The independent effect of liver biochemistries as a prognostic factor in patients with COVID-19 has not been completely addressed. We aimed to evaluate the prognostic value of abnormal liver tests on admission of hospitalized patients with COVID-19.
Materials & methodsWe performed a prospective cohort study including 1611 hospitalized patients with confirmed SARS-CoV-2 infection from April 15, 2020 through July 31, 2020 in 38 different Hospitals from 11 Latin American countries. We registered clinical and laboratory parameters, including liver function tests, on admission and during hospitalization. All patients were followed until discharge or death. We fit multivariable logistic regression models, further post-estimation effect through margins and inverse probability weighting.
ResultsOverall, 57.8% of the patients were male with a mean age of 52.3 years, 8.5% had chronic liver disease and 3.4% had cirrhosis. Abnormal liver tests on admission were present on 45.2% (CI 42.7–47.7) of the cohort (n = 726). Overall, 15.1% (CI 13.4–16.9) of patients died (n = 244). Patients with abnormal liver tests on admission presented higher mortality 18.7% (CI 15.9–21.7), compared to those with normal liver biochemistries 12.2% (CI 10.1–14.6); P < .0001). After excluding patients with history of chronic liver disease, abnormal liver tests on admission were independently associated with death [OR 1.5 (CI 1.1–2.0); P = 0.01], and severe COVID-19 (2.6 [2.0–3.3], P < .0001), both adjusted by age, gender, diabetes, pneumonia and body mass index >30.
ConclusionsThe presence of abnormal liver tests on admission is independently associated with mortality and severe COVID-19 in hospitalized patients with COVID-19 infection and may be used as surrogate marker of inflammation.
Clinicaltrials.govNCT04358380.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causing Coronavirus disease 2019 (COVID-19) has been associated with high mortality. The factors commonly associated with worse prognosis are age greater than 60 years, presence of diabetes, cardiovascular disease, and obesity [1,2]. While SARS-CoV-2 is known to cause mainly pulmonary disease, the emerging literature suggests that many extrapulmonary manifestations of COVID-19 can also be present [3].
The liver might also represent a target organ for SARS-CoV-2 based on the findings that angiotensin-converting enzyme (ACE-2), the major receptor of SARS-CoV-2, is expressed on cholangiocytes [4]. Liver abnormalities could be due to hypoxia-associated metabolic derangements, drug-induced liver injury and hyper-inflammation observed during cytokine storm, causing direct cytopathic damage to the liver [5–7]. A recent systematic review reported a 19% (range 1–53%) pooled prevalence of liver function abnormalities in COVID-19 patients [6,8]. However, studies reporting liver injury in COVID-19 patients are retrospective, with high clinical and statistical heterogeneity [6,9–13]. Most of these studies described a substantial increase in the incidence of liver injury after hospitalization. However, the impact of liver biochemistry parameters on admission, and the clinical course of COVID-19 remains uncertain. Hence, in hospitalized patients with COVID-19, whether abnormal liver tests on admission may be surrogate markers of inflammation and an independent prognostic factor is still unknown.
Because of a later arrival of the COVID-19 pandemic in Latin America, we had a unique opportunity to build a multi-national prospective registry. Thus, we sought to evaluate the effect of abnormal liver parameters on admission on COVID-19 disease severity and mortality.
2Patients and methods2.1Study design, setting and participating centersThis prospective cohort study was performed from April 15, 2020 through July 31, 2020 in 38 different Hospitals from Argentina, Brazil, Chile, Colombia, Dominican Republic, Ecuador, Guatemala, Mexico, Paraguay, Peru and Uruguay. The study was supported and coordinated by the Latin American Association for the Study of the Liver (ALEH), Viral Hepatitis Group of Interest and registered in an open public registry (NCT04358380; www.clinicaltrials.gov). Each Ethics Committee from all the participating centers approved the study protocol, and was exempted from the need for informed consent from patients. The protocol followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [14]. The study followed ethical standards (institutional and national) and those mandated by the Helsinki Declaration of 1975, as revised in 2008. All authors had access to the study data, reviewed, and approved the final version of this manuscript.
2.2Cohort characteristics and study variablesEligibility criteria for enrolment included patients ≥17 years old, hospitalized with SARS-CoV-2 infection confirmed by the real-time polymerase method (RT-PCR) as per the local site-specific protocol. In asymptomatic cases, a nasopharyngeal swab was obtained according to surveillance algorithms from each country (i.e. contact with positive subjects). We also included patients admitted for a different condition and tested positive for COVID-19 during their hospitalization. Patients with high-clinical and epidemiological suspicion of SARS-CoV-2 but without RT-PCR testing were excluded. All eligible patients were enrolled at each clinical site. Study data were prospectively registered into a web-based electronic system. All patients were followed until discharge or death.
2.3Liver function tests and other exposure variables on admission and during hospitalizationWe collected epidemiological, demographic, clinical, routine laboratory, radiological and treatment regimen data on each patient on admission. Data about medication use before hospitalization was collected, including antibiotics, non-steroidal anti-inflammatory drugs, acetaminophen and ACE-2 inhibitors. Laboratory parameters included inflammatory markers such as C-reactive protein and ferritin levels. Lymphopenia was considered when an absolute lymphocyte count was less than 1500/mm3 [15].
To identify specific clinical and laboratory features in patients with abnormal liver biochemistry parameters, enrolled cases were categorized into two groups (with or without abnormal liver biochemistry values on admission). We defined abnormal liver tests as the elevation of total bilirubin, alanine aminotransferase (ALT) or alkaline phosphatase (ALP) over the upper limit of normal (ULN). ULN was defined by the reference value from each institution. Given the lack of consensus on liver injury classification in COVID-19, we categorized the degree of ALT elevation as mild (<2 times ULN), moderate (2–5 times ULN) and severe (>5 times ULN), as it has been previously reported [9]. ALT was selected to represent liver affection rather than AST due to the more predominant extra-hepatic sources of AST, rendering it less liver-specific. Moreover, peak values of routine laboratory tests, including total bilirubin, ALT or ALP, were recorded during hospitalization.
2.4COVID-19 severityThe severity of COVID-19 was classified based on clinical examination results, symptoms, chest radiography and medical support. Severe COVID-19 cases were defined as those who developed acute respiratory distress syndrome (ARDS), required intensive care unit (ICU) monitoring, and/or ventilatory support, as reported elsewhere [15,16].
2.5Primary outcome, sample size calculations and statistical analysisThe primary end-point was overall mortality. Secondary outcome was development of severe COVID-19 during hospitalization. Based on previously reported mortality rates, a mean rate of 4.8% was estimated with a 95% CI 3.9–5.8% [15,17,18]. Assuming a type I error of 5% (P-value <0.05) and a type II error of 0.10 (90% power), and to follow the “1 variable per 10 primary events rule”, to include at least six independent variables in the final logistic regression model, a minimum of 1244 patients would be needed to obtain a minimum of 60 deaths. In addition, we calculated the proportion of patients presenting with abnormal liver tests on admission being 41.1% ± 17.1 [2,17,19].
Categorical data were compared using Fisher’s exact test (2-tailed) or Chi-Square (X2) test as appropriate. Continuous variables were reported with a mean (± standard deviation, SD) or median (Interquartile ranges 25–75%, IQR) and compared with Student’s T or Mann-Whitney U tests according to their respective distributions. We created dummies for ordinal variables were assessed. Multivariable logistic regression was used to evaluate the association between abnormal liver tests and the odds of death (OR) with corresponding 95% confidence intervals (CI) estimated. We first fit univariate models to evaluate crude effects on mortality of prior medical history, clinical and laboratory findings on admission, then outcomes and treatments prescribed during hospitalization. We constructed the final multivariable models including exposure variables with a p-value <0.05 in univariate analysis, using a step-by-step procedure, in order to develop a parsimonious model. The final model’s performance was evaluated, including calibration (Hosmer- Lemeshow goodness-of-fit test) and discrimination power through the area under the receiving operator curve (AUROC). We estimated margins effects after multivariable logistic regression models and inverse probability weighting (IPW) to estimate the attributable risk of abnormal liver function tests on admission, adjusted on the mean values of the other independent covariates. Data were analyzed with STATA 13.0 (StataCorp, Texas, USA).
2.6Sensitivity analysisTwo sensitivity analyses were conducted. As the presence of the underlying liver disease may also play a role in the association of abnormal liver tests with disease severity, we conducted a sensitivity analysis excluding patients with prior chronic liver disease, comprising cirrhosis. Finally, we excluded patients developing SARS-CoV-2 infection during hospitalization.
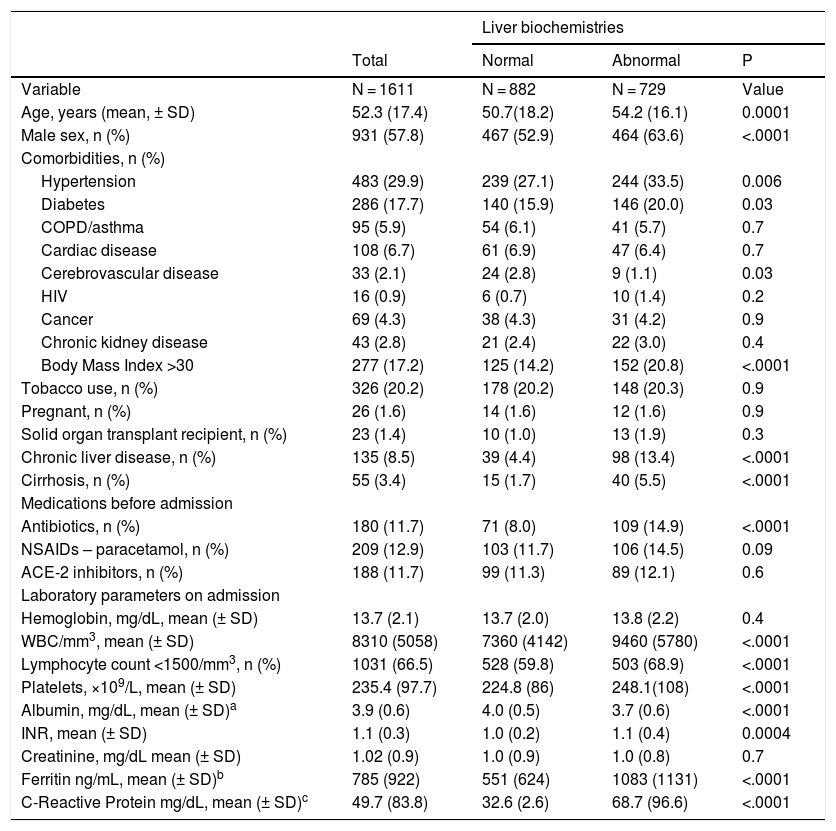
3ResultsA cohort of 1611 patients hospitalized with SARS-CoV-2 infection were enrolled in ALEH´s registry. Baseline characteristics of the study population are displayed in Table 1. Of the entire cohort, 7.7% (CI 6.5–9.2) were admitted for other reasons and acquired SARS-CoV-2 infection during hospitalization (n = 125), and in 3.0% (CI 2.2–4.0) of these patients diagnosis was made >6 days after admission (n = 49). Radiological signs on admission showed pneumonia in 53.6% of the cohort (n = 864). The most frequent radiological finding was bilateral ground-glass opacities and it was present in 35.1% of the patients (n = 536). Common signs and symptoms reported by patients are presented in Supplementary Table 1. Seventy-one (4.4%) patients were asymptomatic at presentation. Overall, 8.5% (CI 7.1–9.9) of the cohort had chronic liver disease (n = 135) and 3.4% (CI 2.5–4.4) had cirrhosis (n = 55). The most common etiologies of chronic liver disease were metabolic-associated fatty liver disease in 86 patients, alcohol induced in 14 cases, chronic hepatitis C in 8 individuals and cholestatic diseases in 10 subjects.
Baseline characteristics of patients hospitalized for COVID-19.
| Liver biochemistries | ||||
|---|---|---|---|---|
| Total | Normal | Abnormal | P | |
| Variable | N = 1611 | N = 882 | N = 729 | Value |
| Age, years (mean, ± SD) | 52.3 (17.4) | 50.7(18.2) | 54.2 (16.1) | 0.0001 |
| Male sex, n (%) | 931 (57.8) | 467 (52.9) | 464 (63.6) | <.0001 |
| Comorbidities, n (%) | ||||
| Hypertension | 483 (29.9) | 239 (27.1) | 244 (33.5) | 0.006 |
| Diabetes | 286 (17.7) | 140 (15.9) | 146 (20.0) | 0.03 |
| COPD/asthma | 95 (5.9) | 54 (6.1) | 41 (5.7) | 0.7 |
| Cardiac disease | 108 (6.7) | 61 (6.9) | 47 (6.4) | 0.7 |
| Cerebrovascular disease | 33 (2.1) | 24 (2.8) | 9 (1.1) | 0.03 |
| HIV | 16 (0.9) | 6 (0.7) | 10 (1.4) | 0.2 |
| Cancer | 69 (4.3) | 38 (4.3) | 31 (4.2) | 0.9 |
| Chronic kidney disease | 43 (2.8) | 21 (2.4) | 22 (3.0) | 0.4 |
| Body Mass Index >30 | 277 (17.2) | 125 (14.2) | 152 (20.8) | <.0001 |
| Tobacco use, n (%) | 326 (20.2) | 178 (20.2) | 148 (20.3) | 0.9 |
| Pregnant, n (%) | 26 (1.6) | 14 (1.6) | 12 (1.6) | 0.9 |
| Solid organ transplant recipient, n (%) | 23 (1.4) | 10 (1.0) | 13 (1.9) | 0.3 |
| Chronic liver disease, n (%) | 135 (8.5) | 39 (4.4) | 98 (13.4) | <.0001 |
| Cirrhosis, n (%) | 55 (3.4) | 15 (1.7) | 40 (5.5) | <.0001 |
| Medications before admission | ||||
| Antibiotics, n (%) | 180 (11.7) | 71 (8.0) | 109 (14.9) | <.0001 |
| NSAIDs – paracetamol, n (%) | 209 (12.9) | 103 (11.7) | 106 (14.5) | 0.09 |
| ACE-2 inhibitors, n (%) | 188 (11.7) | 99 (11.3) | 89 (12.1) | 0.6 |
| Laboratory parameters on admission | ||||
| Hemoglobin, mg/dL, mean (± SD) | 13.7 (2.1) | 13.7 (2.0) | 13.8 (2.2) | 0.4 |
| WBC/mm3, mean (± SD) | 8310 (5058) | 7360 (4142) | 9460 (5780) | <.0001 |
| Lymphocyte count <1500/mm3, n (%) | 1031 (66.5) | 528 (59.8) | 503 (68.9) | <.0001 |
| Platelets, ×109/L, mean (± SD) | 235.4 (97.7) | 224.8 (86) | 248.1(108) | <.0001 |
| Albumin, mg/dL, mean (± SD)a | 3.9 (0.6) | 4.0 (0.5) | 3.7 (0.6) | <.0001 |
| INR, mean (± SD) | 1.1 (0.3) | 1.0 (0.2) | 1.1 (0.4) | 0.0004 |
| Creatinine, mg/dL mean (± SD) | 1.02 (0.9) | 1.0 (0.9) | 1.0 (0.8) | 0.7 |
| Ferritin ng/mL, mean (± SD)b | 785 (922) | 551 (624) | 1083 (1131) | <.0001 |
| C-Reactive Protein mg/dL, mean (± SD)c | 49.7 (83.8) | 32.6 (2.6) | 68.7 (96.6) | <.0001 |
Abbreviation: ACE-2, angiotensin-converting enzyme; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus, INR, international normatized ratio; NSAIDs, non-steroidal anti-inflammatory drugs.
Abnormal liver biochemistry parameters on admission were present on 45.2% of the cohort (CI 42.7–47.7). In those patients with chronic liver disease, the proportion of abnormal liver tests on admission was 71.5% (CI 63.2–78.9). Patients with elevated ALT, total bilirubin and ALP accounted for 35.3%, 6.3% and 19.4%; respectively. Among patients with elevated ALT, 32.6% of the cases presented moderate injury (2–5 times ULN) and 10.7% were severe (>5 times ULN). Moreover, the incidence of elevated ALT during hospitalization was 16.2% (CI 14.4–18.1). When compared to admitted patients with normal liver tests, the group of patients with elevated liver biochemistry values on admission were mostly men (52.9% vs. 63.6%; P < .0001), of older ages (50.7 ± 18.2 vs. 54.2 ± 16.1 years old; P = 0.0001), and presenting a higher proportion of hypertension (27.1% vs. 33.5%; P = 0.006) and body mass index >30 (14.2% vs. 20.8%; P < .0001) (Table 1).
Table 1 also describes laboratory tests results at admission. Compared to the normal liver biochemistry group, patients with abnormal tests results had significantly higher platelet counts, international normatized ratio, and a higher proportion of individuals presented a lymphocyte count <1500/mm3 and lower levels of serum albumin. A higher proportion of patients presenting abnormal liver biochemistry parameters on admission were under antibiotic treatment (P < .0001) (Table 1). During hospitalization, specific COVID-19 treatment was prescribed in 43.8% of the cohort (n = 705) and was more commonly administered to patients with abnormal liver tests on admission (p < 0.0001). Other drugs frequently used for hospitalized patients with COVID-19, were also significantly more commonly prescribed to patients who presented abnormal liver biochemistry values on admission (Supplementary Table 2).
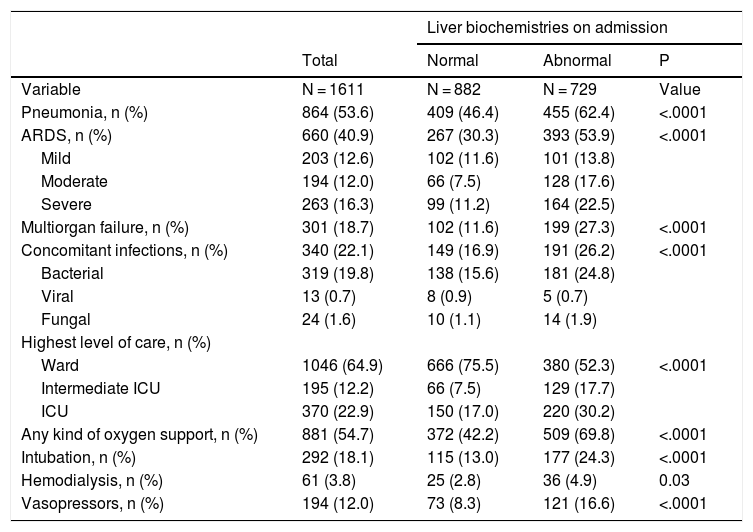
3.2Clinical outcomes of patients with SARS-CoV-2The cumulative mortality rate in the overall cohort was 15.1% (CI 13.4–16.9) after a median time since admission of 10.0 (IQR 4.0–18.5) days (n = 244). Clinical outcomes are reported in Table 2. Severe COVID-19 was developed in 43.8% (CI 41.4–46.3) of the patients. When considering prognostic demographics and prior medical history, increasing age, male gender, obesity, cirrhosis, and diabetes mellitus were significantly associated with a higher risk of mortality (Supplementary Table 3). Adjusted for age patients developing ARDS OR 4.0 (CI 2.5–6.5; P < .0001), requiring mechanical ventilation OR 2.9 (CI 2.5–6.5; P < .0001) or with multiorgan failure OR 5.2 (CI 3.5–7.7; P < .0001) had a higher risk of mortality.
Clinical outcomes of patients hospitalized for COVID-19.
| Liver biochemistries on admission | ||||
|---|---|---|---|---|
| Total | Normal | Abnormal | P | |
| Variable | N = 1611 | N = 882 | N = 729 | Value |
| Pneumonia, n (%) | 864 (53.6) | 409 (46.4) | 455 (62.4) | <.0001 |
| ARDS, n (%) | 660 (40.9) | 267 (30.3) | 393 (53.9) | <.0001 |
| Mild | 203 (12.6) | 102 (11.6) | 101 (13.8) | |
| Moderate | 194 (12.0) | 66 (7.5) | 128 (17.6) | |
| Severe | 263 (16.3) | 99 (11.2) | 164 (22.5) | |
| Multiorgan failure, n (%) | 301 (18.7) | 102 (11.6) | 199 (27.3) | <.0001 |
| Concomitant infections, n (%) | 340 (22.1) | 149 (16.9) | 191 (26.2) | <.0001 |
| Bacterial | 319 (19.8) | 138 (15.6) | 181 (24.8) | |
| Viral | 13 (0.7) | 8 (0.9) | 5 (0.7) | |
| Fungal | 24 (1.6) | 10 (1.1) | 14 (1.9) | |
| Highest level of care, n (%) | ||||
| Ward | 1046 (64.9) | 666 (75.5) | 380 (52.3) | <.0001 |
| Intermediate ICU | 195 (12.2) | 66 (7.5) | 129 (17.7) | |
| ICU | 370 (22.9) | 150 (17.0) | 220 (30.2) | |
| Any kind of oxygen support, n (%) | 881 (54.7) | 372 (42.2) | 509 (69.8) | <.0001 |
| Intubation, n (%) | 292 (18.1) | 115 (13.0) | 177 (24.3) | <.0001 |
| Hemodialysis, n (%) | 61 (3.8) | 25 (2.8) | 36 (4.9) | 0.03 |
| Vasopressors, n (%) | 194 (12.0) | 73 (8.3) | 121 (16.6) | <.0001 |
Abbreviations: ARDS, acute respiratory distress syndrome, ICU, intensive care unit.
Mortality was significantly higher in patients with elevated liver tests results on admission compared to those with normal liver tests values (18.7% vs. 12.2%; P < .0001). Patients with abnormal liver tests on admission required more ICU-level care (p < .0001), developed more frequently severe COVID-19 during hospitalization (57.5% vs. 32.4%, P < .0001), and required more days of hospitalization (10 [IQR 6–16] vs. 7 [IQR 5–12], P < .0001).
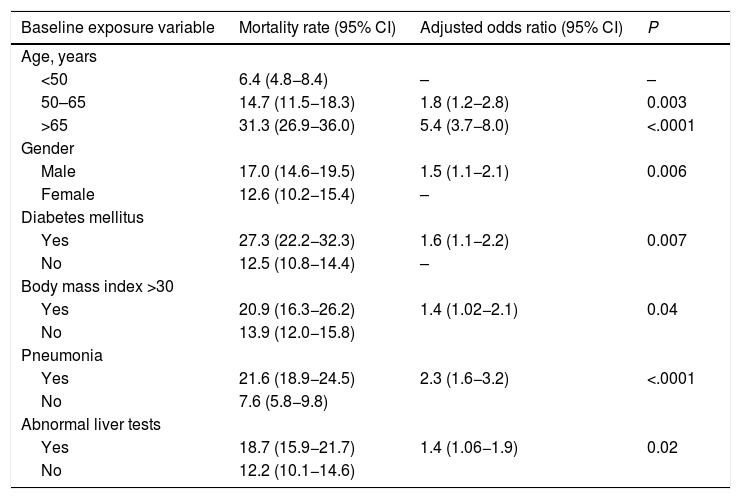
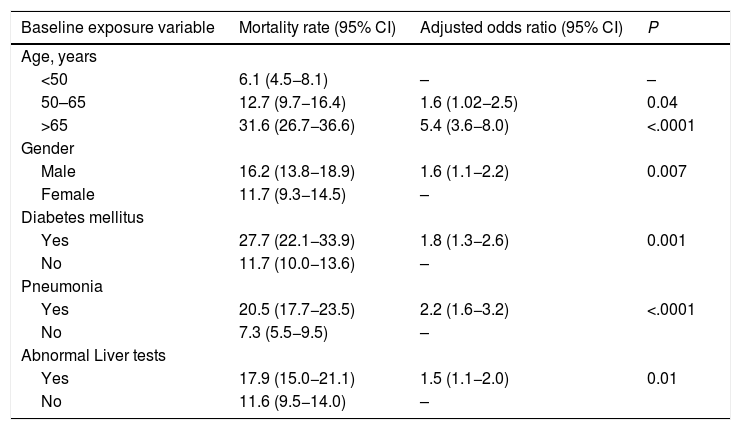
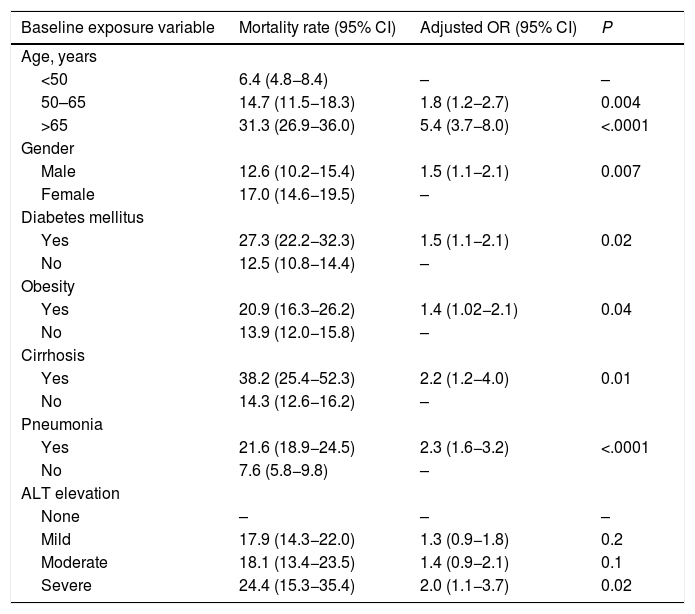
We evaluated the effect of abnormal liver biochemistry values on admission on the risk of mortality adjusted for age, prior medical history, clinical presentation and other laboratory parameters. Abnormal liver tests on admission were significantly associated with death (OR 1.4, CI 1.06–1.9; P = 0.02) (Table 3). After excluding patients with history of chronic liver disease, abnormal liver tests on admission were still independently associated with death (OR 1.5, CI 1.1–2.0; P = 0.01], adjusted for age, gender, diabetes, and pneumonia on admission (Table 4). The model showed adequate calibration (P = 0.19) with an AUROC of 0.76 (CI 0.73–0.78) (Supplementary Figure 1). Moreover, evaluating the dose-dependent effect of elevated ALT on admission as a matter of causality, patients with severe ALT augmentation presented a 2-fold greater odds of death compared to those with mild ALT elevation (OR 2.0, CI 1.1–3.7); P = 0.02) (Table 5). In patients without chronic liver disease, the attributable mortality risk for abnormal liver tests was 3.8% (CI 0.7–7.9%; P = 0.01), considering mean values for other independent covariates. In the IPW analysis, abnormal liver tests had an absolute increased mortality rate of 3.4% (CI −0.01 to 7.0) when compared to those presenting normal liver tests.
Logistic regression analysis evaluating the primary outcome (death) adjusted for variables significantly associated with a higher risk of mortality on admission.
| Baseline exposure variable | Mortality rate (95% CI) | Adjusted odds ratio (95% CI) | P |
|---|---|---|---|
| Age, years | |||
| <50 | 6.4 (4.8−8.4) | – | – |
| 50–65 | 14.7 (11.5−18.3) | 1.8 (1.2−2.8) | 0.003 |
| >65 | 31.3 (26.9−36.0) | 5.4 (3.7−8.0) | <.0001 |
| Gender | |||
| Male | 17.0 (14.6−19.5) | 1.5 (1.1−2.1) | 0.006 |
| Female | 12.6 (10.2−15.4) | – | |
| Diabetes mellitus | |||
| Yes | 27.3 (22.2−32.3) | 1.6 (1.1−2.2) | 0.007 |
| No | 12.5 (10.8−14.4) | – | |
| Body mass index >30 | |||
| Yes | 20.9 (16.3−26.2) | 1.4 (1.02−2.1) | 0.04 |
| No | 13.9 (12.0−15.8) | ||
| Pneumonia | |||
| Yes | 21.6 (18.9−24.5) | 2.3 (1.6−3.2) | <.0001 |
| No | 7.6 (5.8−9.8) | ||
| Abnormal liver tests | |||
| Yes | 18.7 (15.9−21.7) | 1.4 (1.06−1.9) | 0.02 |
| No | 12.2 (10.1−14.6) | ||
Note: Calibration (P = 0.07, Hosmer-Lemeshow test). ROC curve 0.76 (CI 0.74−0.78).
Logistic regression analysis for the primary outcome (death) based on data at admission, excluding patients with chronic liver disease or cirrhosis (n = 137).
| Baseline exposure variable | Mortality rate (95% CI) | Adjusted odds ratio (95% CI) | P |
|---|---|---|---|
| Age, years | |||
| <50 | 6.1 (4.5−8.1) | – | – |
| 50–65 | 12.7 (9.7−16.4) | 1.6 (1.02−2.5) | 0.04 |
| >65 | 31.6 (26.7−36.6) | 5.4 (3.6−8.0) | <.0001 |
| Gender | |||
| Male | 16.2 (13.8−18.9) | 1.6 (1.1−2.2) | 0.007 |
| Female | 11.7 (9.3−14.5) | – | |
| Diabetes mellitus | |||
| Yes | 27.7 (22.1−33.9) | 1.8 (1.3−2.6) | 0.001 |
| No | 11.7 (10.0−13.6) | – | |
| Pneumonia | |||
| Yes | 20.5 (17.7−23.5) | 2.2 (1.6−3.2) | <.0001 |
| No | 7.3 (5.5−9.5) | – | |
| Abnormal Liver tests | |||
| Yes | 17.9 (15.0−21.1) | 1.5 (1.1−2.0) | 0.01 |
| No | 11.6 (9.5−14.0) | – | |
Note: Calibration (P = 0.19, Hosmer-Lemeshow test). ROC curve 0.76 (CI 0.73−0.78).
Logistic regression analysis for the primary outcome (death) evaluating the severity of ALT elevation on admission. Odds Ratios (OR).
| Baseline exposure variable | Mortality rate (95% CI) | Adjusted OR (95% CI) | P |
|---|---|---|---|
| Age, years | |||
| <50 | 6.4 (4.8−8.4) | – | – |
| 50–65 | 14.7 (11.5−18.3) | 1.8 (1.2−2.7) | 0.004 |
| >65 | 31.3 (26.9−36.0) | 5.4 (3.7−8.0) | <.0001 |
| Gender | |||
| Male | 12.6 (10.2−15.4) | 1.5 (1.1−2.1) | 0.007 |
| Female | 17.0 (14.6−19.5) | – | |
| Diabetes mellitus | |||
| Yes | 27.3 (22.2−32.3) | 1.5 (1.1−2.1) | 0.02 |
| No | 12.5 (10.8−14.4) | – | |
| Obesity | |||
| Yes | 20.9 (16.3−26.2) | 1.4 (1.02−2.1) | 0.04 |
| No | 13.9 (12.0−15.8) | – | |
| Cirrhosis | |||
| Yes | 38.2 (25.4−52.3) | 2.2 (1.2−4.0) | 0.01 |
| No | 14.3 (12.6−16.2) | – | |
| Pneumonia | |||
| Yes | 21.6 (18.9−24.5) | 2.3 (1.6−3.2) | <.0001 |
| No | 7.6 (5.8−9.8) | – | |
| ALT elevation | |||
| None | – | – | – |
| Mild | 17.9 (14.3−22.0) | 1.3 (0.9−1.8) | 0.2 |
| Moderate | 18.1 (13.4−23.5) | 1.4 (0.9−2.1) | 0.1 |
| Severe | 24.4 (15.3−35.4) | 2.0 (1.1−3.7) | 0.02 |
Note: Calibration (P = 0.27, Hosmer-Lemeshow test). ROC curve 0.77 (CI 0.75−0.79).
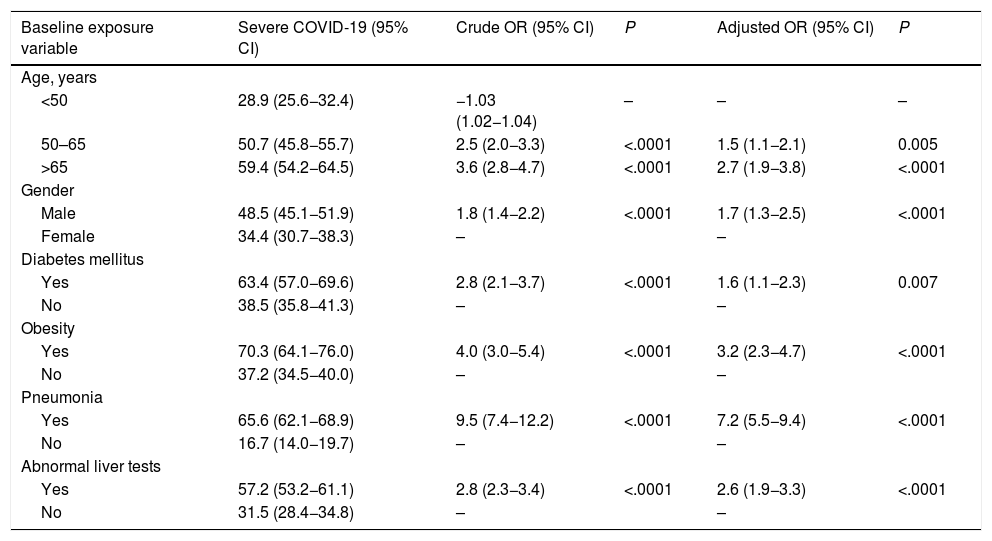
Finally, we analyzed the association of abnormal liver biochemistry values on admission and development of severe COVID-19 during hospitalization. Patients having abnormal liver tests results have a ∼2-fold greater odds of developing severe COVID-19 OR 2.4 (CI 1.9–3.1; P < .0001), adjusted for age, male gender, diabetes mellitus, BMI > 30 and pneumonia (Supplementary Table 4). The model showed an AUROC of 0.83 (CI 0.81–0.85). Sensitivity analysis excluding patients with prior chronic liver disease, abnormal liver tests on admission were still independently associated with severe COVID-19 of 2.6 (CI 1.9–3.3; P < .0001), adjusted for age, gender, diabetes, and pneumonia on admission (Table 6).
Logistic regression analysis for the secondary outcome (severe COVID-19) excluding patients with chronic liver disease or cirrhosis (n = 137). Odds Ratios (OR).
| Baseline exposure variable | Severe COVID-19 (95% CI) | Crude OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|---|
| Age, years | |||||
| <50 | 28.9 (25.6−32.4) | −1.03 (1.02−1.04) | – | – | – |
| 50–65 | 50.7 (45.8−55.7) | 2.5 (2.0−3.3) | <.0001 | 1.5 (1.1−2.1) | 0.005 |
| >65 | 59.4 (54.2−64.5) | 3.6 (2.8−4.7) | <.0001 | 2.7 (1.9−3.8) | <.0001 |
| Gender | |||||
| Male | 48.5 (45.1−51.9) | 1.8 (1.4−2.2) | <.0001 | 1.7 (1.3−2.5) | <.0001 |
| Female | 34.4 (30.7−38.3) | – | – | ||
| Diabetes mellitus | |||||
| Yes | 63.4 (57.0−69.6) | 2.8 (2.1−3.7) | <.0001 | 1.6 (1.1−2.3) | 0.007 |
| No | 38.5 (35.8−41.3) | – | – | ||
| Obesity | |||||
| Yes | 70.3 (64.1−76.0) | 4.0 (3.0−5.4) | <.0001 | 3.2 (2.3−4.7) | <.0001 |
| No | 37.2 (34.5−40.0) | – | – | ||
| Pneumonia | |||||
| Yes | 65.6 (62.1−68.9) | 9.5 (7.4−12.2) | <.0001 | 7.2 (5.5−9.4) | <.0001 |
| No | 16.7 (14.0−19.7) | – | – | ||
| Abnormal liver tests | |||||
| Yes | 57.2 (53.2−61.1) | 2.8 (2.3−3.4) | <.0001 | 2.6 (1.9−3.3) | <.0001 |
| No | 31.5 (28.4−34.8) | – | – | ||
Note: ROC curve 0.83 (CI 0.81−0.85).
Results from this large Latin America prospective cohort study describe that almost half of the patients hospitalized with COVID-19 presented abnormal liver tests on admission. However, most patients just showed mild liver biochemistry parameters elevation at the early stage of SARS-CoV-2 infection. Compared to patients with normal liver tests on admission, those who had abnormal liver test results were more frequently admitted to ICU, had a significantly higher probability of developing severe COVID-19, and higher odds of mortality, highlighting its importance as a marker of disease severity.
Wide variability in liver enzymes deviations in patients with COVID-19 can be secondary to the different considered cutoff values and to the time of liver tests evaluation. In our study, we used liver enzymes cutoffs as defined by the ULN of each participating institution. We intended to describe the impact of liver injury at the early stages of the disease. Thus, while most studies contemplated peak liver test values during hospitalization, we used liver test abnormalities at hospital admission [9,20,21]. The frequency of liver enzymes elevation during SARS-CoV-2 infection is directly related to the progression of the severity of the disease. Some authors have recommended longitudinal monitoring of hepatic transaminases, particularly in patients receiving clinical research treatments [21]. At advanced stages of COVID-19, liver test alterations can have a multifactorial origin. The combination of multiple prescribed drugs and the SARS-CoV-2-induced systemic inflammatory response can lead to liver injury. Thus, evaluating the impact of peak liver test abnormalities during hospitalization can provide misleading conclusions.
In our study, we found that abnormal liver biochemistry values on admission were significantly associated with outcomes during hospitalization such as prolonged length of stay, development of severe COVID-19, and more importantly, death. A previous study from China reported similar findings describing an association between mortality and abnormal liver tests on admission [22]. However, in that study, data were retrospectively collected; patients in the abnormal liver tests group presented a significantly higher proportion of chronic liver disease. Lei at al. analyzed a large retrospective cohort from China, including more than 5700 patients [20]. In this interesting study, the authors concluded that the increase of AST levels had a higher correlation with mortality than other liver injury indicators. We believe that the inclusion of AST as a marker of liver injury is arguable due to the potential extra-hepatic sources of AST rendering it less liver-specific. To avoid potential confounders, we did not include AST in our definition of abnormal liver tests, and we performed a sensitivity analysis excluding patients with chronic liver disease, still observing a significant association to worse outcomes.
Compared to previous studies, our cohort includes a younger population with a lower proportion of associated comorbidities [1,9]. We can speculate that this is the consequence of including patients with mild symptoms of COVID-19 who were hospitalized because they could not isolate themselves at their homes. This allowed us to evaluate the whole spectrum of SARS-CoV-2 infection, from mild cases to those who developed severe COVID-19 and died. Therefore, the prognostic value of liver biochemistry tests would acquire greater relevance. We believe that liver function tests should be included in the initial evaluation of a COVID-19 patient, and may help physicians decide hospitalization or prompt referral irrespectively from radiological pneumonia.
The major strength of our study is the inclusion of a large and geographically diverse population, in which data collection and outcome measures have been prospectively collected. However, we must acknowledge certain limitations in the interpretation of the results of our study. First, the recommended cutoff for defining moderate or severe liver injury is debatable [23,24]. However, we are facing a new disease and its impact on the liver is not yet completely understood. We then graded liver injury by applying cutoff values previously reported in this disease [9]. Second, we did not include other inflammatory markers in our final model. We intended to include affordable and feasible blood tests, together with clinically relevant variables, to build a parsimonious model. Finally, although SARS-CoV-2 infection was diagnosed as per the local site-specific protocol, algorithms followed their local epidemiological situation and available resources.
In summary, in this large multicenter cohort from Latin America, abnormal liver tests on admission were associated with most severe clinical outcomes, including death. This accessible test may be another useful tool to identify patients at increased risk of developing severe COVID-19 who require hospitalization. Moreover, future clinical trials evaluating COVID-19 targeted therapies may consider abnormal liver tests as a stratifying factor for clinical outcomes.
Conflict of interestNone.
FundingNone.
Contributorship statementDr. Mendizabal and Dr. Piñero had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Silva, Mendizabal, Ridruejo, Pagés and Piñero.
Acquisition, analysis, or interpretation of data: all authors.
Drafting of the manuscript: Mendizabal, Piñero, Ridruejo, Silva and Pagés.
Critical revision of the manuscript for important intellectual content: all authors.
Statistical analysis: Piñero, Rubinstein and Mendizabal.
Supervision: Ridruejo, Rubinstein, Silva.
We would like to thank ALEH’s executive office for their invaluable help and support on this project, especially to Macarena Muñoz, María Jesús Marcone and Verónica García Huidobro.
Other authors who collaborated with the data Acquisition: Máximo Cattáneoh,Jonathan Aguirre Valadezi, Germán Rojask, Edgar Ortiz-Brizuela11, Damián Conte (Hospital Privado de Córdoba, Argentina), Natalia Ratusnú (Hospital de Ushuaia, Argentina), Leyla Nazal (Clínica Las Condes, Chile), Diana Escobar (Fundación Valle de Lili, Colombia), Sarai Gonzalez Huezo (Centro Médico Issemym, Mexico).