Background & Aims. It is unclear whether portal vein thrombosis (PVT) unrelated to malignancy is associated with reduced survival or it is an epiphenomenon of advanced cirrhosis. The objective of this study was to assess clinical outcome in cirrhotic patients with PVT not associated with malignancy and determine its prevalence.
Material and methods. Retrospective search in one center from June 2011 to December 2014.
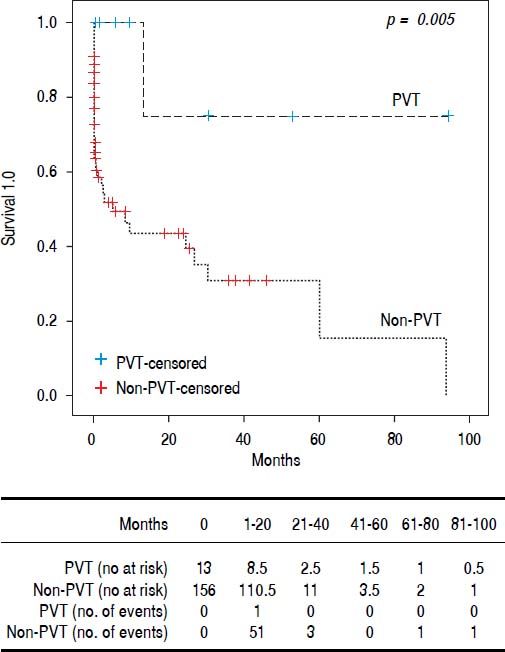
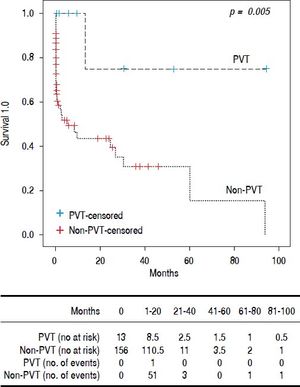
Results. 169 patients, 55 women and 114 men, median age 54 (19-90) years. Thirteen had PVT (7.6%). None of the patients received anticoagulant treatment. The PVT group was younger (49 [25-62] vs. 55 [19-90] years p = 0.025). Child A patients were more frequent in PVT and Child C in Non-PVT. Median Model for End Stage Liver Disease (MELD) score was lower in PVT (12 [8-21] vs. 19 [7-51] p ≤ 0.001) p ≤ 0.001). There was no difference between upper gastrointestinal bleeding and spontaneous bacterial peritonitis in the groups. Encephalopathy grade 3-4 (4 [30.8%] vs. 73 [46.8%] p = 0,007) and large volume ascites (5 [38.5%] vs. 89 [57.1%] p= 0,012) was more common in non-PVT. Survival was better for PVT (16.5 ± 27.9 vs. 4.13 ± 12.2 months p = 0.005). Conclusions: We found that PVT itself does not lead to a worse prognosis. The most reliable predictor for clinical outcome remains the MELD score. The presence of PVT could be just an epiphenomenon and not a marker of advanced cirrhosis.
Liver cirrhosis is a global health problem with an estimated prevalence in the United States of 0.27%.1 In Mexico, it is the fourth and second place in global mortality and mortality in productive age, respectively.2 Several predictors of mortality have been described in cirrhosis, with the most reliable being the Child-Pugh and Model for End Stage Liver Disease (MELD) score.3 The coagulation system in patients with liver disease suffers several changes, going to pro-thrombotic or predisposition to bleeding depending on the predominating factors.4 Nowadays, there is a wide availability of imaging techniques used routinely in patients with cirrhosis to assess complications. This has caused an increase in the diagnosis of portal vein thrombosis (PVT) not related to malignancy. Portal vein thrombosis has been reported from 1 to 22% in different studies.5,6 Increased gastrointestinal hemorrhage and intestinal infarction has been linked to PVT.7 However it is unclear whether non-malignancy related PVT is associated with reduced survival or if it is epiphenomenon of advanced liver disease. Some studies have found similar survival rates and even lower mortality in patients with PVT.8,9 In Mexico the prevalence of PVT or the clinical outcome in these patients is unknown. Therefore, we decided to conduct this study assessing as the primary objective its relevance on clinical outcome: such as upper gastrointestinal bleeding, spontaneous bacterial peritonitis (SBP), grade 3 or 4 encephalopathy, large volume ascites and overall survival and secondary outcome the prevalence of PVT not associated to malignancy.
Material and MethodsWe conducted a retrospectively search from June 2011 to December 2014 of patients treated with the ICD 10 diagnosis of “fibrosis and liver cirrhosis” or “other cirrhosis” in a single center (Hospital Universitario, UANL). Liver cirrhosis was diagnosed by physical examination, laboratory exams, radiologic study and/or histopathology. We excluded patients with known malignancy; incomplete medical record or those patients who did not have at least one follow up at this institution. Hepatocellular carcinoma and other primary malignancies were excluded with physical examination, upper endoscopy, and radiologic examination (computed tomography). In our patients, no other cause of PVT was suspected.
For each patient we registered age, gender, etiology of cirrhosis, CHILD and MELD score, hemoglobin, platelets, albumin, globulin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total and indirect bilirubin, glucose, creatinine, coagulation panel, diagnosis of PVT and last follow up at the hospital. The diagnosis of PVT was made by Doppler ultrasonography (GE Logic 7, 2007) or contrast-enhanced tomography, both interpreted by a radiologist. Portal vein thrombosis was defined when there was endoluminal material with partial or total absence of flow or the presence of cavernous transformation.
Data were retrieved on an Excel spread (Microsoft Office 2013) and analyzed with SPSS software Version 20 for Windows (IBM Corp. 1989-2011). Normality was studied using the Shapiro-Wilk test. Categorical variables were presented as percentages and frequencies and continuous variables as means and standard deviations or medians and minimum and maximum range. Student’s t test and the Mann-Whitney U test were used to compare continuous variables according to normality. Categorical variables were compared with χ2. Kaplan-Meier was used to assess survival and frequency of clinical outcomes. A two tailed-P value < 0.05 was considered statistically significant.
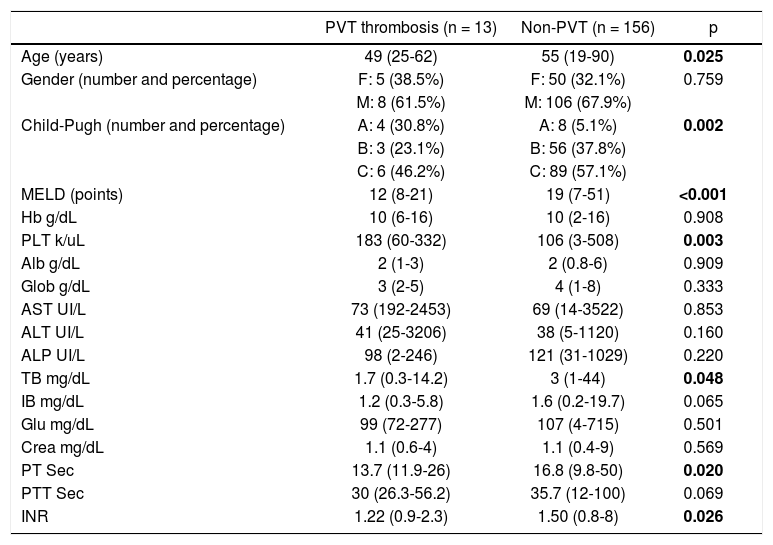
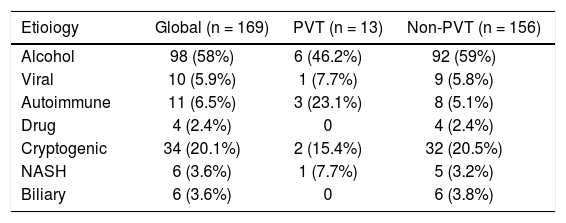
ResultsGeneral characteristicsWe identified 189 patients, however, we excluded 15 patients with a diagnosis of malignancy and 5 with an incomplete medical record. Therefore, we included 169 patients. Fifty-five women and 114 men, median age was 54 (19-90) years. Thirteen patients (7.6%) presented PVT, 8 had a diagnosis made by ultrasonography and contrast enhanced tomography and 5 only with ultrasonography. Nine patients had total thrombosis, 3 had partial thrombosis and 1 had cavernous degeneration. None of the patients with PVT received anticoagulation treatment. The analysis was divided in two groups, patients with PVT and patients without PVT. The patients with PVT were younger (49 vs. 55 years p = 0.025), and male gender was predominant in both groups (8 [61.5%] and 106 [67.9%], respectively) without being statistically significant (Table 1). The predominant etiology of cirrhosis was alcohol in both groups (Table 2). Child A was more frequent in the PVT group (4 [30.8%] vs. 8 [5.1%]), and Child C in the non-PVT group (6 [46.2%] vs. 89 [57.1%]). MELD score mean was lower among PVT patients (12 [8-21] vs. 19 [7-51] p= <0.001). Prothrombin time, INR and total bilirubin also were lower in PVT patients. Finally, platelet levels were higher in PVT patients (183,000 [60,000-332,000] vs. 106 [3,000-508,000] p= 0.003).
Demographic characteristics.
| PVT thrombosis (n = 13) | Non-PVT (n = 156) | p | |
|---|---|---|---|
| Age (years) | 49 (25-62) | 55 (19-90) | 0.025 |
| Gender (number and percentage) | F: 5 (38.5%) | F: 50 (32.1%) | 0.759 |
| M: 8 (61.5%) | M: 106 (67.9%) | ||
| Child-Pugh (number and percentage) | A: 4 (30.8%) | A: 8 (5.1%) | 0.002 |
| B: 3 (23.1%) | B: 56 (37.8%) | ||
| C: 6 (46.2%) | C: 89 (57.1%) | ||
| MELD (points) | 12 (8-21) | 19 (7-51) | <0.001 |
| Hb g/dL | 10 (6-16) | 10 (2-16) | 0.908 |
| PLT k/uL | 183 (60-332) | 106 (3-508) | 0.003 |
| Alb g/dL | 2 (1-3) | 2 (0.8-6) | 0.909 |
| Glob g/dL | 3 (2-5) | 4 (1-8) | 0.333 |
| AST UI/L | 73 (192-2453) | 69 (14-3522) | 0.853 |
| ALT UI/L | 41 (25-3206) | 38 (5-1120) | 0.160 |
| ALP UI/L | 98 (2-246) | 121 (31-1029) | 0.220 |
| TB mg/dL | 1.7 (0.3-14.2) | 3 (1-44) | 0.048 |
| IB mg/dL | 1.2 (0.3-5.8) | 1.6 (0.2-19.7) | 0.065 |
| Glu mg/dL | 99 (72-277) | 107 (4-715) | 0.501 |
| Crea mg/dL | 1.1 (0.6-4) | 1.1 (0.4-9) | 0.569 |
| PT Sec | 13.7 (11.9-26) | 16.8 (9.8-50) | 0.020 |
| PTT Sec | 30 (26.3-56.2) | 35.7 (12-100) | 0.069 |
| INR | 1.22 (0.9-2.3) | 1.50 (0.8-8) | 0.026 |
* F: female. M: male, MELD: Model for End Stage Liver Disease. Hb: hemoglobin. PLT: platelets. Alb: albumin. TB: total bilirubin. IB: Indirect bilirubin. Glu: glucose. Crea: creatinine. PT: prothrombin time. PTT: partial thromboplastin time. INR: international normalized ratio.
Etioloqv of cirrhosis.
| Etioiogy | Global (n = 169) | PVT (n = 13) | Non-PVT (n = 156) |
|---|---|---|---|
| Alcohol | 98 (58%) | 6 (46.2%) | 92 (59%) |
| Viral | 10 (5.9%) | 1 (7.7%) | 9 (5.8%) |
| Autoimmune | 11 (6.5%) | 3 (23.1%) | 8 (5.1%) |
| Drug | 4 (2.4%) | 0 | 4 (2.4%) |
| Cryptogenic | 34 (20.1%) | 2 (15.4%) | 32 (20.5%) |
| NASH | 6 (3.6%) | 1 (7.7%) | 5 (3.2%) |
| Biliary | 6 (3.6%) | 0 | 6 (3.8%) |
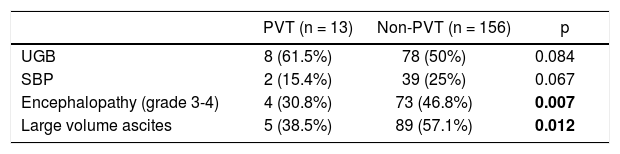
We performed Kaplan-Meier analysis for upper gastrointestinal bleeding, spontaneous bacterial peritonitis, encephalopathy, large volume ascites and global survival. The frequency of upper gastrointestinal bleeding (8 [61.5%] vs. 78 [50%]) and spontaneous bacterial peritonitis (2 [15.4%] vs. 39 [25%]) was not statistically different between groups (p = 0.84 and p = 0.67 respectively). However, the rate of encephalopathy (grade 3-4) requiring inhospital treatment was better in the PVT group (4 [30.8%] vs. 73 [46.8%] p= 0.007). Large volume ascites was also less frequent in the PVT group (5 [38.5%] vs. 89 [57.1%] p= 0.012) (Table 3). One patient (8%) in PVT and 56 (36%)) in non-PVT died during follow up (p = 0.063). Global survival was better for patients with PVT (16.5 ± 27.9 vs. 4.13 ± 12.2 months p = 0.005) (Figure 1).
Acute decompensating precipitants that lead to organ failure in patients with cirrhosis have a mortality of 30%.10 Importantly, mortality is higher in previously compensated patients than in those with previous decompensation. Precipitating factors such as infections, PVT, surgery, and hepatocellular carcinoma generally trigger decompensating events.11 Several factors can lead to the development of PVT in patients with cirrhosis, primarily from a reduction in portal blood flow and hypercoagulability.12
The aim of this study was to assess the relevance on clinical outcome in cirrhotic patients with PVT not associated with malignancy and its prevalence. In Mexico, to our knowledge, there are no studies assessing this outcome. The prevalence in our study was 7.6%, similar to the range previously reported in the literature.5,13,14 Contrary to previous studies,15–17 we found that patients with PVT are younger.
Male gender and alcohol etiology were predominant, similar to what has been previously found in our country.18,19 This is due to the trends of alcohol consumption in Mexico.19 We found more patients being classified as cryptogenic cirrhosis compared to the literature,9,16,20 but in our country, most of the patients are evaluated without specialized studies due to economic burden, therefore there could be less patients with this diagnosis. The patients in the PVT group had lower Child-Pug and MELD score compared with the patients in non-PVT. This could be explained in part because patients with non-PVT are more likely to visit a hospital due to symptomatology caused by cirrhosis complications following poor hepatic reserve. However, we did not explore the presence of abdominal pain because of the retrospective design. Nonetheless, the MELD score in the patients in our group without PVT was significantly higher compared to previous studies.9,15,20 PT, INR and total bilirubin were lower in the PVT group while the platelet was count higher, this could be due to the state of advanced disease in patients without PVT.
Upper gastrointestinal bleeding (UGB) did not differ between groups, even though it has been described as a common presentation among patients with PVT;7,21 thus, we do not consider PVT a risk factor for UGB as previously reported.7 The frequency of spontaneous bacterial peritonitis was the same, and to date, there is only a case report describing this finding in patients with PVT.22 Encephalopathy requiring in-hospital treatment was more frequent in patients without PVT contrasting previous studies.9,17 This could be explained due to the disparity of MELD score in both groups. In our study, we did not assess recanalization after diagnosis of PVT; these patients have been described to achieve less frequently hepatic encephalopathy.23 Nonetheless, it has been proposed that patients with PVT develop high levels of ammonia due to porto-systemic shunts leading to sub-clinical neurological abnormalities compatible with minimal hepatic encephalopathy.24 Contrary to our findings, these patients do not seem to be at more risk for presenting hepatic encephalopathy. Large volume ascites was also more frequent in non-PVT patients. Moderate ascites has been described previously in patients with PVT;9,15 however, we believe this finding could be the result of advanced liver stage in the non-PVT group. Global mortality was less for patients with PVT, a finding consistent with previous reports.9,15
Recently, Hugenholtz, et al. remarked the importance of anticoagulation in patients with cirrhosis in different settings including PVT;25 however, we found that PVT by itself does not carry a worse prognosis in terms of survival, UGB, SBP, large volume ascites or encephalopathy, even if it does not receive anticoagulation treatment.8 The reliable variable to predict outcomes remains the MELD score. Most of our patients had MELD scores above 14 and all of our findings could be explained by diminished hepatic function. The presence of PVT could be just an epiphenomenon and not a marker of advanced liver disease.
To our knowledge, this is the first study to report the prevalence of PVT in Mexico, and confirms many findings described previously about PVT. However, our study also has some limitations. It is a single center retrospective study, we did not assess recanalization of PVT and we did not have the information of mutations that predispose to thrombosis.4,17 This study opens the scenario to calculate the size for prospective studies and randomized clinical trials to evaluate the benefits and harms of anticoagulation treatment.
Abbreviations- •
ALP: alkaline phosphatase.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
MEDL: Model for End Stage Liver Disease.
- •
PVT: portal vein thrombosis.
- •
UGB: upper gastrointestinal bleeding.
None.
Financial SupportNone.