The global prevalence of non-alcoholic fatty liver disease (NAFLD) is approximately 25%, with Hispanic populations at greatest risk. We describe the prevalence of NAFLD in a cohort of Guatemalan adults and examine whether exposure to a protein-energy supplement from conception to two years is associated with lower prevalence of NAFLD.
Materials and methodsFrom 1969 to 1977, four villages in Guatemala were cluster-randomized to receive a protein-energy supplement (Atole) or a no-protein, low-energy beverage (Fresco). We conducted a follow-up of participants from 2015 to 2017. We assessed blood samples (n=1093; 61.1% women; aged 37–53 years) for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and estimated NAFLD prevalence using the liver fat score. We used generalized linear and logistic models to estimate the difference-in-difference effect of Atole from conception to two years on NAFLD.
ResultsMedian ALT and AST were 19.7U/L (interquartile range, IQR: 14.1, 27.4) and 26.0U/L (IQR: 21.4, 32.8), respectively. The median NAFLD liver fat score was 0.2 (IQR: −1.2, 1.6) in women and −1.2 (IQR: −2.2, 0.5) in men (p<0.0001). The prevalence of NAFLD was 67.4% among women and 39.5% among men (p<0.0001). The association between Atole exposure from conception to two years and NAFLD was not significant (OR: 0.90, 95% CI: 0.50–1.63).
ConclusionsNAFLD prevalence among Guatemalan adults exceeds the global average. Protein-energy supplementation in early life was not associated with later NAFLD. There is a need for further studies on the causes and onset of NAFLD throughout the life course.
Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome. The long-term consequences of NAFLD include increased risk of end-stage liver disease, liver cancer, and cardiovascular disease [1]. The global prevalence of NAFLD has risen to 25%, with some of the highest estimates reported in South and Central America [2,3]. In Guatemala, one study reported a prevalence of 56.5% [4]. Known risk factors for NAFLD include genetics, age, nutrition, and obesity. Individuals with adipogenic genes such as Patatin-like phospholipase domain-containing protein 3 (PNPLA3), which is common in Hispanics, have a higher risk for NAFLD [5].
The clinical manifestation of NAFLD in children and the presence of steatosis at birth in some newborns suggest that its origins may lie in utero [6–9]. Maternal restriction of calories and protein have been associated with increased hepatic fat in offspring in animal models [10]. In rats, maternal restriction of protein during pregnancy and lactation is associated with hepatic steatosis in offspring [11–15]. Furthermore, a high fat diet intensified the effects of perinatal protein restriction on liver outcomes [12,14]. The combination of early life undernutrition and later exposure to an obesogenic environment, characteristic of many countries undergoing the nutrition transition, may therefore contribute to the risk of developing NAFLD [16].
The impact of undernutrition in early life on later NAFLD has been studied in two cohorts. Exposure to the Great Chinese Famine in early life was associated with an increased risk of NAFLD in adulthood [17,18]. In a study using the Helsinki Birth Cohort (n=1587), individuals who were in the smallest body mass index (BMI) tertile in early childhood and obese as adults had the greatest risk of NAFLD [19]. Other studies have found associations between NAFLD and proxies of in utero malnutrition (such as being small for gestational age and of low-birth weight), as well as accelerated growth in the first three months in early life [20–22]. The effect of protein-energy intake in early life on later NAFLD has not been described in humans [23].
In the 1960s and 70s, the Institute of Nutrition of Central America and Panama (INCAP) conducted a nutrition supplementation trial in four villages in southeastern Guatemala to assess the effect of improved nutrition on child growth and development. Children in the improved nutrition group had greater total intake of protein (9g/day) and energy (100kcal/day) compared to the control group [24,25]. The population in these villages was undernourished; about 45% of children under three years had severe stunting and 86% had any stunting at 24 months [25,26]. At follow-up in 2015–2017, there was a high prevalence of cardiometabolic diseases in the cohort, including 32% with obesity and 67% with metabolic syndrome [27]. We assessed the prevalence of NAFLD and related markers of NAFLD, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST), in the cohort in mid-adulthood. Additionally, we investigated the impact of exposure to a protein-energy supplement from conception to age two years on NAFLD. This period of early life, also known as the first 1000 days, is a time of rapid development and a crucial window for influencing later health [28]. We hypothesized that exposure to increased protein and energy nutrition during early life would result in decreased risk of NAFLD later in life.
2Material and methods2.1Study populationBetween January 1, 1969 and February 28, 1977, two pairs of size-matched villages were randomized to receive either Atole (a protein and energy-containing supplement) or Fresco (a no-protein low-energy supplement). Atole was a nutritional supplement (6.4g protein, 0.4g fat, and 90kcal per 100mL) made from dry skimmed milk, sugar, and Incaparina, a vegetable protein mixture developed by INCAP. Fresco contained sugar water and flavoring (33kcal per 100mL) and had no protein. Atole and Fresco were fortified to have matching micronutrient content. The supplements were available to everyone in each village at a central location twice every day. Study staff in the original trial recorded supplement intake for pregnant and lactating women and children under 7 years. The full study design has been previously published [29].
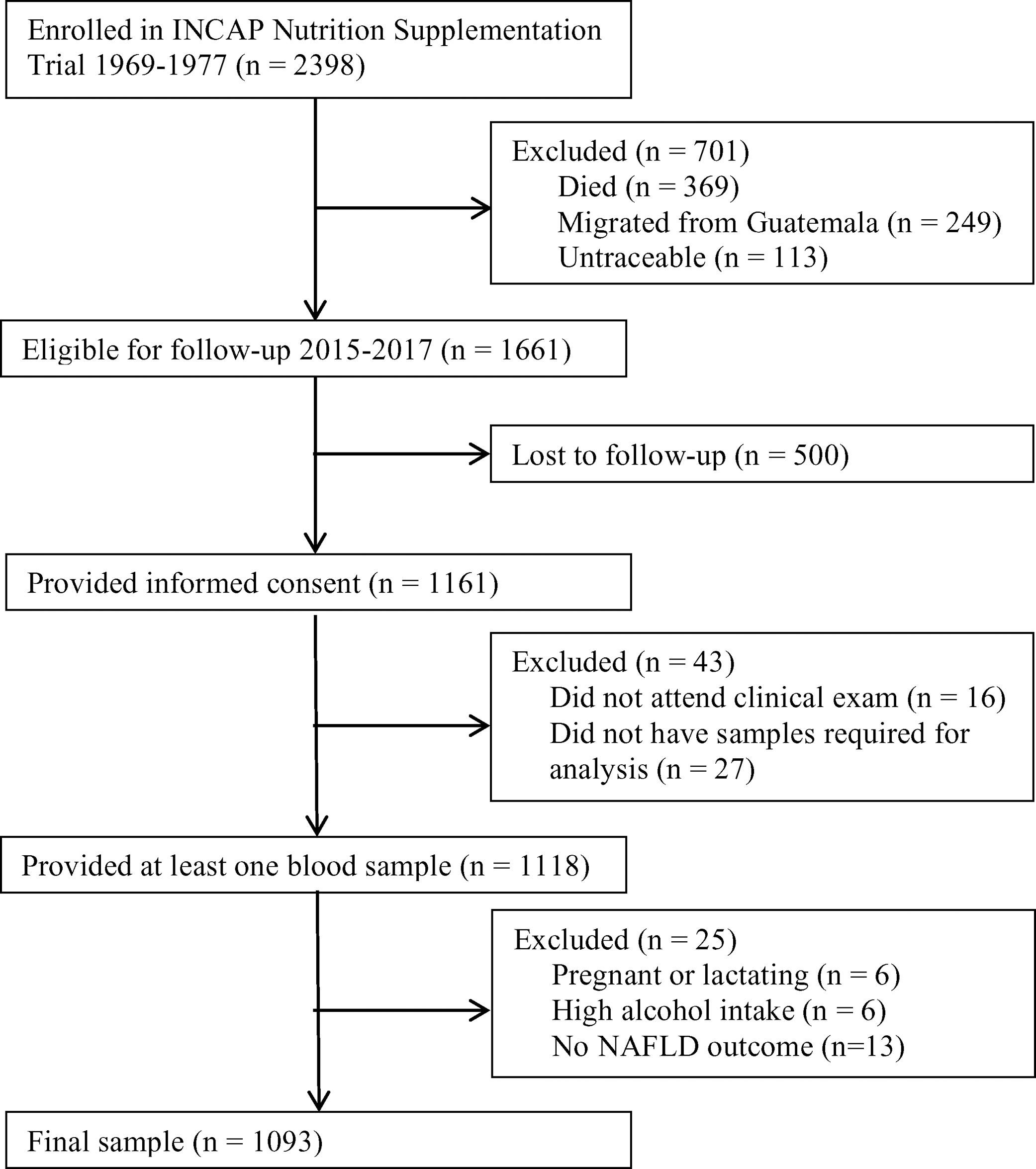
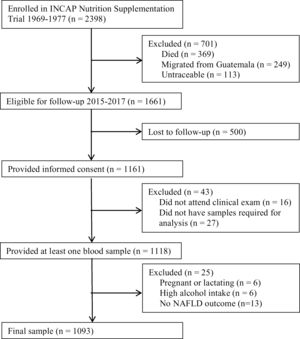
A follow-up was conducted in 2015–2017 to examine the cardiometabolic health of the cohort. Of the original 2398 participants enrolled in the nutrition-supplementation trial, 1161 were not lost to follow-up and provided informed consent. Of these participants, 1118 provided at least one plasma sample (Fig. 1). Characteristics of those lost to follow-up in comparison to those who participated have previously been published [27].
Flow diagram. Flow diagram of original 1969–1977 INCAP Guatemala study to 2015–2017 data collection and analysis. Of the original 2398 participants 369 had died, 249 had migrated outside Guatemala, and 113 were untraceable resulting in 1661 who were eligible for enrollment in 2015–2017. Of these, 1118 provided informed consent and provided at least one sample during the clinical exam. During analysis we excluded an additional 6 for pregnancy/lactation status, 6 for high alcohol intake, and 13 for not having data on the non-alcoholic fatty liver disease (NAFLD) outcome for a final analytic sample size of 1093.
Of the 1118 individuals who provided plasma samples, for the present study we excluded six who were pregnant or lactating. Since alcohol consumption also impacts liver function and can lead to steatosis, we additionally excluded six individuals who reported alcohol consumption>21 drinks/week in men and >14 drinks/week in women [1]. Finally, we excluded 13 individuals for missing the NAFLD outcome.
This study and the informed consent process were approved by the Institutional Review Boards of Emory University (Atlanta, GA) and INCAP (Guatemala City, Guatemala).
2.2Data collection and lab assaysIn addition to the birth village and date of birth, the original study collected data on maternal characteristics such as age, height, and schooling years. In the 2015–2017 wave of data collection, we obtained data on participant characteristics such as years of schooling, socioeconomic (SES) status, residence in Guatemala City, and self-reported alcohol intake. Trained field workers and phlebotomists collected anthropometric measurements and fasting blood samples, as previously described [27].
Plasma samples were frozen for storage at −80°C until being shipped on dry ice to a biomarker core laboratory in the United States. The samples were thawed at 4°C in batches, each containing approximately 40 plasma samples. Plasma ALT and AST values were assessed using the AU480 analyzer (Beckman Coulter Diagnostics, Fullerton CA, US) using enzymatic methods (Sekisui Diagnostics P.E.I. Inc., Canada). Insulin was assayed using immunoturbidimetric methods (Kamiya Biomedical Company, WA, US).
2.3Variable specification2.3.1ExposureWe determined exposure to Atole or Fresco by birth village. We used the child's birth date and the trial start and end dates to determine the age of exposure as previously described [27]. Early exposure was defined as 1000 days from conception (assumed to be 266 days before the birth date to approximate the average length of pregnancy) to age two years. In our main analysis, we considered individuals who were exposed for the entire period from conception to age two years to be fully exposed and those who were exposed for only part of that time to not be exposed. For sensitivity analysis, we also created a three-category exposure variable: those who were fully exposed, those who were partially exposed, and those without any exposure from conception to age two years.
2.3.2NAFLD and related outcomesWe used the NAFLD liver fat score equations developed by Kotronen, et al. to determine the presence of hepatic steatosis (liver fat score) and its quantity (liver fat percent) [30]. The equations include metabolic syndrome, type 2 diabetes, fasting insulin, fasting AST, and the AST/ALT ratio. A higher score indicates greater steatosis. We used the optimal cut off point of −0.640 for the NALFD liver fat score to define the presence of suspected NAFLD. This cut off was determined by Kotronen, et al. using the Youden index [30].
We defined metabolic syndrome by the presence of at least three of the following criteria: (1) central obesity (waist circumference>88cm in women, >102cm in men), (2) elevated fasting glucose (≥100mg/dL) or use of diabetes medication, (3) elevated triglycerides (≥150mg/dL) or statin use, (4) low high density lipoprotein (HDL cholesterol (<40mg/dL in men, <50mg/dL in women), and (5) hypertension (≥85mm Hg diastolic) or hypertension medication use [31]. We defined type 2 diabetes as a fasting plasma glucose ≥126mg/dL, a post challenge glucose of ≥200mg/dL or the use of diabetes medication [32]. We calculated the AST/ALT ratio by dividing AST by ALT.
2.3.3CovariatesWe calculated body mass index (BMI) by weight in kilograms divided by height in meters squared [33]. We calculated the waist-to-height ratio by dividing the waist circumference in centimeters by height in centimeters. We categorized childhood and adulthood socioeconomic status into tertiles. We had previously derived socioeconomic status from a principal component analysis of household characteristics and consumer durable goods [27]. We characterized current residence dichotomously as Guatemala City or other to capture rural vs urban location. We included age at follow-up and completed grades of schooling for mothers and their children as continuous variables.
2.4Statistical analysisWe calculated median and interquartile range (IQR) values for continuous variables and percent for categorical variables. We used Wilcoxon Rank Sum tests to compare differences in medians between men and women for continuous clinical covariates and NAFLD related outcomes. We used a Pearson chi-square test to compare NAFLD, type 2 diabetes, and metabolic syndrome percent in men and women.
We used multiple imputation to deal with missing data for maternal height (20.5% missing), maternal years of schooling (3.5% missing), and maternal age (1.6% missing). We used the fully conditional specification method for five imputations. To impute missing values, we used predictive mean matching for continuous variables and the logistic regression method for ordinal variables. We included all predictor and outcome variables that were not linear effects of each other as predictors in the imputation model [34].
We used generalized regression models to estimate the difference-in-difference intent-to-treat effect of Atole vs Fresco from conception to age two years, net of the differences attributable to village or birth year effects. We estimated the difference-in-difference effect by an interaction term between the type of exposure (Atole vs. Fresco) and the timing of exposure (during the full first 1000 days versus other). The base model included birth village in the form of three dummy variables (this also serves to account for treatment assignment of Atole vs Fresco), a dichotomous variable to account for duration of exposure (full vs other), birth year, maternal height (as an indicator of maternal nutrition status), maternal years of schooling, sex, and childhood SES. We additionally controlled for several adult sociodemographic and clinical factors including SES status, years of schooling, residence, BMI, waist-to-height ratio, and height. We considered sex as a potential effect modifier. We tested for stratum heterogeneity by examining the significance of the third-order interaction between sex, exposure type and duration.
We conducted all statistical analysis in SAS version 9.4. We used PROC GENMOD, for modeling and PROC MI and PROC MIANALYZE for multiple imputation. Statistical significance was determined by p<0.05 and p-values were two-sided.
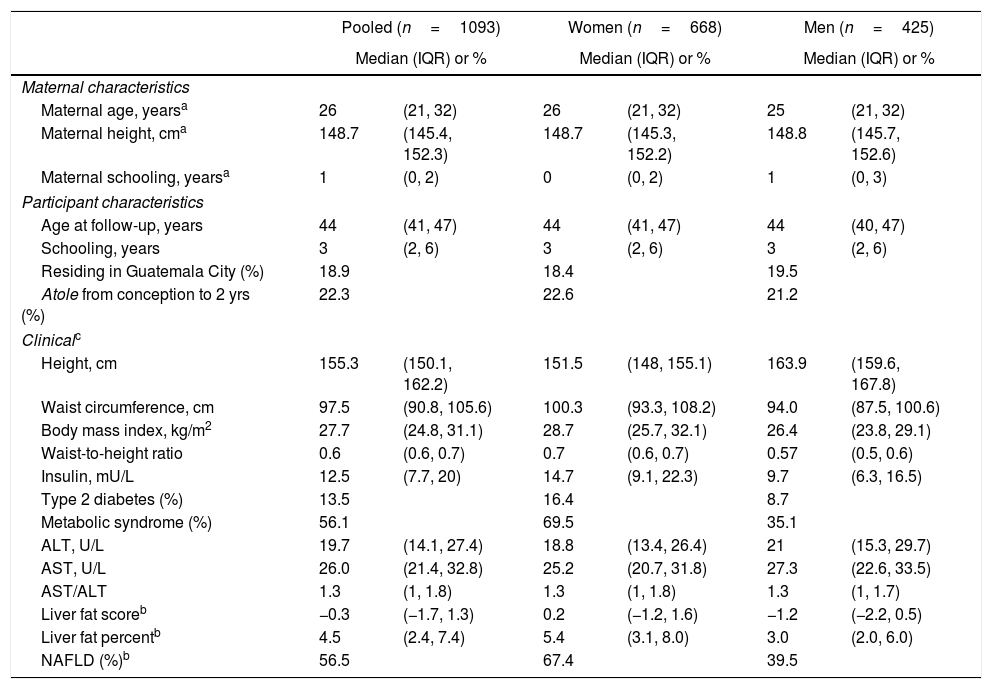
3ResultsThe final sample size was 1093 individuals (61.1% women) (Table 1). Selected characteristics of the study population are in Table 1. The median age at follow-up was 44 years (IQR 41–47). Twenty-two percent of participants were exposed to Atole during the full period from conception to age two years. An additional 17.8% were exposed to Atole for part of the period from conception to age 2 years.
Sociodemographic and clinical characteristics (n=1093).
| Pooled (n=1093) | Women (n=668) | Men (n=425) | ||||
|---|---|---|---|---|---|---|
| Median (IQR) or % | Median (IQR) or % | Median (IQR) or % | ||||
| Maternal characteristics | ||||||
| Maternal age, yearsa | 26 | (21, 32) | 26 | (21, 32) | 25 | (21, 32) |
| Maternal height, cma | 148.7 | (145.4, 152.3) | 148.7 | (145.3, 152.2) | 148.8 | (145.7, 152.6) |
| Maternal schooling, yearsa | 1 | (0, 2) | 0 | (0, 2) | 1 | (0, 3) |
| Participant characteristics | ||||||
| Age at follow-up, years | 44 | (41, 47) | 44 | (41, 47) | 44 | (40, 47) |
| Schooling, years | 3 | (2, 6) | 3 | (2, 6) | 3 | (2, 6) |
| Residing in Guatemala City (%) | 18.9 | 18.4 | 19.5 | |||
| Atole from conception to 2 yrs (%) | 22.3 | 22.6 | 21.2 | |||
| Clinicalc | ||||||
| Height, cm | 155.3 | (150.1, 162.2) | 151.5 | (148, 155.1) | 163.9 | (159.6, 167.8) |
| Waist circumference, cm | 97.5 | (90.8, 105.6) | 100.3 | (93.3, 108.2) | 94.0 | (87.5, 100.6) |
| Body mass index, kg/m2 | 27.7 | (24.8, 31.1) | 28.7 | (25.7, 32.1) | 26.4 | (23.8, 29.1) |
| Waist-to-height ratio | 0.6 | (0.6, 0.7) | 0.7 | (0.6, 0.7) | 0.57 | (0.5, 0.6) |
| Insulin, mU/L | 12.5 | (7.7, 20) | 14.7 | (9.1, 22.3) | 9.7 | (6.3, 16.5) |
| Type 2 diabetes (%) | 13.5 | 16.4 | 8.7 | |||
| Metabolic syndrome (%) | 56.1 | 69.5 | 35.1 | |||
| ALT, U/L | 19.7 | (14.1, 27.4) | 18.8 | (13.4, 26.4) | 21 | (15.3, 29.7) |
| AST, U/L | 26.0 | (21.4, 32.8) | 25.2 | (20.7, 31.8) | 27.3 | (22.6, 33.5) |
| AST/ALT | 1.3 | (1, 1.8) | 1.3 | (1, 1.8) | 1.3 | (1, 1.7) |
| Liver fat scoreb | −0.3 | (−1.7, 1.3) | 0.2 | (−1.2, 1.6) | −1.2 | (−2.2, 0.5) |
| Liver fat percentb | 4.5 | (2.4, 7.4) | 5.4 | (3.1, 8.0) | 3.0 | (2.0, 6.0) |
| NAFLD (%)b | 56.5 | 67.4 | 39.5 | |||
Abbreviations: ALT=alanine aminotransferase; AST=aspartate aminotransferase; AST/ALT=ratio between AST and ALT; NAFLD=non-alcoholic fatty liver disease; IQR=interquartile range.
Maternal age (n=1075), maternal height (n=869), maternal schooling (n=1054) and post-challenge glucose (n=1011) were missing values.
Calculated using fasting insulin, type 2 diabetes, metabolic syndrome, fasting AST, and fasting AST/ALT ratio [30].
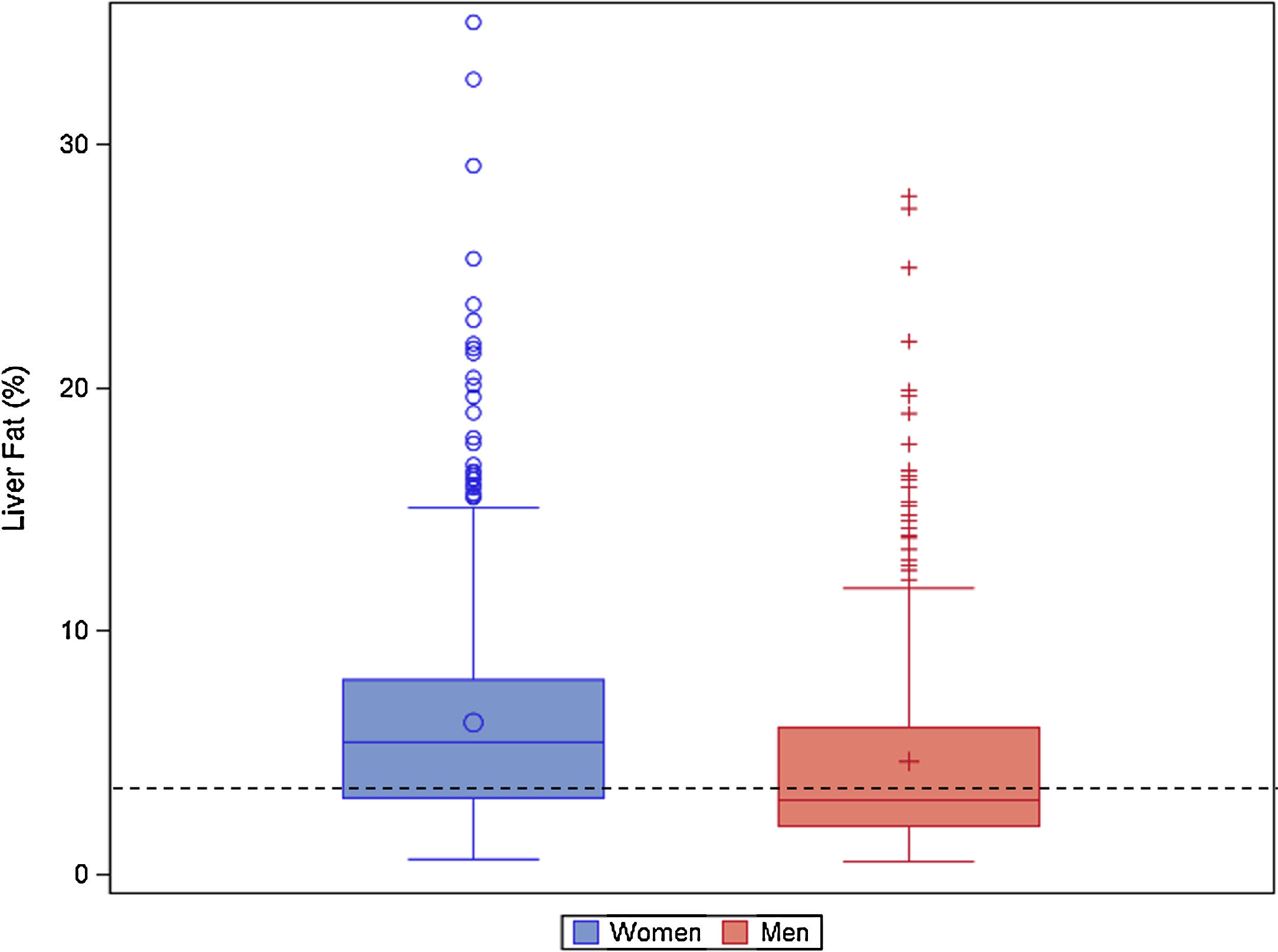
The median NAFLD liver fat score was 0.2 (IQR: −1.2, 1.6) in women and −1.2 (IQR: −2.2, 0.5) in men (p<0.0001). The median liver fat percent was 5.4% (IQR: 3.1, 8.0) in women and 3.0% (IQR: 2.0, 6.) in men (p<0.0001) (Table 1; Fig. 2). Median ALT was 19.7U/L (IQR: 14.0, 27.4), median AST was 26.0U/L (IQR: 21.3, 32.8), and the median AST/ALT ratio was 1.3 (IQR 1.0, 1.8). Men had significantly higher ALT and AST values (p<0.0001). The prevalence of NAFLD was 56.5% and was higher in women (67.4%) than men (39.5%; p<0.0001).
Liver fat percent boxplot by sex. Histogram of liver fat percent by sex (blue(0)=women, red(+)=men) in the Guatemala INCAP study cohort 2015–2017 (n=668 women, 425 men). The liver fat percent is calculated using presence of metabolic syndrome, type 2 diabetes, fasting insulin, fasting aspartate aminotransferase(AST), and fasting AST/ALT ratio.[30] The dotted black line indicates the 5% cut off for NAFLD.
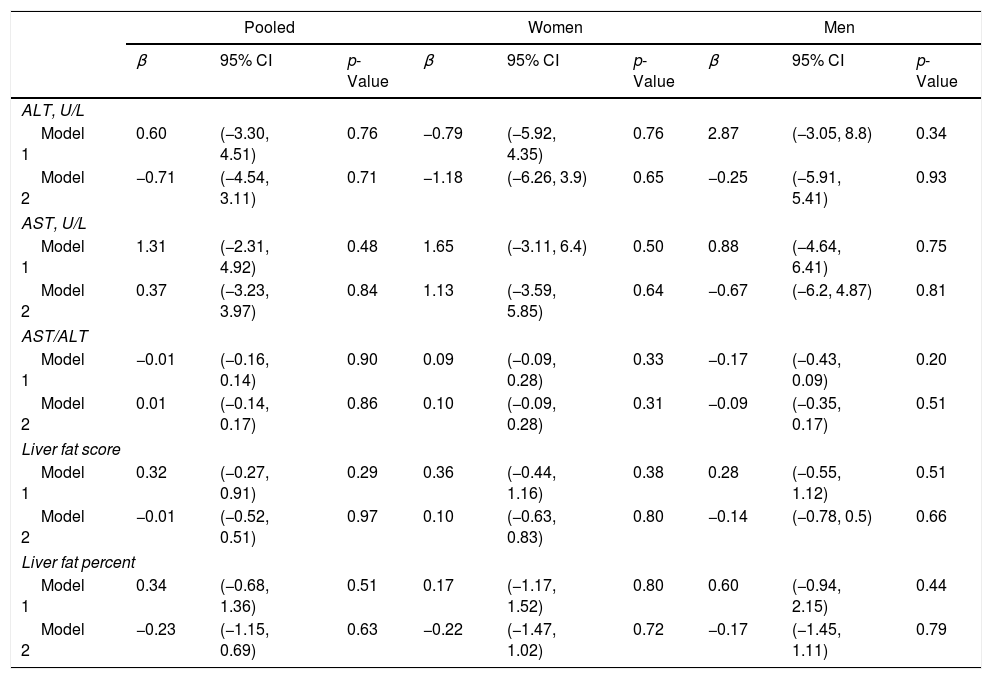
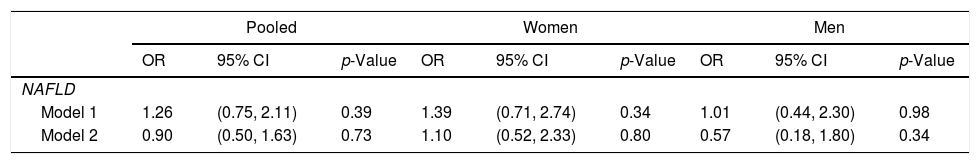
The assocations between exposure to Atole in early life and NAFLD-related parameters are provided in Table 2. The estimates for ALT, AST, AST/ALT ratio, liver fat score, and liver fat percent were not statistically significant in the base model or with additional adjustment for adult factors. Similarly, the odds ratio for NAFLD was not significant in the base model (OR: 1.26, 95% CI: 0.75–2.11) or after accounting for adult factors (OR: 0.90, 95% CI: 0.50–1.63) (Table 3). There was no significant stratum heterogeneity by sex. In sensitivity analysis, we found no significant differences in the models for those with partial vs no exposure and full vs no exposure.
Adjusteda linear difference-in-difference estimates for exposure to Atole from conception to age 2 years vs other, by non-alcoholic fatty liver disease related outcomes (n=1093).
| Pooled | Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p-Value | β | 95% CI | p-Value | β | 95% CI | p-Value | |
| ALT, U/L | |||||||||
| Model 1 | 0.60 | (−3.30, 4.51) | 0.76 | −0.79 | (−5.92, 4.35) | 0.76 | 2.87 | (−3.05, 8.8) | 0.34 |
| Model 2 | −0.71 | (−4.54, 3.11) | 0.71 | −1.18 | (−6.26, 3.9) | 0.65 | −0.25 | (−5.91, 5.41) | 0.93 |
| AST, U/L | |||||||||
| Model 1 | 1.31 | (−2.31, 4.92) | 0.48 | 1.65 | (−3.11, 6.4) | 0.50 | 0.88 | (−4.64, 6.41) | 0.75 |
| Model 2 | 0.37 | (−3.23, 3.97) | 0.84 | 1.13 | (−3.59, 5.85) | 0.64 | −0.67 | (−6.2, 4.87) | 0.81 |
| AST/ALT | |||||||||
| Model 1 | −0.01 | (−0.16, 0.14) | 0.90 | 0.09 | (−0.09, 0.28) | 0.33 | −0.17 | (−0.43, 0.09) | 0.20 |
| Model 2 | 0.01 | (−0.14, 0.17) | 0.86 | 0.10 | (−0.09, 0.28) | 0.31 | −0.09 | (−0.35, 0.17) | 0.51 |
| Liver fat score | |||||||||
| Model 1 | 0.32 | (−0.27, 0.91) | 0.29 | 0.36 | (−0.44, 1.16) | 0.38 | 0.28 | (−0.55, 1.12) | 0.51 |
| Model 2 | −0.01 | (−0.52, 0.51) | 0.97 | 0.10 | (−0.63, 0.83) | 0.80 | −0.14 | (−0.78, 0.5) | 0.66 |
| Liver fat percent | |||||||||
| Model 1 | 0.34 | (−0.68, 1.36) | 0.51 | 0.17 | (−1.17, 1.52) | 0.80 | 0.60 | (−0.94, 2.15) | 0.44 |
| Model 2 | −0.23 | (−1.15, 0.69) | 0.63 | −0.22 | (−1.47, 1.02) | 0.72 | −0.17 | (−1.45, 1.11) | 0.79 |
Abbreviations: ALT=alanine aminotransferase; AST=aspartate aminotransferase; AST/ALT=ratio between AST and ALT; CI=confidence interval; SES=socioeconomic status.
Model 1 (base model) adjusted for birth village, duration of exposure, birth year, maternal height, maternal years of schooling, sex (in pooled models), and childhood SES. Model 2 additionally adjusted for adult SES, years of schooling, residence, body mass index, waist-to-height ratio, and height.
Adjusted binomial logistic difference-in-difference OR for exposure to Atole for full period from conception to age 2 y vs. other, for NAFLD (n=1093).a
| Pooled | Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| NAFLD | |||||||||
| Model 1 | 1.26 | (0.75, 2.11) | 0.39 | 1.39 | (0.71, 2.74) | 0.34 | 1.01 | (0.44, 2.30) | 0.98 |
| Model 2 | 0.90 | (0.50, 1.63) | 0.73 | 1.10 | (0.52, 2.33) | 0.80 | 0.57 | (0.18, 1.80) | 0.34 |
Abbreviations: OR=odds ratio; CI=confidence interval; NAFLD=non-alcoholic fatty liver disease; SES=socioeconomic status.
Model 1 (base model) adjusted for birth village, duration of exposure, birth year, maternal height, maternal years of schooling, sex (in pooled models), and childhood SES. Model 2 additionally adjusted for adult SES, years of schooling, residence, body mass index, waist-to-height ratio, and height.
We describe the prevalence of NAFLD among a cohort that participated in an improved protein-energy supplementation trial during early life in Guatemala. Their prevalence of NAFLD in mid-adulthood was 56.5% and was significantly higher in women compared to men. Exposure to the protein-energy supplement from conception to two years was not associated with NAFLD in mid-adulthood.
The NAFLD prevalence we report is consistent with the only other study on NAFLD prevalence in Guatemala, despite the use of different NAFLD criteria [4]. Rivera-Andrade used the Fatty Liver Index and found a NAFLD prevalence of 66.5% and 50.6% in women and men over 40 years old, respectively [4]. NAFLD is usually more common in men, although this difference is less pronounced with age [35]. Women in our study had a higher prevalence of metabolic syndrome and type 2 diabetes, which could partially explain their higher NAFLD prevalence, especially since we used a NAFLD score that includes type 2 diabetes and metabolic syndrome in its calculation.
In a previous publication using the same population, the Atole supplement had mixed effects on cardiometabolic outcomes. It was associated with increased odds of obesity (OR: 1.99, 95% CI: 1.16–3.41), reduced odds of diabetes (OR: 0.47, 95% CI: 0.22–0.99), and was not significantly associated with metabolic syndrome [27]. The diverging associations between early-life nutrition and later cardiometabolic conditions may be due to various interlinked mechanisms, including the role of protein in directing the development of metabolically active tissues [36].
Additionally, we may have failed to observe a significant association between early-life protein-energy supplementation and adulthood NAFLD because the lifetime cumulative effect of other factors such as BMI trajectory or diet that dominate over the effect of early life exposure to Atole. Excluding those with obesity, which might overpower any effects from early life, did not meaningfully change our estimates.
Animal models have linked early life protein and energy restriction to later NAFLD and described potential pathways through which this occurs. In rats, maternal protein restriction results in upregulation of the transcription factors sterol regulatory element binding protein (SREBP-1c), carbohydrate-responsive element-binding protein (ChREBP) and peroxisome proliferator-activated receptor-γ (PPAR-γ). This effects downstream genes important to lipid metabolism and insulin signaling [11,14,15]. At the organ level, growth restricted offspring exhibit fewer but larger hepatic lobules and enzymatic alterations, which, along with a decreased pancreatic b-cell mass, lead to insulin resistance [37,38]. In the liver, insulin resistance inhibits b-oxidation and leads to decreased output of lipids. This, along with the increase of free fatty acid influx into the liver due to insulin resistance, results in steatosis of the liver.
There are several strengths in our study. One strength is the sample size and use of multiple imputation to account for missing data to maximize model size. Although attrition is a concern as about 50% of the original cohort were in the 2015–2017 wave of data collection, there is no evidence that attrition has affected the validity of this study. Ford, et al. found that attrition was not differential with respect to Atole exposure from conception to age two years. Additionally, individuals lost to follow-up were similar to those who participated in the 2015–2017 study, except for sex [27].
Another strength of this study is the use of the composite NAFLD liver fat score. ALT levels alone have poor sensitivity and specificity in adults [39,40]. Therefore, scores like the NAFLD liver fat score are useful as non-invasive predictors of the disease [30]. The original NAFLD liver fat score was developed in a large Finnish cohort in 2009 so it is possible it is not transferable to other populations. However, when compared to three other NAFLD prediction scores, the NAFLD liver fat score had the best non-invasive prediction score for NAFLD and associated mortality including in Hispanics [41].
There are a few limitations in our study. The ideal exposure period may only be a subset of the first 1000 days such as during liver organogenesis in utero, which begins at four weeks and continues throughout gestation. In our study, it is not possible to examine exposure during pregnancy and early childhood separately due to the limited sample size; 3.7% (n=40) of the original cohort were exposed to Atole only in pregnancy. Additionally, sufficient data were not available in our cohort on variables that have been previously associated with NAFLD including gestational age and birthweight of which 63% and 57% were missing, respectively. Among those with data available about 10% were born pre-term (<37 weeks gestation) or with low birthweight (<2.5kg).
5ConclusionsUnderstanding the developmental origins of NAFLD and other metabolic conditions is particularly important because of the growing prevalence of these diseases [10]. The high prevalence of NAFLD in Guatemala highlights the need for population-based studies that will help identify the underlying mechanisms of the onset and development of NAFLD throughout the life course, including and beyond early-life nutritional exposure.AbbreviationsNAFLD non-alcoholic fatty liver disease alanine aminotransferase aspartate aminotransferase Institute of Nutrition of Central America and Panama interquartile range odds ratio body mass index socioeconomic status
This work was supported by the National Institutes of Health [HD075784].
Conflict of interestMBV reports personal fees from Intercept, grants from Immuron, grants and personal fees from Target Pharmasolutions, personal fees from Boehringer Ingelheim, grants and personal fees from Bristol Myers Squibb, personal fees from Prosciento, and personal fees from Novo Nordisk during the conduct of the study.
We thank Andrea J Sharma her feedback.





![Liver fat percent boxplot by sex. Histogram of liver fat percent by sex (blue(0)=women, red(+)=men) in the Guatemala INCAP study cohort 2015–2017 (n=668 women, 425 men). The liver fat percent is calculated using presence of metabolic syndrome, type 2 diabetes, fasting insulin, fasting aspartate aminotransferase(AST), and fasting AST/ALT ratio.[30] The dotted black line indicates the 5% cut off for NAFLD. Liver fat percent boxplot by sex. Histogram of liver fat percent by sex (blue(0)=women, red(+)=men) in the Guatemala INCAP study cohort 2015–2017 (n=668 women, 425 men). The liver fat percent is calculated using presence of metabolic syndrome, type 2 diabetes, fasting insulin, fasting aspartate aminotransferase(AST), and fasting AST/ALT ratio.[30] The dotted black line indicates the 5% cut off for NAFLD.](https://static.elsevier.es/multimedia/16652681/0000001900000004/v2_202007040825/S1665268120300417/v2_202007040825/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)




