Introduction and aim. New criteria for acute kidney injury (AKI) in cirrhosis have been proposed, but its prognostic significance is unclear. This study aims to evaluate the prognostic significance of the AKI criteria in cirrhotic patients hospitalized for acute decompensation.
Material and methods. This is a prospective cohort study. AKI was defined as an increase in creatinine (Cr) levels ≥ 0.3 mg/dL in 48 h or ≥ 50% of the basal value in the last 7d. AKI was divided into stages 1 (elevation: < 2x basal), 2 (2 or 3x), and 3 (> 3x).
Results. In this study, 227 patients aged 53.9 ± 11.5 years were included, of whom 37% had AKI (28% AKI1, 5% AKI2, and 4% AKI3). Thirty percent of the patients died or were transplanted within 90 days from causes related to the presence of ascites at hospital admission and higher values of Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) scores, but not to the presence of AKI. In a regression analysis conducted to assess the effect of the final Cr level in patients with AKI, 90-day mortality was associated with ascites, higher CLIF-SOFA score, and AKI with final Cr level ≥ 1.5 mg/dL. The patients with AKI with Cr levels ≥ 1.5 mg/dL showed lower transplant-free survival rates than those without AKI, and those with AKI1 with final Cr level < 1.5 mg/dL.
Conclusions. Early AKI was frequent and associated with 90-day mortality or transplantation only when the final Cr level was ≥ 1.5 mg/dL. Distinct approaches are needed for patients with AKI1 according to final Cr.
Renal dysfunction is a frequent and challenging complication of liver cirrhosis, and its definition has been extensively revised.1 Twenty years ago, the proposed criteria to establish a significant reduction in glomerular filtration rate in cirrhotic patients, which by that time was defined as hepatorenal syndrome, were:
- •
Serum creatinine level higher than 1.5 mg/dL or
- •
24-h creatinine depuration of < 40 mL/min, excluding other causes of renal insufficiency.2
Recently, the acute kidney injury (AKI) network staging system was created, which is based on studies that showed that the risk of death and renal substitution therapy increases at each stage3,4 and that AKI is associated with a greater risk of death.5 Therefore, a small increase in serum creatinine level (e.g., 0.3 mg/dL or 50% higher than the basal) was established to be enough to diagnose AKI.1
Despite the fact that the concept of AKI has been especially elaborated for patients at intensive care units (ICUs), this concept has been used for patients admitted for acute decompensation of cirrhosis. This is because, in addition to hepatorenal syndrome, this group of patients may develop renal insufficiency due to a series of other causes, particularly volume depletion, bacterial infections, administration of nephrotoxic drugs, exacerbation of chronic renal diseases, or a combination of factors.6,7 Although clinical practice guidelines for AKI clearly states that etiology is important for therapy, it is not required for the diagnosis and staging of AKI.1
Currently, AKI is classified into three stages according to the magnitude of increase in serum creatinine level as follows: stage 1 (elevation of creatinine level: < 2 times the basal value), stage 2 (2 or 3 times the basal value), and stage 3 (3 times the basal value).1 Fagundes, et al. proposed the following new classification into 3 groups of risk:
- •
Patients with stage AKI 1 with a creatinine peak ≥ 1.5 mg/dL.
- •
Patients with stage 1 with a creatinine peak > 1.5 mg/dL, and
- •
Patients with stage 2 or 3 disease.8
In addition, in 2015, the Ascites International Club published the new resolutions for diagnosis and management of AKI in cirrhotic patients, including the cutoff point of 1.5 mg/dL for stage.19
Considering that the prognostic significance of the new diagnostic criteria for AKI in cirrhosis is still unclear, this study aimed to describe the clinical characteristics of a group of individuals admitted for acute decompensation of the disease, identify the characteristics associated with AKI and 90-day mortality, and finally, determine the prognostic significance of new criteria for AKI in this population.
Material and MethodsStudy SampleFrom January 2011 to October 2014, a prospective cohort study was performed, which included individuals consecutively admitted to the emergency service of a Brazilian tertiary hospital for acute decompensation of cirrhosis. The following exclusion criteria were used: admission for elective procedures, admissions not related to liver cirrhosis complications, hospitalization for less than 48 h, hepatocellular carcinoma outside Milan criteria, absence of laboratory data, and questionable diagnosis of liver cirrhosis. Repeated hospital admissions were excluded, and the most recent admission was considered for this analysis. All the patients were initially admitted in the emergency unit. The decision to transfer the patient to the ward or ICU was made by the assistant physician according to the severity of acute decompensation. The diagnosis of cirrhosis was established histologically (when available) or based on a combination of clinical, imaging, and laboratory findings in patients with evidence of portal hypertension.
MethodsAcute liver decompensation was defined as the acute development of hepatic encephalopathy, large-volume ascites, gastrointestinal hemorrhage, bacterial infection, or any combination of these. AKI was defined on the first 48 h of admission as an increase in creatinine level ≥ 0.3 mg/dL in 48 h or ≥ 50% of the basal value, presumably occurring in the last 7 days. The last value of serum creatinine within the last 3 months before admission (when available) or the creatinine value at admission was used as baseline creatinine. The final creatinine was that of admission, in case of an available creatinine prior to hospitalization or 48-h creatinine when admission creatinine was considered the baseline creatinine. AKI was divided into stage 1 (elevation of creatinine level: < 2 times the basal), stage 2 (2 or 3 times), and stage 3 (> 3 times).9 All patients were evaluated within 24 h after admission and, subsequently, within 48 h after admission by one of the researchers involved in the study. The following clinical variables were collected: age, sex, ethnicity, cirrhosis etiology, current complications of cirrhosis, classification of renal dysfunction at admission, infection within the first 48 h of hospitalization, and regular use of propranolol.
All individuals were subjected to laboratory evaluation at admission and 48 h, and the following were assessed: creatinine, sodium, albumin, international normalized ratio (INR), total bilirubin level, platelet count, and C-reactive protein (CRP).
Active alcoholism was defined as an average overall consumption of 21 or more drinks per week for men and 14 or more drinks per week for women during the 4 weeks before enrollment (one standard drink is equal to 12 g absolute alcohol).10 The patients were monitored during hospitalization, and 90-day mortality was assessed through telephone enquiries in case of hospital discharge.
Individuals with suspicion of bacterial infection at hospital admission were subjected to clinical examination in order to confirm this diagnosis and establish the main primary source of infection. The diagnosis of infection was performed according the guidelines established by the Centers for Disease Control.11 Diagnostic paracentesis was performed in all patients with ascites at admission. Spontaneous bacterial peritonitis (SBP) was diagnosed when the neutrophil count in the ascitic fluid was ≥ 250 neutrophils/mm3 in the absence of an intra-abdominal source of infection, regardless of negative culture result.12 All the patients with SBP received ceftriaxone and intravenous albumin adjusted for weight, in the first and third days after diagnosis.
Hepatic encephalopathy was graded according to the West-Haven criteria.13 When present, a precipitant factor was investigated and lactulose administration was initiated, with adjusted dosage as needed. All patients with acute variceal bleeding received intravenous octreotide and an antibiotic (oral norfloxacin or intravenous ceftriaxone), and were subjected to emergency therapeutic endoscopy after stabilization. The severity of liver disease was estimated according to the Child-Pugh classification system14 and through the model for end-stage liver disease (MELD),15 calculated based on laboratory tests performed at admission. Acute-on-Chronic Liver Failure (ACLF) and the Chronic Liver Failure-Sequential Organ Failure Assessment (CLIF-SOFA) were defined as proposed by the European Association for the Study of Liver-Chronic Liver Failure (EASL-CLIF) Consortium.16
Statistical analysesPatients were divided into two groups according to the presence or absence of AKI. The groups were compared regarding clinical and laboratory variables.
The normality of the distribution of variables was determined by using the Kolmogorov-Smirnov test. The numerical variables with normal distribution were expressed as mean and standard deviation (SD), and compared by using the Student t test. The numerical variables with non-normal distributions were expressed as median and compared by using the Mann-Whitney U test. The qualitative variables were represented by frequency (%); and for its analysis, the χ2 and Fisher exact tests were used, as necessary. Bivariate and multivariate analyses were performed to identify the variables associated with AKI and 90-day death or liver transplantation. Multiple logistic regression analysis (forward stepwise regression) was used to investigate the factors independently associated with 90-day death or liver transplantation. Kaplan-Meier curves were constructed after logistic regression analysis as a way of demonstrating the impact of different AKI categories in transplant-free survival. Differences in survival between the groups were compared by using the log-rank test.
Statistical analysis was performed by Statistical Package for the Social Sciences software version 22.0 (IBM SPSS statistics, Chicago, Illinois, EUA). Results were considered significant when P values < 0.05.
The study protocol is in agreement with the ethical guidelines of the Declaration of Helsinki and was approved by the local ethics research committee, under No. 252.709.
ResultsCharacteristics of the sampleFrom January 2011 to October 2014, 336 admissions due to acute decompensation of hepatic cirrhosis were registered. When evaluated based only on the most recent hospitalization, 252 patients were considered for inclusion in the study. Twenty-two patients who were hospitalized for less than 48 h were excluded. Another three individuals who did not have laboratory data were excluded. Therefore, 227 patients were eligible for the study.
The mean age was 53.9 ± 11.5 years, 72.7% described themselves as Caucasian or White and a male predominance was observed (72.7%). The mean MELD score was 16.84 ± 6.51, 41.3% of the patients were classified as Child-Pugh C and 24.7% presented ACLF (Grade 1 in 18.5%, Grade 2 in 4.4% and Grade 3 in 1.8%). Active alcoholism was reported by 37.0% of the patients. The main cirrhosis etiologies were alcohol intake (36.1%) and hepatitis C virus (HCV) (15.9%), whereas 22.9% presented both alcoholism and HCV. Previous cirrhosis decompensation was reported by 62.2% of patients.
Variables associated with AKIAt admission, AKI was observed in 37.0% of the patients (stages 1, 2, and 3 in 27.8%, 4.8%, and 4.4%, respectively). Among the individuals who presented with AKI, 85.7% had pre renal insufficiency and 14.3% had hepatorenal syndrome. Patients with HRS type 1 with creatinine ≥ 1.5 mg/dL receive intravenous albumin for 48 h and, if they do not respond and there is no other obvious cause for renal dysfunction, they receive terlipressin-albumin. Among patients with hepatorenal syndrome type 1 in the first 3 days of hospital admission, none have survived despite medical efforts.
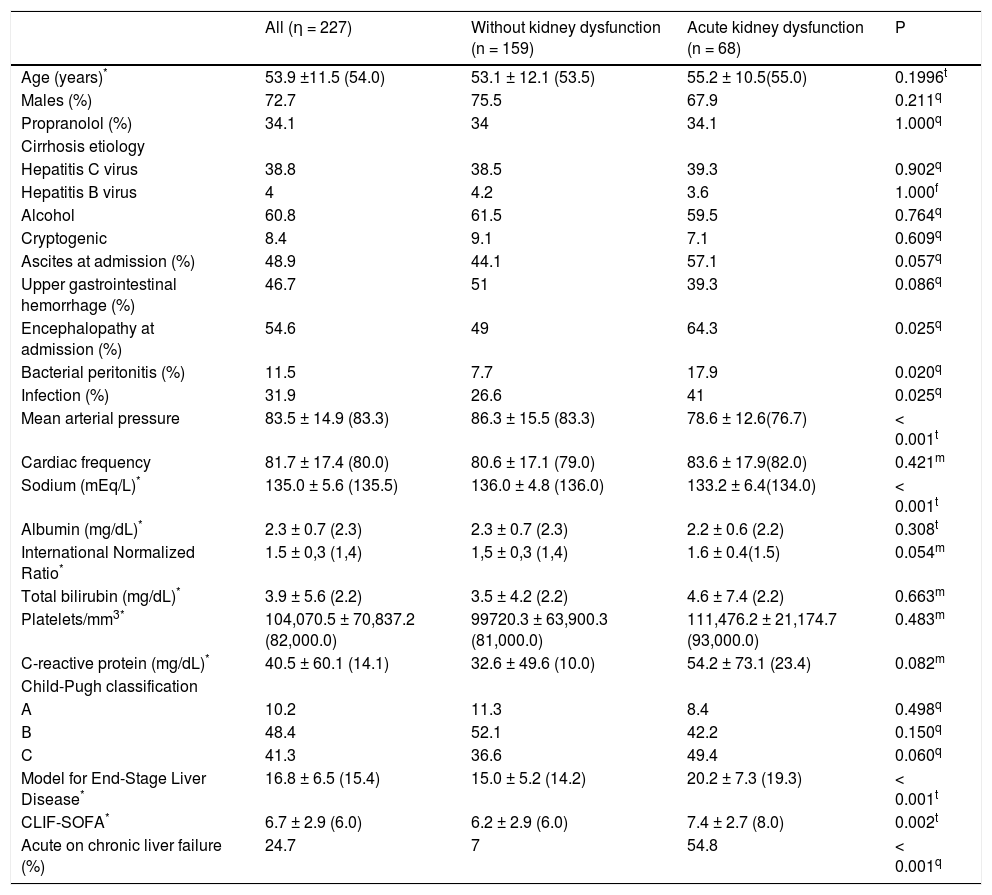
As compared to patients without AKI, the AKI group had greater proportion of individuals with hepatic encephalopathy at admission (64.3% vs. 49.0%; P = 0.025), SBP (17.9% vs. 7.7%; P = 0.020), and bacterial infection (41.0% vs. 26.6%; P = 0.025). Regarding laboratory variables, individuals with AKI showed lower mean sodium levels (133.2 ± 6.4 vs. 136.0 ± 4.8 mEq/L; P < 0.001). As expected, patients with AKI showed greater mean MELD score (20.2 ± 7.3 vs. 15.0 ± 5.2; P < 0.001) and CLIF-SOFA score (7.4% ± 2.7 vs. 6.2% ± 2.9; P = 0.002), and higher proportion of individuals with ACLF (54.8% vs. 7.0%; P < 0.001) (Table 1).
Characteristics of the 227 individuals hospitalized for acute decompensation of hepatic cirrhosis, according to the presence of acute kidney dysfunction†at admission.
| All (η = 227) | Without kidney dysfunction (n = 159) | Acute kidney dysfunction (n = 68) | P | |
|---|---|---|---|---|
| Age (years)* | 53.9 ±11.5 (54.0) | 53.1 ± 12.1 (53.5) | 55.2 ± 10.5(55.0) | 0.1996t |
| Males (%) | 72.7 | 75.5 | 67.9 | 0.211q |
| Propranolol (%) | 34.1 | 34 | 34.1 | 1.000q |
| Cirrhosis etiology | ||||
| Hepatitis C virus | 38.8 | 38.5 | 39.3 | 0.902q |
| Hepatitis Β virus | 4 | 4.2 | 3.6 | 1.000f |
| Alcohol | 60.8 | 61.5 | 59.5 | 0.764q |
| Cryptogenic | 8.4 | 9.1 | 7.1 | 0.609q |
| Ascites at admission (%) | 48.9 | 44.1 | 57.1 | 0.057q |
| Upper gastrointestinal hemorrhage (%) | 46.7 | 51 | 39.3 | 0.086q |
| Encephalopathy at admission (%) | 54.6 | 49 | 64.3 | 0.025q |
| Bacterial peritonitis (%) | 11.5 | 7.7 | 17.9 | 0.020q |
| Infection (%) | 31.9 | 26.6 | 41 | 0.025q |
| Mean arterial pressure | 83.5 ± 14.9 (83.3) | 86.3 ± 15.5 (83.3) | 78.6 ± 12.6(76.7) | < 0.001t |
| Cardiac frequency | 81.7 ± 17.4 (80.0) | 80.6 ± 17.1 (79.0) | 83.6 ± 17.9(82.0) | 0.421m |
| Sodium (mEq/L)* | 135.0 ± 5.6 (135.5) | 136.0 ± 4.8 (136.0) | 133.2 ± 6.4(134.0) | < 0.001t |
| Albumin (mg/dL)* | 2.3 ± 0.7 (2.3) | 2.3 ± 0.7 (2.3) | 2.2 ± 0.6 (2.2) | 0.308t |
| International Normalized Ratio* | 1.5 ± 0,3 (1,4) | 1,5 ± 0,3 (1,4) | 1.6 ± 0.4(1.5) | 0.054m |
| Total bilirubin (mg/dL)* | 3.9 ± 5.6 (2.2) | 3.5 ± 4.2 (2.2) | 4.6 ± 7.4 (2.2) | 0.663m |
| Platelets/mm3* | 104,070.5 ± 70,837.2 (82,000.0) | 99720.3 ± 63,900.3 (81,000.0) | 111,476.2 ± 21,174.7 (93,000.0) | 0.483m |
| C-reactive protein (mg/dL)* | 40.5 ± 60.1 (14.1) | 32.6 ± 49.6 (10.0) | 54.2 ± 73.1 (23.4) | 0.082m |
| Child-Pugh classification | ||||
| A | 10.2 | 11.3 | 8.4 | 0.498q |
| Β | 48.4 | 52.1 | 42.2 | 0.150q |
| C | 41.3 | 36.6 | 49.4 | 0.060q |
| Model for End-Stage Liver Disease* | 16.8 ± 6.5 (15.4) | 15.0 ± 5.2 (14.2) | 20.2 ± 7.3 (19.3) | < 0.001t |
| CLIF-SOFA* | 6.7 ± 2.9 (6.0) | 6.2 ± 2.9 (6.0) | 7.4 ± 2.7 (8.0) | 0.002t |
| Acute on chronic liver failure (%) | 24.7 | 7 | 54.8 | < 0.001q |
Logistic regression analysis (forward conditional) was performed including the following variables with P < 0.050 in the bivariate analysis: hepatic encephalopathy, infection at admission, mean arterial pressure (MAP), and sodium levels. The variables intimately associated to renal dysfunction such as creatinine levels, MELD score, CLIF-SOFA score, and ACLF were not included in the multivariate analysis. We identified MAP (odds ratio [OR], 0.965; 95% confidence interval [CI], 0.943-0.987; P = 0.002) and sodium levels (OR, 0.920; 95% CI, 0.871-0.971; P = 0.003) as independently associated to AKI.
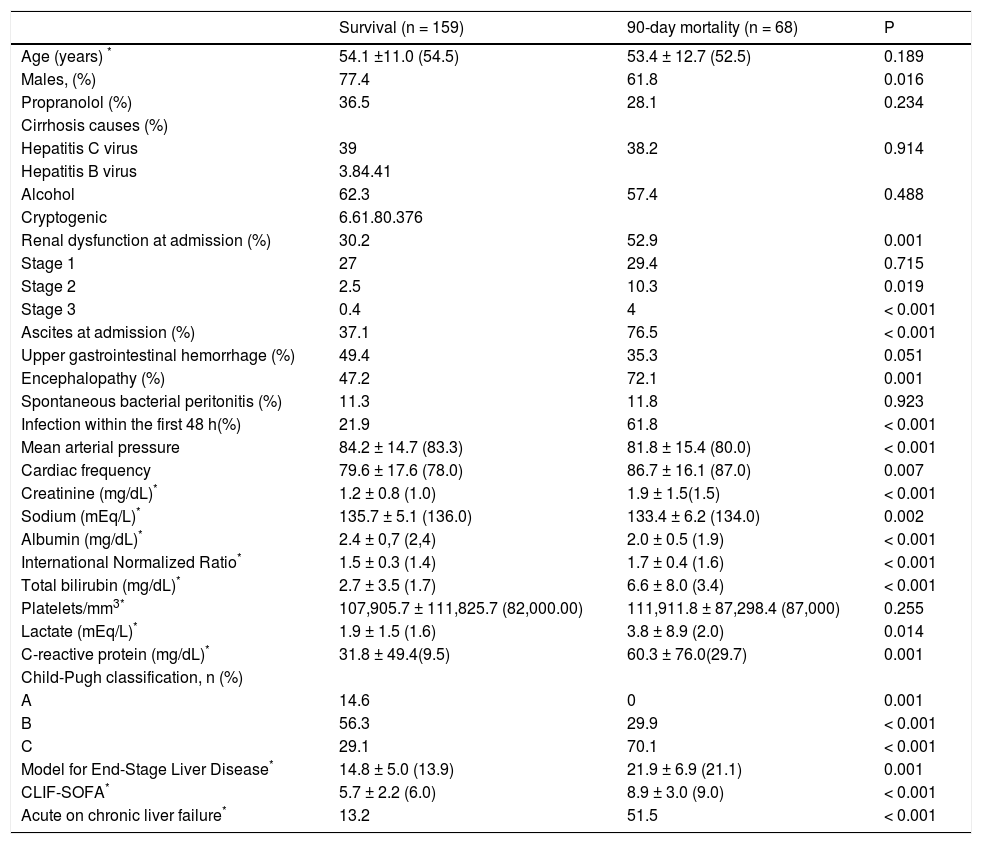
Variables associated with 90-day death or liver transplantationAmong the individuals included in the study, 63 (27.7%) died and 5 (2.2%) were transplanted within 90 days after hospitalization. In the bivariate analysis (Table 2), the 90-day death/liver transplantation was associated with a lower proportion of male patients (61.8% vs. 77.4%; P = 0.016) and a higher proportion of individuals with encephalopathy (72.1% vs. 47.2%; P = 0.001), ascites (76.5% vs. 37.1%; P < 0.001), bacterial infections (61.8% vs. 21.9%; P < 0.001), Child-Pugh class C (70.1% vs. 29.1%; P < 0.001), ACLF (51.5% vs. 13.2%; P < 0.001), and AKI (52.9% vs. 30.2%; P = 0.001). In addition, patients with poor outcome showed greater creatinine values (1.5 mg/dL vs. 1.0 mg/dL; P < 0.001), INR (1.6 vs. 1.4; P < 0.001), total bilirubin level (3.4 mg/dL vs. 1.7 mg/dL; P < 0.001), CRP level (29.7 mg/L vs. 9.5 mg/L; P = 0.001), lactate level (2.0 vs. 1.6 mEq/L; P = 0.014); MELD score (21.9 ± 6.9 vs. 14.8 ± 5.0; P < 0.001), and CLIF-SOFA score (8.1 ± 3.01 vs. 5.7 ± 2.2; P < 0.001), in addition to lower levels of albumin (2.0 ± 0.5 g/dL vs. 2.4 ± 0.7 g/dL; P < 0.001) and sodium (134.0 mEq/L vs. 136.0 mEq/L; P = 0.002). When individuals who died or were transplanted within 90 days after admission were compared to those who survived, no differences were observed regarding age, propranolol use, cirrhosis etiology, spontaneous bacterial peritonitis, and platelet count.
Factors associated with 90-day mortality among patients hospitalized for acute decompensation of cirrhosis.
| Survival (n = 159) | 90-day mortality (n = 68) | P | |
|---|---|---|---|
| Age (years) * | 54.1 ±11.0 (54.5) | 53.4 ± 12.7 (52.5) | 0.189 |
| Males, (%) | 77.4 | 61.8 | 0.016 |
| Propranolol (%) | 36.5 | 28.1 | 0.234 |
| Cirrhosis causes (%) | |||
| Hepatitis C virus | 39 | 38.2 | 0.914 |
| Hepatitis B virus | 3.84.41 | ||
| Alcohol | 62.3 | 57.4 | 0.488 |
| Cryptogenic | 6.61.80.376 | ||
| Renal dysfunction at admission (%) | 30.2 | 52.9 | 0.001 |
| Stage 1 | 27 | 29.4 | 0.715 |
| Stage 2 | 2.5 | 10.3 | 0.019 |
| Stage 3 | 0.4 | 4 | < 0.001 |
| Ascites at admission (%) | 37.1 | 76.5 | < 0.001 |
| Upper gastrointestinal hemorrhage (%) | 49.4 | 35.3 | 0.051 |
| Encephalopathy (%) | 47.2 | 72.1 | 0.001 |
| Spontaneous bacterial peritonitis (%) | 11.3 | 11.8 | 0.923 |
| Infection within the first 48 h(%) | 21.9 | 61.8 | < 0.001 |
| Mean arterial pressure | 84.2 ± 14.7 (83.3) | 81.8 ± 15.4 (80.0) | < 0.001 |
| Cardiac frequency | 79.6 ± 17.6 (78.0) | 86.7 ± 16.1 (87.0) | 0.007 |
| Creatinine (mg/dL)* | 1.2 ± 0.8 (1.0) | 1.9 ± 1.5(1.5) | < 0.001 |
| Sodium (mEq/L)* | 135.7 ± 5.1 (136.0) | 133.4 ± 6.2 (134.0) | 0.002 |
| Albumin (mg/dL)* | 2.4 ± 0,7 (2,4) | 2.0 ± 0.5 (1.9) | < 0.001 |
| International Normalized Ratio* | 1.5 ± 0.3 (1.4) | 1.7 ± 0.4 (1.6) | < 0.001 |
| Total bilirubin (mg/dL)* | 2.7 ± 3.5 (1.7) | 6.6 ± 8.0 (3.4) | < 0.001 |
| Platelets/mm3* | 107,905.7 ± 111,825.7 (82,000.00) | 111,911.8 ± 87,298.4 (87,000) | 0.255 |
| Lactate (mEq/L)* | 1.9 ± 1.5 (1.6) | 3.8 ± 8.9 (2.0) | 0.014 |
| C-reactive protein (mg/dL)* | 31.8 ± 49.4(9.5) | 60.3 ± 76.0(29.7) | 0.001 |
| Child-Pugh classification, n (%) | |||
| A | 14.6 | 0 | 0.001 |
| B | 56.3 | 29.9 | < 0.001 |
| C | 29.1 | 70.1 | < 0.001 |
| Model for End-Stage Liver Disease* | 14.8 ± 5.0 (13.9) | 21.9 ± 6.9 (21.1) | 0.001 |
| CLIF-SOFA* | 5.7 ± 2.2 (6.0) | 8.9 ± 3.0 (9.0) | < 0.001 |
| Acute on chronic liver failure* | 13.2 | 51.5 | < 0.001 |
P: χ2 test, Fisher exact test, Student t test, and Mann-Whitney U test.
The logistic regression analysis included the following variables with P < 0.010 in the bivariate analysis: AKI, ascites, encephalopathy, infection, cardiac frequency, sodium level, albumin level, INR, Child-Pugh class C, MELD score, CLIF-SOFA score, and ACLF. In this analysis, 90-day death/liver transplantation was related to ascites at admission (OR, 4.071; 95% CI, 1.860-8.907; P < 0.001), and higher values of CLIF-SOFA (OR, 1.652; 95% CI, 1.392-1.961; P < 0.001), but not to AKI.
A subsequent analysis was performed to evaluate the effect of the final creatinine level in patients with AKI (AKI absent or with final creatinine < 1.5 mg/dL vs. AKI present with final creatinine level ≥ 1.5 mg/dL). In the bivariate analysis, 90-day death/liver transplantation was significantly higher among patients with AKI and final creatinine ≥ 1.5 mg/dL as compared to those with AKI and final creatinine < 1.5 mg/dL (58.8% vs. 21.6%; P < 0.001).
We also performed a new regression analysis that included AKI with a final creatinine level ≥ 1.5 mg/dL, and the remaining variables with a P value < 0.10. Ninety-day death/liver transplantation was independently associated with the presence of ascites (OR, 3.768; 95% CI, 1.694-8.379; P = 0.001), higher CLIF-SOFA score (OR, 1.568; 95% CI, 1.319-1.864; P < 0.001), and AKI with a final Cr ≥1.5 mg/dL (OR, 2.602; 95% CI, 1.133-5.974; P = 0.024).
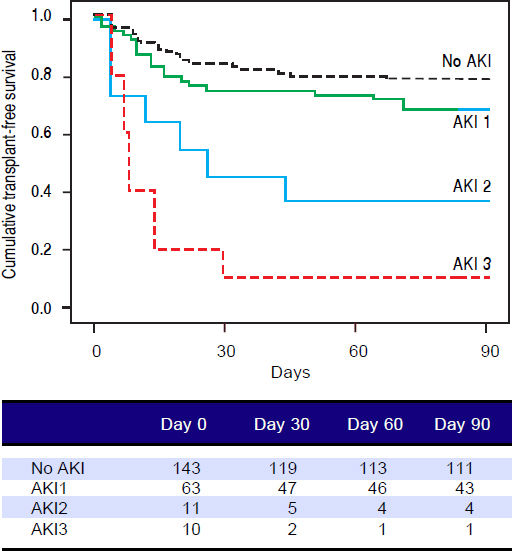
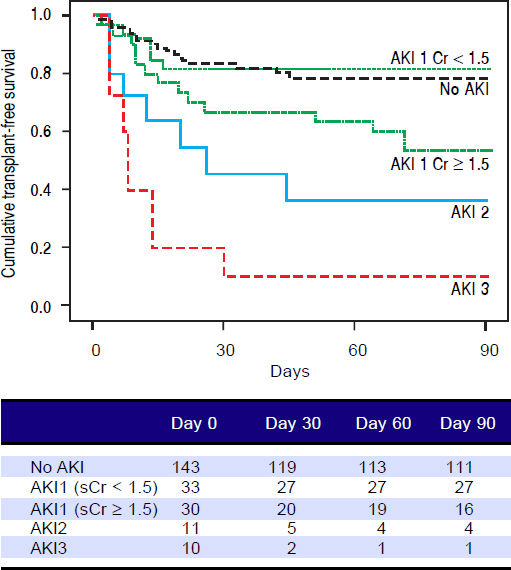
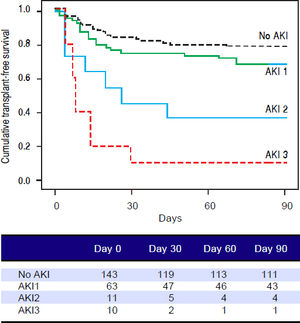
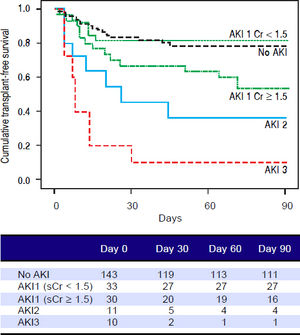
Figures 1 and 2 exhibited the Kaplan-Meier curves for mortality during the follow-up period, according to the presence and stage of AKI. There were no differences in 90-day transplant-free survival probability when patients without AKI were compared to those with AKI stage 1 (77.6% vs. 68.3%; P = 0.147). Furthermore, we observed a higher transplant-free survival probability among patients without AKI than among those with AKI stage 2 (77.6% vs. 36.4%; P < 0.001) and stage 3 (77.6% vs. 10.0%; P < 0.001). Individuals with AKI were compared according to stage (dysfunction degree) with a difference in transplant-free survival being observed between individuals in stages 1 and 2 (68.3% vs. 36.4%; P = 0.022), but not between stages 2 and 3 (36.4% vs. 10.0%; P = 0.124). When patients with AKI stage 1 and final creatinine < 1.5 mg/dL were compared with those without AKI, no differences were observed in 90-day transplant-free survival (81.8% vs. 76.6%; P = 0.669). On the other hand, individuals with AKI stage 1 and final creatinine level ≥ 1.5 mg/dL showed significantly lower transplant-free survival rates than those without AKI (53.3% vs. 77.6%; P = 0.006). Transplant-free survival rates were also significantly lower in patients with AKI stage 1 and final creatinine ≥ 1.5 mg/dL as compared to those with AKI and final creatinine < 1.5 mg/dL (53.3% vs. 81.8%; P = 0.025) (Figure 2).
Ninety-day cumulative survival rate of cirrhotic patients according to the stages of acute kidney injury (AKI). Surviva was significantly lower in the patients with AKI stages 2 and 3 than in those without AKI (P < 0.001, log-rank test). When individuals were compared according to the AKI stage, those with AKI stage 1 had higher survival than those with stage 2 (P = 0.022). No differences were observed between those in stages 2 and 3.
Ninety-day cumulative survival rate of the cirrhotic patients according to the stages of acute kidney injury (AKI). Survival was significantly lower in patients with AKI stage 1 and final creatinine level ≥1.5 mg/dL than in those without AKI (P = 0.006, log-rank test). This difference can aso be observed between patients with AKI stage 1 and final creatinine values ≥ or < 1.5 mg/dL (P = 0.025).
Creatine is a nitrogenous organic acid synthesized in the liver before being stored in muscles where it is phosphorylated as creatinine. Creatinine production varies slightly from day to day and depends on muscle mass, protein intake, sex, age and ethnicity.17 In cirrhotic patients, serum creatinine may overestimate renal function for several reasons, as hepatic dysfunction may result in a decrease in creatinine synthesis, including protein calorie malnutrition and loss of muscular mass, which are common during cirrhosis.17,18
AKI, according to the International Club of Ascites consensus definition,9 was found in 37% of our patients. The two main causes of AKI were prerenal insufficiency and hepatorenal syndrome, as described in the literature.17 In previous studies, AKI prevalence ranges from 33% to 60% among individuals with decompensated cirrhosis and varies slightly according to the diagnostic criteria used19–22 and the cause of decompensation.21 AKI occurred in 11% of patients with UGIH, 34% of patients with SBP, 27% patients with non-SBP bacterial infections, and in 40% to 49% of critically ill cirrhotic patients who required intensive therapy.23,24
In the multivariate analysis, AKI was independently associated with lower MAP and sodium values. Similarly, Wong, et al. observed lower values of MAP (81 ± 16 vs. 85 ± 15 mmHg; P = 0.04) and sodium (131 ± 7 vs. 134 ± 6 mmol/L; P = 002) among individuals who developed AKI than among those without renal dysfunction.25 Ruiz-DelArbol, et al. did not find any association between serum sodium and renal dysfunction, but described a significant reduction in MAP among individuals with AKI, suggesting that hemodynamic instability contributes to the worsening of renal function.26 Therefore, maintaining the effective circulating volume is a strategy to prevent and treat renal dysfunction.
Identifying clinical characteristics in the first 48 h after admission that could predict 90-day mortality is a useful strategy in the management of cirrhotic patients, as it allows early interventions that can influence prognosis. In the present study, the presence of ascites and higher CLIF-SOFA scores, but not AKI, were independently associated with 90-day death/liver transplantation in the initial regression analysis. However, when we took into consideration AKI with higher final creatinine levels, a subsequent regression analysis showed that AKI with a final Cr ≥ 1.5 mg/dL along with ascites and CLIF-SOFA were independent predictors of 90-day death/liver transplantation.
The CLIF-SOFA score has been recently proposed by the EASL-CLIF consortium16 and validated by our group.27 Moreau, et al. described a 51.2% 90-day death/liver transplantation in patients with ACLF and that elevated CLIF-SOFA values would be independently associated with mortality.16 These findings were very similar to those found in our study. The presence of ascites is regarded as a predictor of renal dysfunction,28 and its association with mortality has been clearly described by Silva, et al., where the Kaplan-Meier 90-day survival probability was much higher (91.6%) for cirrhotic patients without ascites and ACLF at admission, and only 21.9% for patients with both complications.27
Wong, et al. prospectively evaluated 337 patients with cirrhosis and infection and observed a greater proportion of patients with AKI who died within 30 days (34% vs. 7%).22 Belcher, et al. evaluated 192 cirrhotic patients with renal dysfunction and observed that progression of AKI was associated with mortality (OR, 3.8; 95% CI, 1.3-11.1). A relevant question would be whether increased serum creatinine level is ≥ 0.3 mg/dL but < 1.5 mg/dL (AKI 1A) would be associated with higher mortality than that in patients with unchanged serum creatinine or increased serum creatinine level < 0.3 mg/dL.29 The cutoff for creatinine value of 1.5 mg/dL was used in the traditional definition of hepatorenal syndrome.2 Further dividing patients with stage 1 AKI into two groups (1A: creatinine level < 1.5 mg/dL and 1B: creatinine level ≥ 1.5 mg/dL) is a physiopathological approach, as it combines dynamic reduction in glomerular filtration rate (GFR), given by the increase in serum creatinine level, associated with a deep reduction in GFR, according to classical definition.8 In our study, when patients without AKI were compared with those with AKI stage 1 and final creatinine level < 1.5 mg/dL, we did not observe any difference in 90-day transplant-free survival. Fagundes, et al. evaluated 375 patients admitted due to decompensated cirrhosis and also observed similar probabilities of survival between patients without AKI and patients with AKI and creatinine level ≤ 1.5 mg/dL (88 vs. 84%, respectively; P = 0.52).8 Piano, et al. evaluated 233 patients with ascites and observed that patients with AKI1 and serum creatinine level < 1.5 mg/dL presented lower mortality (P = 0.03) and progression rates (P = 0.01), and better improvement rate (P = 0.025) than patients with AKI1 and creatinine level ≥ 1.5 mg/dL.30 In our study, individuals with AKI stage 1 and final creatinine level ≥ 1.5 mg/dL showed significantly higher transplant-free survival than those without AKI (53.3% vs. 77.6%; P = 0.006). Transplant-free survival rates were also significantly lower in patients with AKI stage 1 and final creatinine ≥ 1.5 mg/dL as compared to those with AKI and final creatinine < 1.5 mg/dL (53.3% vs. 81.8%; P = 0.025), which is similar to that described by Piano.30 Bucsics, et al. evaluated a retrospective cohort where all the individuals were decompensated cirrhotic patients with ascites. The authors demonstrated that renal dysfunction was an important predictor of 30-day mortality, even in patients with AKI 1 and creatinine levels < 1.5 mg/dL.20 In our study, the variable AKI was only independently associated with mortality when using the cutoff level of 1.5 mg/dL.
Despite the valuable findings on AKI in cirrhotic patients, we have to address some limitations to this study. The relatively small number of patients with AKI may have influenced the results - especially concerning their classification in subgroups and the regression analysis. In fact, previous studies on the same subject have included a similar number of patients,19,30 and need external validation on different populations. Additionally, some studies have included heterogeneous population in distinct clinical scenarios (regular ward or intensive care unit),8 while our study prospectively evaluated only patients admitted in the emergency room. Secondly, we do not have data on the evolution of renal dysfunction during hospitalization. Our aim was to evaluate the impact of early AKI during hospitalization. Information on late development of AKI and progression rate of renal dysfunction in patients with early AKI would be of interest. Another important consideration is that in the present study none of the AKI episodes were considered to be related to acute tubular necrosis (ATN) by the hepatology team. In fact all patients who did not respond to volume expansion had a full work up to exclude other causes of AKI including renal ultrasound, urine analysis looking for proteinuria, hematuria, cylindruria. We also usually performed fractional excretion of sodium (FENa) although its performance is very limited in patients with cirrhosis. Even though ATN is a relatively common cause of AKI in studies including patients with cirrhosis, the majority of ATN episodes occur in hospital related AKI, patients in ICU and secondary to nephrotoxic agents such as contrast media and antimicrobials. Our study prospectively included patients evaluated within hours of hospitalization, solely in the emergency room, clinical context where ATN is very uncommon and that probably justifies the results.
In conclusion, early AKI was frequent in cirrhotic patients hospitalized for acute decompensation and was associated with 90-day death/liver transplantation only when the final creatinine level was ≥ 1.5 mg/dL. These findings indicate the need of distinct approaches for patients with AKI 1 according to final creatinine level.
Financial SupportNo financial support was received for the completion of this study.
Conflict of InterestThe authors declare that they have no conflicts of interest.
AcknowledgmentsWork presented orally as Free Theme at the XXIII Brazilian Congress of Hepatology.