Higher rates of psychiatric disorders are reported among cirrhotic patients. This study examines the demographic and clinical outcomes post-liver transplant (LT) among cirrhotic patients with a major psychiatric diagnosis (cases) compared to those without psychiatric diagnosis (controls).
Materials and methodsRetrospective case control design was used among 189 cirrhotic patients who had undergone LT at Methodist University Hospital Transplant Institute, Memphis, TN between January 2006 and December 2014. Multivariable regression and Cox proportional hazard regression were conducted to compare allograft loss and all-cause mortality.
ResultsThe study sample consisted of a matched cohort of 95 cases and 94 controls with LT. Females and those with Hepatic Encephalopathy (HE) were more likely to have psychiatric diagnosis. Patients with hepatocellular carcinoma (HCC) were twice as likely to have allograft loss. Psychiatric patients with HCC had two and a half times (HR 2.54; 95% CI: 1.20–5.37; p = 0.015) likelihood of all-cause mortality. Data censored at 1-year post-LT revealed that patients with psychiatric diagnosis have a three to four times higher hazard for allograft loss and all-cause mortality compared to controls after adjusting for covariates, whereas when the data is censored at 5 year, allograft loss and all-cause mortality have two times higher hazard ratio.
ConclusionsThe Cox proportional hazard regression analysis of censored data at 1 and 5 year indicate higher allograft loss and all-cause mortality among LT patients with psychiatric diagnosis. Patients with well-controlled psychiatric disorders who undergo LT need close monitoring and medication adherence.
Cirrhosis is a potentially fatal condition with hepatitis C, alcohol use disorder, and non-alcoholic steatohepatitis being the predominant etiologies in the United States [1–4]. End-stage liver disease (ESLD) manifestations, such as variceal bleeding, hepatic encephalopathy (HE), and ascites, result in significant morbidity and oftentimes mandate evaluation for liver transplant (LT) [5,6]. A significant concern surrounding transplantation involves the mental health of transplant candidates. Major psychiatric disorders, such as depression and anxiety, have been implicated in contributing to greater rates of morbidity and mortality among transplant patients [7,8]. Liver disease and psychiatric illness have been proposed to be associated with each other based on observed increased incidences of psychiatric disorders among patients with liver disease, which are often compounded by a history of alcohol and drug use [9–12]. Psychiatric abnormalities, such as neuroses, affective disorders, withdrawal syndromes, and personality disorders, were diagnosed in 75% of the patients with alcoholic liver disease, compared to among 26.7% of the nonalcoholic cirrhotic patients [10].
Trumper and Appleby reported 39% of pre-heart and/or liver transplant cases suffered from anxiety or depressive disorders [13]. Patients on the waitlist for LT with self-reported depression, anxiety, and trauma had significantly poorer health-related quality of life (HRQoL) [14]. Similarly, depression and anxiety impairing HRQoL were found in chronic liver disease patients and in those with cirrhosis [15–17]. Conversely, a retrospective study among US veterans found that patients with bipolar disorders had a higher prevalence of liver disease compared to matched controls (21.5% vs. 3.5%) [18]. Psychiatric disorders are definitely potential contraindications for patients seeking liver transplantation. Other common contraindications include comorbid medical conditions, dementia, drug addiction, therapeutic noncompliance, and limited family or social support [8,19,20]. Presence of such factors can contribute to adverse post-LT outcomes, and in some cases, disqualify patients from transplant evaluations and listing.
The literature on post-LT outcomes among cirrhotic patients with psychiatric diagnosis remain inconclusive. For one, current literature has not established a clear link between psychiatric diagnosis and differential survival rates in post-LT patients. In addition, there are mixed findings on the impact of mental health on allograft loss and mortality in LT recipients. A few studies examining pre-LT anxiety and depression did not find any impact on post-LT survival [21,22]. However, one study found that depression in early post-LT was associated with increased mortality [7]. However, yet another study reported depressive symptoms among patients awaiting liver or kidney transplants were in fact associated with a three- to four-fold decreased risk of both graft failures and mortality [23]. On the other hand, Dew et al. found that depression significantly increased risks of graft loss and mortality, while anxiety did not result in the same significant association [24]. Therefore, the reported association between the psychiatric diagnoses and post-LT outcomes is inconsistent.
This study contributes to the body of literature on this topic by focusing on three objectives: (1) to assess the factors associated with psychiatric diagnosis among patients with cirrhosis; (2) to compare the clinical and healthcare utilization outcomes in cirrhotic patients with and without psychiatric diagnosis; and (3) to examine association of demographic and clinical factors with graft- and patient-survival post-LT among patients with and without psychiatric diagnosis. Identifying factors associated with post-LT graft and patient-survival will provide a greater understanding on the protocols needed to improve post-LT outcomes among patients with psychiatric diagnosis. With an ever-expanding list of patients awaiting transplant and high prevalence of psychiatric illness, this study will provide insights into post-LT outcomes. Additional knowledge on these issues will ensure longevity and optimal care of the LT post-procedure as well as mitigate the risks of non-compliance by the recipient.
2MethodsUsing retrospective case control design, we identified a cohort of 900 patients [≥18 years old] who had undergone LT at Methodist University Hospital (MUH) Transplant Institute, Memphis, TN between January 2006 and December 2014. Inclusion criteria for this study were cases who had a major psychiatric diagnosis (depression, bipolar, or schizophrenia) pre-transplant and underwent LT during the study period. Exclusion criteria included undergoing simultaneous liver and kidney transplants, acute liver failure, or undergoing a re-transplant of the liver. The control group of the study consisted of patients who underwent LT at MUH during the same 2006–2014 time period but were not diagnosed with any major psychiatric disorders. Using listing model for end-stage liver disease (MELD), we divided the cases into five classes (Class 1 = MELD <9, Class 2 = MELD 10–19, Class 3 = MELD 20–29, Class 4 = MELD 30–39, and class 5 = MELD 40 or higher). Controls were matched to the cases based on age at transplant, etiology of liver disease, listing MELD class, and by presence or absence of hepatocellular carcinoma (HCC).
Review of these patients’ medical histories were conducted through electronic medical records (EMR) that included hepatology clinic notes, social work assessments pre-transplant, and psychiatric consultations, when available, to identify the patients with major psychiatric illness. Electronic medical records of patients were reviewed to extract the psychiatric diagnoses, treatments rendered, whether they be medications, cognitive/behavioral therapy, and follow-ups with a psychiatrist. Liver disease was recorded and categorized as: hepatitis C (HCV), alcoholic cirrhosis (ETOH), hepatocellular carcinoma (HCC), non-alcoholic steatohepatitis (NASH), autoimmune liver diseases such as autoimmune hepatitis (AIH), primary sclerosing cholangitis (PSC), and primary biliary cholangitis (PBC); and others which included all other etiologies of liver disease. We also collected patients’ demographics, liver-related symptoms such as HE, comorbid clinical conditions, liver histology, imaging studies, and serologic tests. In addition, we recorded the use of tobacco, alcohol, and illicit drugs. Liver disease severity score, Model of End-Stage Liver Disease (MELD) [25] was calculated. All clinical outcomes and all-cause mortality were extracted from the patients’ EMR throughout their post-LT course. The IRBs of the University of Tennessee Health Science Center and the University of Memphis approved the study and waived the requirement for informed consent.
2.1Statistical analysisData on baseline patient characteristics were analyzed in two ways based on the type of variable. Categorical variables were reported as frequencies and percentages whereas continuous variables were summarized with measures of central tendency and variability. Thus, these variables were reported either as frequencies, means and standard deviations (SD) or medians, and interquartile range (IQR). Comparisons between psychiatric (cases) and non-psychiatric patients (controls) were analyzed through chi-square tests for categorical variables while continuous variables were analyzed using Student’s t-test or Mann Whitney U test, as appropriate. The case and control groups were compared on demographic, etiology of liver disease, liver-related disease, comorbid conditions, MELD, and behavioral factors. The two groups were also assessed for the clinical and healthcare utilization outcomes post-LT. The Scientific Registry of Transplant Recipients indicates differential outcomes among LT patients at 1-, 3-, and 5 years, ranging from 71% to 89% for allograph survival and 74%–91% for overall survival [26]. Therefore, we decided to censor data at 1-, 3-, and 5 years for our primary outcomes, allograft loss and all-cause mortality. Secondary outcomes included the number of readmissions post-LT after 12 months and after 13–36 months, and the number of psychiatric readmissions at the 12 months and 13–36 months. An additional secondary outcome was the incidence of biopsy proven acute cellular rejection.
Associated risk factors for psychiatric diagnosis were identified through stepwise selection logistic regression. Univariate and multivariable logistic regression were conducted to assess factors that contribute to differences in post-LT outcomes in individuals with major psychiatric diagnosis. Finally, a model that included sex and HE was identified and evaluated through multivariable logistic regression for their association with the psychiatric diagnosis. In addition, we ran Cox proportional hazard regression analyses to compare allograft loss and all-cause mortality for cases and controls groups. We also compared allograft loss and all-cause mortality between cases and controls at year, 3 years, and 5 years using univariate Cox proportional hazard regression, as well as multivariate Cox proportional hazard regression controlling for age, gender, race and listing MELD score. All statistical analyses were conducted using SAS (version 9.4) [27].
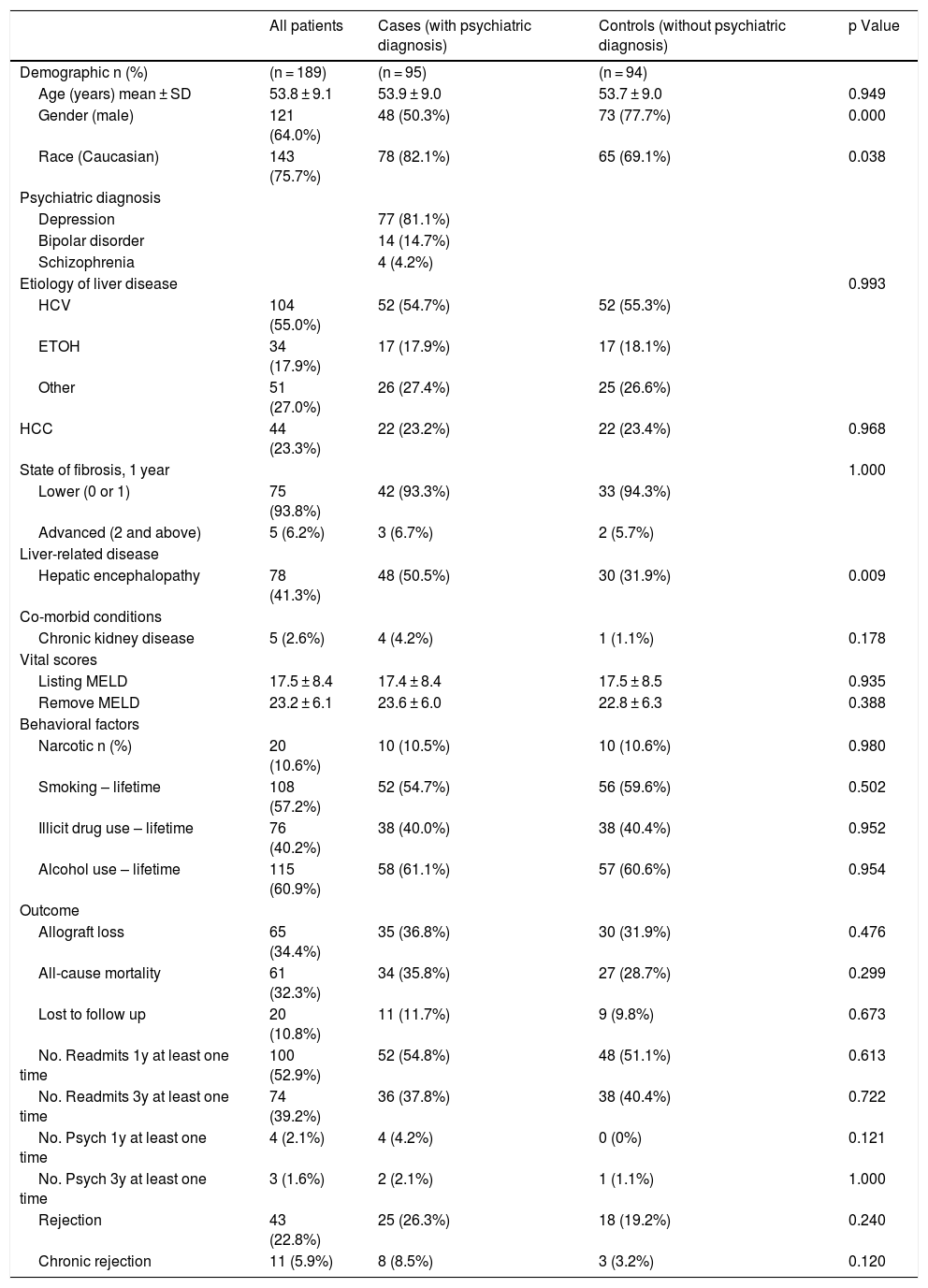
3ResultsThe sample size in this study comprised of 189 cirrhotic patients with a mean age of 53.8 ± 9.1 years. Of the 189 patients, the majority were male (64.0%) and Caucasian (75.7%). While there were 95 patients with major psychiatric diagnosis (cases), the control group comprised of 94 patients. Of the 95 cases, a majority (n = 77) carried diagnosis of major depression, 14 had bipolar disorder, and 4 had schizophrenia. Among the liver disease etiologies, HCV infection and alcohol use were the most common, at 54.7% and 17.9%, respectively, among patients with psychiatric diagnosis. Over one-fifth (23.2%) of cases had been diagnosed with hepatocellular carcinoma (HCC) among the cases. The mean listing MELD score at baseline for those with psychiatric diagnosis was 17.4 ± 8.4. At baseline, 50.5% of cases had been diagnosed with HE compared to 31.9% of controls (Table 1).
Demographic and clinical variables of all cirrhotic patients with and without psychiatric diagnosis (n = 189).
| All patients | Cases (with psychiatric diagnosis) | Controls (without psychiatric diagnosis) | p Value | |
|---|---|---|---|---|
| Demographic n (%) | (n = 189) | (n = 95) | (n = 94) | |
| Age (years) mean ± SD | 53.8 ± 9.1 | 53.9 ± 9.0 | 53.7 ± 9.0 | 0.949 |
| Gender (male) | 121 (64.0%) | 48 (50.3%) | 73 (77.7%) | 0.000 |
| Race (Caucasian) | 143 (75.7%) | 78 (82.1%) | 65 (69.1%) | 0.038 |
| Psychiatric diagnosis | ||||
| Depression | 77 (81.1%) | |||
| Bipolar disorder | 14 (14.7%) | |||
| Schizophrenia | 4 (4.2%) | |||
| Etiology of liver disease | 0.993 | |||
| HCV | 104 (55.0%) | 52 (54.7%) | 52 (55.3%) | |
| ETOH | 34 (17.9%) | 17 (17.9%) | 17 (18.1%) | |
| Other | 51 (27.0%) | 26 (27.4%) | 25 (26.6%) | |
| HCC | 44 (23.3%) | 22 (23.2%) | 22 (23.4%) | 0.968 |
| State of fibrosis, 1 year | 1.000 | |||
| Lower (0 or 1) | 75 (93.8%) | 42 (93.3%) | 33 (94.3%) | |
| Advanced (2 and above) | 5 (6.2%) | 3 (6.7%) | 2 (5.7%) | |
| Liver-related disease | ||||
| Hepatic encephalopathy | 78 (41.3%) | 48 (50.5%) | 30 (31.9%) | 0.009 |
| Co-morbid conditions | ||||
| Chronic kidney disease | 5 (2.6%) | 4 (4.2%) | 1 (1.1%) | 0.178 |
| Vital scores | ||||
| Listing MELD | 17.5 ± 8.4 | 17.4 ± 8.4 | 17.5 ± 8.5 | 0.935 |
| Remove MELD | 23.2 ± 6.1 | 23.6 ± 6.0 | 22.8 ± 6.3 | 0.388 |
| Behavioral factors | ||||
| Narcotic n (%) | 20 (10.6%) | 10 (10.5%) | 10 (10.6%) | 0.980 |
| Smoking – lifetime | 108 (57.2%) | 52 (54.7%) | 56 (59.6%) | 0.502 |
| Illicit drug use – lifetime | 76 (40.2%) | 38 (40.0%) | 38 (40.4%) | 0.952 |
| Alcohol use – lifetime | 115 (60.9%) | 58 (61.1%) | 57 (60.6%) | 0.954 |
| Outcome | ||||
| Allograft loss | 65 (34.4%) | 35 (36.8%) | 30 (31.9%) | 0.476 |
| All-cause mortality | 61 (32.3%) | 34 (35.8%) | 27 (28.7%) | 0.299 |
| Lost to follow up | 20 (10.8%) | 11 (11.7%) | 9 (9.8%) | 0.673 |
| No. Readmits 1y at least one time | 100 (52.9%) | 52 (54.8%) | 48 (51.1%) | 0.613 |
| No. Readmits 3y at least one time | 74 (39.2%) | 36 (37.8%) | 38 (40.4%) | 0.722 |
| No. Psych 1y at least one time | 4 (2.1%) | 4 (4.2%) | 0 (0%) | 0.121 |
| No. Psych 3y at least one time | 3 (1.6%) | 2 (2.1%) | 1 (1.1%) | 1.000 |
| Rejection | 43 (22.8%) | 25 (26.3%) | 18 (19.2%) | 0.240 |
| Chronic rejection | 11 (5.9%) | 8 (8.5%) | 3 (3.2%) | 0.120 |
Regarding drug use, cases and controls had similar rates of narcotic use (10.5% vs. 10.6%), lifetime smoking use (54.7% vs. 59.6%), lifetime illicit drug use (40.0% vs. 40.4%), and lifetime alcohol use (61.1% vs. 60.6%). Of the 95 patients with psychiatric diagnosis, 36.8% experienced allograft loss, whereas 31.9% controls reported graft loss. While all-cause mortality rate was 35.8% among patients with psychiatric diagnosis, 28.7% of the controls reported mortality post-LT. In terms of patients lost to follow-up, 11.7% of the cases were lost to follow-up compared to 9.8% of the controls. With regards to readmissions, 54.8% of cases reported readmissions in the past year at least 1 time compared to 51.1% of controls. Finally, 26.3% of cases reported rejection with 8.5% demonstrating chronic rejections, compared to controls where, 19.2% and 3.2% reported rejection and chronic rejections, respectively (Table 1).
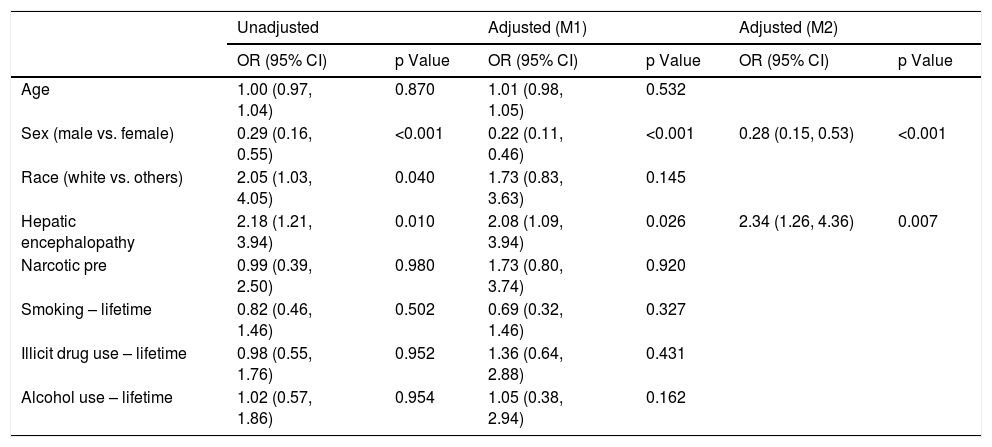
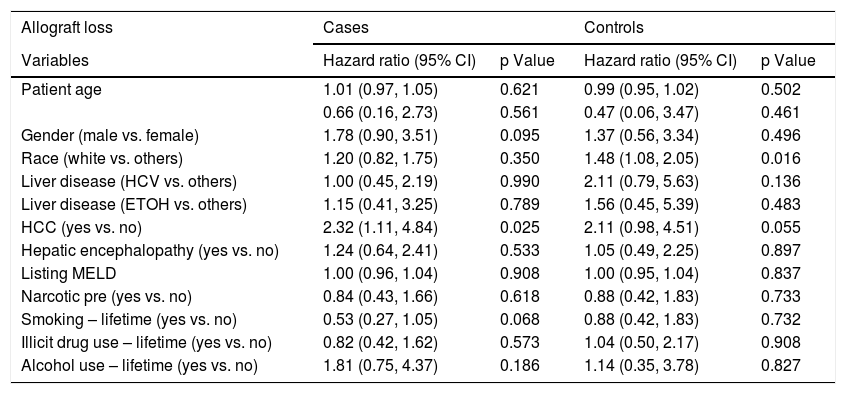
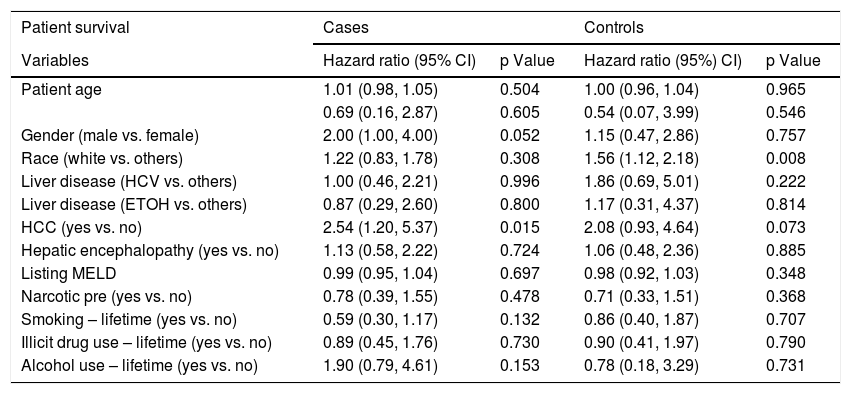
Logistic regression analysis found that there were two statistically significant risk factors (sex and HE) associated with psychiatric diagnosis among cirrhotic patients. After adjusting for other co-variates, males were less likely than females (OR 0.28; 95% CI: 0.15, 0.53; p = <.001) to have psychiatric diagnosis. Patients with HE were twice as likely to be associated with psychiatric diagnosis (OR 2.34; 95% CI: 1.26, 4.36; p = 0.007) (Table 2). Based on the Cox regression analysis, it was found that psychiatric patients with hepatocellular carcinoma (HCC) were twice as likely to have allograft loss (HR 2.32; 95% CI: 1.11, 4.84; p = 0.025). Similarly, among controls, patients with HCC were equally likely to have allograft loss (HR 2.11; 95% CI: 0.98, 4.51; p = 0.055) (Table 3). Cases with HCC had two and a half times (HR 2.54; 95% CI: 1.20, 5.37; p = 0.015) likelihood of all-cause mortality (Table 4). Among controls, whites had one a half times (HR 1.56; 95% CI 1.12, 2.18; p = 0.008) more likelihood of all-cause mortality.
Factors associated with psychiatric diagnosis among patients with cirrhosis (n = 189).
| Unadjusted | Adjusted (M1) | Adjusted (M2) | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Age | 1.00 (0.97, 1.04) | 0.870 | 1.01 (0.98, 1.05) | 0.532 | ||
| Sex (male vs. female) | 0.29 (0.16, 0.55) | <0.001 | 0.22 (0.11, 0.46) | <0.001 | 0.28 (0.15, 0.53) | <0.001 |
| Race (white vs. others) | 2.05 (1.03, 4.05) | 0.040 | 1.73 (0.83, 3.63) | 0.145 | ||
| Hepatic encephalopathy | 2.18 (1.21, 3.94) | 0.010 | 2.08 (1.09, 3.94) | 0.026 | 2.34 (1.26, 4.36) | 0.007 |
| Narcotic pre | 0.99 (0.39, 2.50) | 0.980 | 1.73 (0.80, 3.74) | 0.920 | ||
| Smoking – lifetime | 0.82 (0.46, 1.46) | 0.502 | 0.69 (0.32, 1.46) | 0.327 | ||
| Illicit drug use – lifetime | 0.98 (0.55, 1.76) | 0.952 | 1.36 (0.64, 2.88) | 0.431 | ||
| Alcohol use – lifetime | 1.02 (0.57, 1.86) | 0.954 | 1.05 (0.38, 2.94) | 0.162 | ||
M1: Including all the variables in the table.
M2: Variables after stepwise selection.
Factors associated with post-LT allograft loss among cirrhotic patients with and without psychiatric diagnosis.
| Allograft loss | Cases | Controls | ||
|---|---|---|---|---|
| Variables | Hazard ratio (95% CI) | p Value | Hazard ratio (95% CI) | p Value |
| Patient age | 1.01 (0.97, 1.05) | 0.621 | 0.99 (0.95, 1.02) | 0.502 |
| 0.66 (0.16, 2.73) | 0.561 | 0.47 (0.06, 3.47) | 0.461 | |
| Gender (male vs. female) | 1.78 (0.90, 3.51) | 0.095 | 1.37 (0.56, 3.34) | 0.496 |
| Race (white vs. others) | 1.20 (0.82, 1.75) | 0.350 | 1.48 (1.08, 2.05) | 0.016 |
| Liver disease (HCV vs. others) | 1.00 (0.45, 2.19) | 0.990 | 2.11 (0.79, 5.63) | 0.136 |
| Liver disease (ETOH vs. others) | 1.15 (0.41, 3.25) | 0.789 | 1.56 (0.45, 5.39) | 0.483 |
| HCC (yes vs. no) | 2.32 (1.11, 4.84) | 0.025 | 2.11 (0.98, 4.51) | 0.055 |
| Hepatic encephalopathy (yes vs. no) | 1.24 (0.64, 2.41) | 0.533 | 1.05 (0.49, 2.25) | 0.897 |
| Listing MELD | 1.00 (0.96, 1.04) | 0.908 | 1.00 (0.95, 1.04) | 0.837 |
| Narcotic pre (yes vs. no) | 0.84 (0.43, 1.66) | 0.618 | 0.88 (0.42, 1.83) | 0.733 |
| Smoking – lifetime (yes vs. no) | 0.53 (0.27, 1.05) | 0.068 | 0.88 (0.42, 1.83) | 0.732 |
| Illicit drug use – lifetime (yes vs. no) | 0.82 (0.42, 1.62) | 0.573 | 1.04 (0.50, 2.17) | 0.908 |
| Alcohol use – lifetime (yes vs. no) | 1.81 (0.75, 4.37) | 0.186 | 1.14 (0.35, 3.78) | 0.827 |
Factors associated with post-LT all-cause mortality among cirrhotic patients with and without psychiatric diagnosis.
| Patient survival | Cases | Controls | ||
|---|---|---|---|---|
| Variables | Hazard ratio (95% CI) | p Value | Hazard ratio (95%) CI) | p Value |
| Patient age | 1.01 (0.98, 1.05) | 0.504 | 1.00 (0.96, 1.04) | 0.965 |
| 0.69 (0.16, 2.87) | 0.605 | 0.54 (0.07, 3.99) | 0.546 | |
| Gender (male vs. female) | 2.00 (1.00, 4.00) | 0.052 | 1.15 (0.47, 2.86) | 0.757 |
| Race (white vs. others) | 1.22 (0.83, 1.78) | 0.308 | 1.56 (1.12, 2.18) | 0.008 |
| Liver disease (HCV vs. others) | 1.00 (0.46, 2.21) | 0.996 | 1.86 (0.69, 5.01) | 0.222 |
| Liver disease (ETOH vs. others) | 0.87 (0.29, 2.60) | 0.800 | 1.17 (0.31, 4.37) | 0.814 |
| HCC (yes vs. no) | 2.54 (1.20, 5.37) | 0.015 | 2.08 (0.93, 4.64) | 0.073 |
| Hepatic encephalopathy (yes vs. no) | 1.13 (0.58, 2.22) | 0.724 | 1.06 (0.48, 2.36) | 0.885 |
| Listing MELD | 0.99 (0.95, 1.04) | 0.697 | 0.98 (0.92, 1.03) | 0.348 |
| Narcotic pre (yes vs. no) | 0.78 (0.39, 1.55) | 0.478 | 0.71 (0.33, 1.51) | 0.368 |
| Smoking – lifetime (yes vs. no) | 0.59 (0.30, 1.17) | 0.132 | 0.86 (0.40, 1.87) | 0.707 |
| Illicit drug use – lifetime (yes vs. no) | 0.89 (0.45, 1.76) | 0.730 | 0.90 (0.41, 1.97) | 0.790 |
| Alcohol use – lifetime (yes vs. no) | 1.90 (0.79, 4.61) | 0.153 | 0.78 (0.18, 3.29) | 0.731 |
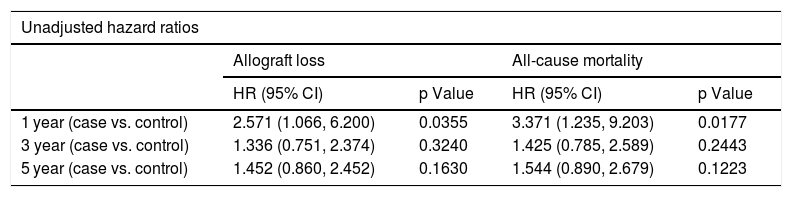
Table 5 shows the results from Cox proportional hazard regression for allograft loss and all-cause mortality. When we censored the survival data at 1 year, 3 years, and 5 years after LT, the results showed that the patients with psychiatric disorder have a two to three times higher hazard compared to controls both in allograft loss (HR 2.571; 95% CI: 1.066, 6.200, p-value 0.0355) and all-cause mortality (HR 3.371; 95% CI: 1.235,-9.203, p-value 0.0177). After adjusting for covariates, the hazard increases to three times for allograft loss (HR 3.230; 95% CI: 1.308, 7.975; p-value 0.0110) and four times for all-cause mortality (HR 4.230; 95% CI: 1.235, 9.203; p-value 0.0177) at the 1-year mark. We also found there is a significant effect of psychiatric disorder on both allograft loss and all-cause mortality after adjusting for covariates when the data are censored at 5 years. The adjusted analysis is significant for both allograft loss (p = 0.0336) and all-cause mortality (p = 0.0335), reflecting over one and half times higher hazard for psychiatric patients relative to controls.
Cox proportional hazard regression analysis for allograft loss and all-cause mortality.
| Unadjusted hazard ratios | ||||
|---|---|---|---|---|
| Allograft loss | All-cause mortality | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| 1 year (case vs. control) | 2.571 (1.066, 6.200) | 0.0355 | 3.371 (1.235, 9.203) | 0.0177 |
| 3 year (case vs. control) | 1.336 (0.751, 2.374) | 0.3240 | 1.425 (0.785, 2.589) | 0.2443 |
| 5 year (case vs. control) | 1.452 (0.860, 2.452) | 0.1630 | 1.544 (0.890, 2.679) | 0.1223 |
| Adjusted hazard ratiosa | ||||
|---|---|---|---|---|
| Allograft loss | All-cause mortality | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| 1 year (case vs. control) | 3.230 (1.308, 7.975) | 0.0110 | 4.230 (1.520, 11.773) | 0.0058 |
| 3 year (case vs. control) | 1.599 (0.878, 2.913) | 0.1249 | 1.715 (0.921, 3.192) | 0.0889 |
| 5 year (case vs. control) | 1.806 (1.047, 3.114) | 0.0336 | 1.951 (1.102, 3.454) | 0.0218 |
This study sought to compare the post-LT outcomes in cirrhotic patients with and without psychiatric diagnosis with the intent of identifying factors that may play a role in any observed differences between these outcomes. In a sample of 189 cirrhotic patients with a history of liver transplantation, a few significant differences were observed in baseline demographic characteristics and clinical factors associated with psychiatric diagnosis among patients with cirrhosis. Although non-adherence to pharmacotherapy is common among individuals with psychiatric disorders [28], chronic rejection (a surrogate of adherence) and lost to follow-up were not statistically significant in our study. Psychotropic medications generally require dose reduction pre-transplant as a consequence of impaired hepatic and renal clearance; however, in the post-LT period, medication metabolism is improved, and dose adjustments are again needed [29]. During the early post-transplant period, care is relinquished to the transplant team and non-transplant providers are usually uncomfortable adjusting medications for LT patients [30]. At our institution, the first 6 months of post-LT care is managed by the transplant surgeons, potentially leading to delays in adjustment of psychotropic medications and follow-up appointments with primary care doctors and/or physiatrists. Additionally, psychopathologic symptoms tend to worsen 1–2 years after LT, as the recipients endure both physical changes due to medical complications and psychological problems as they adjust to their new body integrity and dependence on new medications and medical care [31]. We hypothesize that these factors contribute to poor allograft and overall survival outcomes in the cases. However, the retrospective nature of the study did not allow us to establish these associations. Our findings were in contrast to another study which found that LT patients with clinically diagnosed major depressive disorder had an initial trend of greater survival rates for the first 8–9 years post-LT [22]. While the study was not conclusive on the reason behind this finding, they hypothesized that individuals with depression might seek medical care more frequently due to “defensive pessimism,” which postulates that these patients may be more attentive to physical warning signs and engage in clinical consultations at a higher rate [32].
We found that presence of HE to be significantly different between transplant recipients with and without psychiatric diagnosis. Hepatic encephalopathy can include a wide range of non-specific neurological and psychiatric manifestations, such as alterations in attention, memory, psychomotor speed, sleep, as well as confusion, stupor and coma [33]. Overt HE remains a diagnosis of exclusion, as medications effect, alcohol abuse, and psychiatric disorders can present similar symptoms [34]. Minimal and covert HE (early stage) require specific tests [34] that are not always available or used routinely in all liver transplant centers. More so, testing is non-specific and requires its use on individuals without confounding factors [34]. Traditionally, HE has been considered as a fully reversible condition with treatment; however, new data suggests persistent learning impairment after recovery of an episode of overt HE [35]. The majority of the cases in this study suffered from depression, a condition that has a reported higher prevalence in females [36]. We presume there has been an element of overlap between HE and depression and/or over-diagnosis of HE in our female patient cohort. Again, the retrospective nature of this study limited our ability to dissect more details about the diagnosis of HE in this population.
The increased rates of allograft loss and all-cause cause mortality in cases with HCC was an unexpected finding. Although potential explanations for poor outcomes include delayed and lower quality of care in this group of patients [37], our institution follows the United Network for Organ Sharing guidance for LT for HCC [38]; specifically, Milan [39] and USCF downstaging criteria [40]. The lower allograft and patient survival in HCC has been reported in other studies [41,42] and is possibly related to tumor biology, rate of recurrence after LT, and limited use of inhibitors of the mammalian target of rapamycin (mToR) [42]. Tumor biology and effectiveness of pre-LT loco-regional therapy were not evaluated in this study.
Another major finding of this study is that when data is censored at 1-year post-LT, patients with psychiatric diagnosis have a three to four times higher hazard for both allograft loss and all-cause mortality compared to controls after adjusting for covariates. Similar findings were observed when data is censored at 5-year mark, albeit at lower level (two times) hazard for both allograft loss and all-cause mortality. Our findings are in line with Corruble et al. study, which reported that depressive symptoms were significantly associated with 22% higher hazard of post-LT all-cause mortality [7].
The large sample size of cirrhotic patients with psychiatric diagnosis was a significant strength of this study. However, as with any retrospective study conducted with observations and data from a single institution, there are certain inherent limitations. The nature of the study prevented accounting for certain patient outcomes, including their quality of life, and/or functional status. Furthermore, the non-significant differences between patients may be attributed to the selection bias, possibly, carefully selected individuals with controlled psychiatric symptoms and with appropriate pharmacotherapy and counseling. Since our cohort included only those patients who received liver transplants from a single hospital that houses one of the largest liver transplant programs in the country, our results may not be generalizable to patient populations who do not have the similar access to this type of care. Likewise, missing data regarding care provided outside the institution was another potential limitation of this study.
The significance of this study can be attributed to the findings that the presence of a psychiatric diagnosis such as, depression, bipolar disorder, and schizophrenia, did result in significant adverse allograft loss and all-cause mortality when the data is censored at 1-year and 5-year post-LT. This is important because a higher percentage of liver transplant patients have co-occurring psychiatric disorders [43], who may require counselling or other pharmacotherapeutic inteverntions pre-LT for improved post-LT outcomes. The presence of psychiatric disorders adds complexity to the already demanding liver transplant care. Patients with well-controlled psychiatric disorders should not be denied access to transplant but followed closely by transplant hepatologist and psychiatrists for symptom monitoring and medications adjustment both pre- and post-LT. However, additional large-scale studies containing data from multiple hospitals are needed to further validate these findings. Furthermore, prospective studies on the development of psychiatric illnesses that arise during the waitlist period and post-liver transplant in patients also merit consideration in the future.AbbreviationsESLD End stage liver disease Hepatic encephalopathy Liver transplant Health-related quality of life Methodist University Hospital Hepatocellular carcinoma Hepatitis C Model for End-stage Liver Disease Alcoholic cirrhosis Non-alcoholic steatohepatitis Autoimmune liver diseases Primary sclerosing cholangitis Primary biliary cholangitis
HCG and BA conceptualized the study and BA collected the data. YJ, SKK, and SKS did the statistical analysis and interpretation. SKK and HA wrote the first draft of the manuscript. All authors participated in intellectual input, critical revisions, and approval of the manuscript.
FundingThis study was not funded by any external funding source.
Conflict of interestThe authors have no conflicts of interest to declare.