variables that have been shown to independently predict HCC recurrence, such as AFP and explant findings including vascular microinvasion, the sum of the largest viable tumor, and the number of viable tumors. The RETREAT score has demonstrated good discriminatory power in previous studies. In the original development cohorts, the score achieved a c-statistic or AUROC of 0.82 (95 % CI, 0.77-0.86). Subsequently, the RETREAT score was validated using the United Network for Organ Sharing (UNOS) database, where it achieved an AUROC of 0.77 for predicting HCC recurrence [19]. In our cohort, the discriminatory power of the RETREAT score, assessed using c-statistics, was 0.743 (95 % CI 0.634-0.851). Although slightly lower than the original development and validation cohorts, the discriminatory power of the RETREAT score in our cohort remains substantial. These results suggest that the RETREAT score can provide valuable prognostic information for assessing the risk of HCC recurrence in our specific patient population.
Halazun et al. [13] proposed the MORAL score, using both morphological criteria and biological markers, including the neutrophil-lymphocyte ratio (NLR) and AFP. Their model consists of two components: the Pre-MORAL score, which assesses preoperative predictors, and the Post-MORAL score, which considers postoperative factors. In the original study, the Pre-MORAL score, based on an NLR ≥ 5, AFP > 200 ng/mL, and nodule diameter > 3 cm, demonstrated excellent preoperative prediction of recurrence-free survival with an AUROC of 0.82. However, in your cohort, this level of predictive accuracy was not replicated, and the AUROC for the Pre-MORAL score was lower at 0.692 (95 % CI 0.592-0.792). For the Post-MORAL score, which incorporates grade 4 differentiation, microvascular invasion, number of nodules > 3, and tumor diameter > 3 cm, the original study reported an AUROC of 0.88 (95 % CI 0.83-0.93) for predicting HCC recurrence. In your cohort, the AUROC for the Post-MORAL score was 0.733 (95 % CI 0.628-0.837), indicating a lower discriminatory power compared to the original findings. The authors also developed the MORAL combo, which combines the Pre-MORAL and Post-MORAL scores, resulting in an AUROC of 0.91 (95 % CI 0.87-0.95) for HCC recurrence in their study. In your study, the AUROC for the MORAL combo was 0.749 (95 % CI 0.643-0.854). These results suggest that the performance of the MORAL scores in predicting HCC recurrence may vary across different cohorts. While the original study reported promising results, it is important to consider the specific characteristics and context of your cohort, which may contribute to the differences observed in the AUROC values.
The PLR has been proposed as a potential predictor of HCC recurrence following liver transplantation. A study published in 2015 demonstrated that patients with a PLR ≥ 125 had more advanced tumors and a higher risk of recurrence, with an AUROC of 0.627 [20]. Furthermore, a systematic review and meta-analysis involving 899 patients indicated that a high PLR (>150) was associated with an increased risk of HCC recurrence after LT [21]. However, it is important to interpret these findings with caution due to the heterogeneity of the included studies. In your cohort, the AUROC for PLR as a predictor of HCC recurrence was 0.566 (95 % CI 0.457-0.675), suggesting a relatively lower discriminatory power compared to previous studies. The molecular mechanisms underlying the association between PLR and HCC recurrence remain unknown [22]. It is worth noting that while PLR has been proposed as a potential predictor, its predictive value may vary across different cohorts and populations.
The risk of disease recurrence after LT for HCC is multifactorial [23] and predictive models for recurrence risk of HCC after LT remain an unmet need [24,25]. Therefore, it is crucial to conduct studies that validate candidate models in external cohorts. This validation process is essential as it allows for a more comprehensive evaluation of the models' performance and ensures their applicability across different patient populations. Validating these models in external cohorts enables a more robust and reliable assessment, leading to an improved selection of patients and a more effective allocation of grafts in clinical practice. It is important to note that these regression models provide an estimation of the average risk within a population, rather than predicting an individual's specific risk [24]. Individual patient characteristics, as well as other clinical factors, can significantly influence the actual risk of HCC recurrence after LT.
For example, a study using the UNOS database (N=12,771 patients) found that women have a 25 % lower risk than men of experiencing HCC recurrence [26], even after accounting for etiology of cirrhosis, AFP level at the time of transplantation, tumor diameter or presence of vascular invasion. The influence of the ABO blood group system on HCC recurrence after LT was evaluated in a French multicenter study, which showed that group A recipients have a higher risk of recurrence compared with recipients of other ABO blood groups [27]. While some research has proposed a potential link between obesity and the stimulation of tumor proliferation, as well as an increased risk of recurrence following LT for HCC [28], a study comparing outcomes after LT for HCC in patients with and without NASH [29] no significant differences in recurrence rates were observed between the two groups.
Therefore, it is essential to approach these models with caution and consider them as tools that inform the average population risk rather than providing personalized predictions. To advance the field and improve patient outcomes, further research and validation studies are needed to develop more accurate and personalized predictive models for HCC recurrence after LT. These models have the potential to enhance patient selection, optimize resource allocation, and contribute to improved clinical decision-making in the context of liver transplantation.
In our study, the incidence of HCC recurrence after LT was found to be 8.4 %, which is consistent with the findings of a Brazilian multicenter study that analyzed 1,069 patients [30]. The median time to onset of recurrence was 19.8 months. HCC recurrence after LT had an important impact on survival, with a median of one year after the diagnosis [18]. In our cohort, the 5-year survival rate for patients with HCC recurrence was 28.4 %, which was considerably lower compared to the 75.6 % survival rate observed in patients without recurrence (p < 0.001). These findings underscore the importance of effective strategies for the prevention and early detection of HCC recurrence after LT. The results of this study reinforce the need for comprehensive surveillance protocols and interventions aimed at reducing the risk of HCC recurrence after LT. Efforts should be directed towards identifying high-risk patients, optimizing treatment strategies, and developing novel approaches to improve long-term outcomes for patients with HCC undergoing LT.
5ConclusionsIn conclusion, this study assessed several predictive scores for HCC recurrence after LT and found that the R3-AFP [16] model, UCLA nomogram [12], AFP model [11], RETREAT [14], Combo MORAL, and Post-MORAL models [13] demonstrated a moderate predictive capacity for HCC recurrence. These models provided valuable clinical utility in identifying patients at risk of recurrence. Importantly, no significant differences were observed among these models in their ability to predict recurrence. In addition, it should be noted that the pre-MORAL [13] and PLR [15] scores performed poorly in this study, indicating their limited efficacy in predicting HCC recurrence after LT. Overall, the results emphasize the complexity of predicting HCC recurrence after LT and the importance of utilizing multiple factors and models to assess the risk. Further studies and external validations are necessary to develop more robust and reliable predictive models that can assist in personalized patient management and improve long-term outcomes in the field of HCC transplantation.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availabilityAll data relevant to the study are included in the article.
Author contributionsABMB: study concept and design, analysis and interpretation of data, drafting the manuscript, critical revision of the manuscript for important intellectual content.
SR: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. CAM: critical revision of the manuscript for important intellectual content. AMFJ: critical revision of the manuscript for important intellectual content. MVF: acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. MM: critical revision of the manuscript for important intellectual content.
We thank the Liver Transplant Group of Santa Casa de Misericórdia de Porto Alegre (Guido Cantisani team).