N-acetyl-p-aminophenol (APAP)-induced liver injury is a major clinical challenge worldwide. The present study investigated the molecular role of microRNA (miR)-338-3p in the development of APAP-induced acute liver injury.
Materials and methodsB6 mice were treated with an miR-338-3p agomir, antagomir, and intraperitoneally injected with APAP 24h later to induce acute liver injury. Histological analysis was performed to evaluate the degree of liver injury. The gene expression of miR-338-3p and its downstream regulators was measured by reverse transcription-quantitative PCR and western blot. The miR target was validated using a luciferase reporter assay.
ResultsThe results revealed that miR-338-3p was significantly upregulated following the intraperitoneal administration of APAP. Augmenting miR-338-3p alleviated acute liver injury caused by APAP overdose, while silencing of miR-338-3p exhibited a detrimental effect. Moreover, miR-338-3p inhibited the expression of pro-inflammatory cytokines by preventing the aberrant activation of inflammatory signaling pathways, including the nuclear factor kappa-B (NF-κB)/mitogen-activated protein kinase (MAPK) signaling pathway. Furthermore, calcium/calmodulin-dependent protein kinase IIα (CAMK IIα) was identified as a direct target of miR-338-3p.
ConclusionThe present study demonstrated that miR-338-3p inhibited inflammation in APAP-induced acute liver injury.
microRNAs (miRNAs/miRs) are a class of non-coding RNA, 20–30 nucleotides in length, that modulate gene expression by binding to complementary mRNA sequences and consequently prevent translation or result in mRNA degradation [1]. To date, >10,000 miRNAs have been identified in mammals, and their sequences are highly conserved across species. Moreover, each miRNA may induce the degradation or silencing of hundreds of target sequences due to their tolerance for imperfect base pairing [2]. Therefore, miRNAs are involved in a number of cellular processes, including liver injury and the subsequent liver failure or regeneration [3].
Previous studies have investigated the dynamic miRNA expression profile during liver injury or failure, and emerging evidence has demonstrated that miRNAs play an important role in the underlying pathogenesis [4]. For instance, in a murine model of N-acetyl-p-aminophenol (APAP)-induced liver injury, a subtoxic dose of APAP triggered significant changes in hepatic and serum miRNA expression levels, while traditional clinical indicators of liver failure, including alanine aminotransaminase (ALT) and aspartate transaminase (AST), remained unchanged [5,6]. In a mouse model of chronic ethanol consumption, several miRNAs were reported to mediate hepatic metabolism and onset of liver regeneration following chronic liver injury through the regulation of nuclear factor kappa-B (NF-κB) [7].
miR-338-3p has been reported to influence cellular activities such as proliferation, differentiation and apoptosis [8]. Previous studies have revealed that miR-338-3p expression is significantly downregulated in various types of [9–12], suggesting that miR-338-3p may serve as a tumor suppressor. In vitro and in vivo studies have reported that the downregulation of miR-338-3p promoted angiogenesis in hepatocellular carcinoma [13]. Additionally, miR-338-3p inhibits cell proliferation in hepatocellular carcinoma by targeting forkhead box P4 [14], sphingosine kinase 2 [15] and hypoxia-induced factor 1α [16]. While the role of miR-338-3p has been well-characterized in liver cancer and cancer cell lines, little is known about the involvement of miR-338-3p in APAP-induced liver injury. Therefore, the present study investigated the specific role of miR-338-3p in the process of this disease.
2Materials and methods2.1APAP-induced liver injury model.Wild-type male C57BL/6J (B6) mice (age, 8–10 weeks) were purchased from the Shanghai Slac Laboratory. All animal protocols were approved by the Institutional Animal Care and Use Committee of The First Affiliated Hospital of Xi’an Jiaotong University. APAP was dissolved in pre-warmed phosphate buffered saline (PBS; pH 7.4) prior to administration. 40 mice were randomly divided into four groups: the control group was administered PBS; the model group was injected intraperitoneally at a dose of 300mg/kg APAP; the miR-338-3p agomir group received an intravenous injection of 10mmol/l miR-338-3p agomir with APAP administration 24h later; the miR-338-3p antagomir group received an intravenous injection of 10mmol/l miR-338-3p antagomir with APAP administration 24h later. The Entranster™ in vivo transfection reagent were purchased from Engreen Biosystem Ltd. and was used according to the manufacturer's instructions. All mice were euthanized with diethyl ether. Serum and liver tissue samples were harvested, and a portion of the liver tissue was immediately fixed in 10% buffered formalin for subsequent histological analysis.
2.2Serum correlation factors measurementsALT and AST activities were determined using commercial AST and ALT assay kits (Nanjing jiancheng Biotech) according to the manufacturer's instructions. Interleukin-1β (IL-1β), Interleukin-6 (IL-6) tumor necrosis factor-α (TNF-α) as well as the chemokine C-C motif chemokine ligand 2 (CCL2) were detected by ELISA according to the manufacturer's instructions.
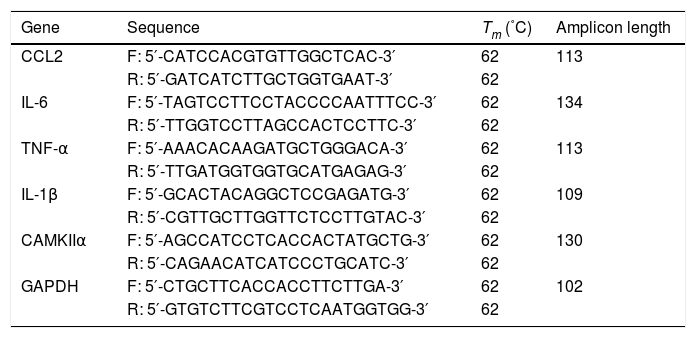
2.3RNA isolation and reverse transcription-quantitative PCRTotal RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The TaqMan miRNA assay kit (Thermo Fisher Scientific, Inc.) was used to quantify the gene expression of miR-338-3p using the reference small nuclear RNA Sno202 as the internal control. cDNA was synthesized using the Prime Script RT Reagent kit (Takara Bio, Inc.). qPCR was subsequently performed using SYBR Premix Ex Taq qPCR kit (Takara Bio, Inc.). The primers used for qPCR are presented in Table 1. mRNA expression levels were normalized to the internal reference gene GAPDH.
Primers used for quantitative PRC.
| Gene | Sequence | Tm (˚C) | Amplicon length |
|---|---|---|---|
| CCL2 | F: 5′-CATCCACGTGTTGGCTCAC-3′ | 62 | 113 |
| R: 5′-GATCATCTTGCTGGTGAAT-3′ | 62 | ||
| IL-6 | F: 5′-TAGTCCTTCCTACCCCAATTTCC-3′ | 62 | 134 |
| R: 5′-TTGGTCCTTAGCCACTCCTTC-3′ | 62 | ||
| TNF-α | F: 5′-AAACACAAGATGCTGGGACA-3′ | 62 | 113 |
| R: 5′-TTGATGGTGGTGCATGAGAG-3′ | 62 | ||
| IL-1β | F: 5′-GCACTACAGGCTCCGAGATG-3′ | 62 | 109 |
| R: 5′-CGTTGCTTGGTTCTCCTTGTAC-3′ | 62 | ||
| CAMKIIα | F: 5′-AGCCATCCTCACCACTATGCTG-3′ | 62 | 130 |
| R: 5′-CAGAACATCATCCCTGCATC-3′ | 62 | ||
| GAPDH | F: 5′-CTGCTTCACCACCTTCTTGA-3′ | 62 | 102 |
| R: 5′-GTGTCTTCGTCCTCAATGGTGG-3′ | 62 |
The liver samples were homogenized in liquid nitrogen, and the homogenate was subsequently lysed on ice for 60min in RIPA lysis buffer (W062-1-1) (Nanjing jiancheng Biotech). Total protein (25μg/lane) was separated via SDS-PAGE on a 10% gel. Proteins were then transferred onto polyvinylidene difluoride membranes. The membranes were incubated with primary antibodies overnight at 4°C. Following primary antibody incubation, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies. The protein bands were visualized using an enhanced chemiluminescence detection system. The signal intensity was quantified using Image J software.
2.5miR-338-3p target validationThe web-based tools TargetScan (http://www.targetscan.org/mamm_31/), miRBase (http://www.mirbase.org/) and miRWalk (http://mirwalk.umm.uni-heidelberg.de/) were used to predict miR-338-3p targets, which were subsequently validated by a luciferase reporter assay. 293T cells were seeded in 96-well plates and transfected with a mixture of 5ng 3′UTR luciferase reporter vector or empty vector together with the miR-338-3p mimic or miR-338-3p inhibitor (Shanghai GenePharma Co., Ltd.) using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's instructions. The transfected cells were lysed 24h after transfection and the cell lysates were analyzed using the luciferase activity assay. Firefly luciferase activity was normalized to Renilla luciferase activity. The calcium/calmodulin-dependent protein kinase II α (CAMKIIα)-3′-UTR was cloned into the psiCHECK-2 vector (Promega Corporation). The following primers were used: CAMKIIα-3′ UTR forward, 5′ -CCGCTCGAGTGCTTCCCTCGCAAACT-3′ and reverse, 5′-TTAGCGGCCGCTGGCTCTTCCTCCCCTAA-3′.
2.6Histological analysisLiver tissues fixed in neutral buffered formalin were embedded in paraffin and cut into 5-μm thick sections. The sections were stained with hematoxylin and eosin (H&E) to evaluate necrosis. The sections were subsequently examined using a LEICA DM5000B microscope (Leica Microsystems GmbH) (magnification, ×200).
2.7Statistical analysisStatistical analyses were performed using GraphPad Prism software (version 6; GraphPad Software, Inc.). One-way ANOYA was used for comparison among groups. Data are expressed as the mean±standard error of the mean of at least three independent experiments. P<0.05 was considered to indicate a statistically significant difference.
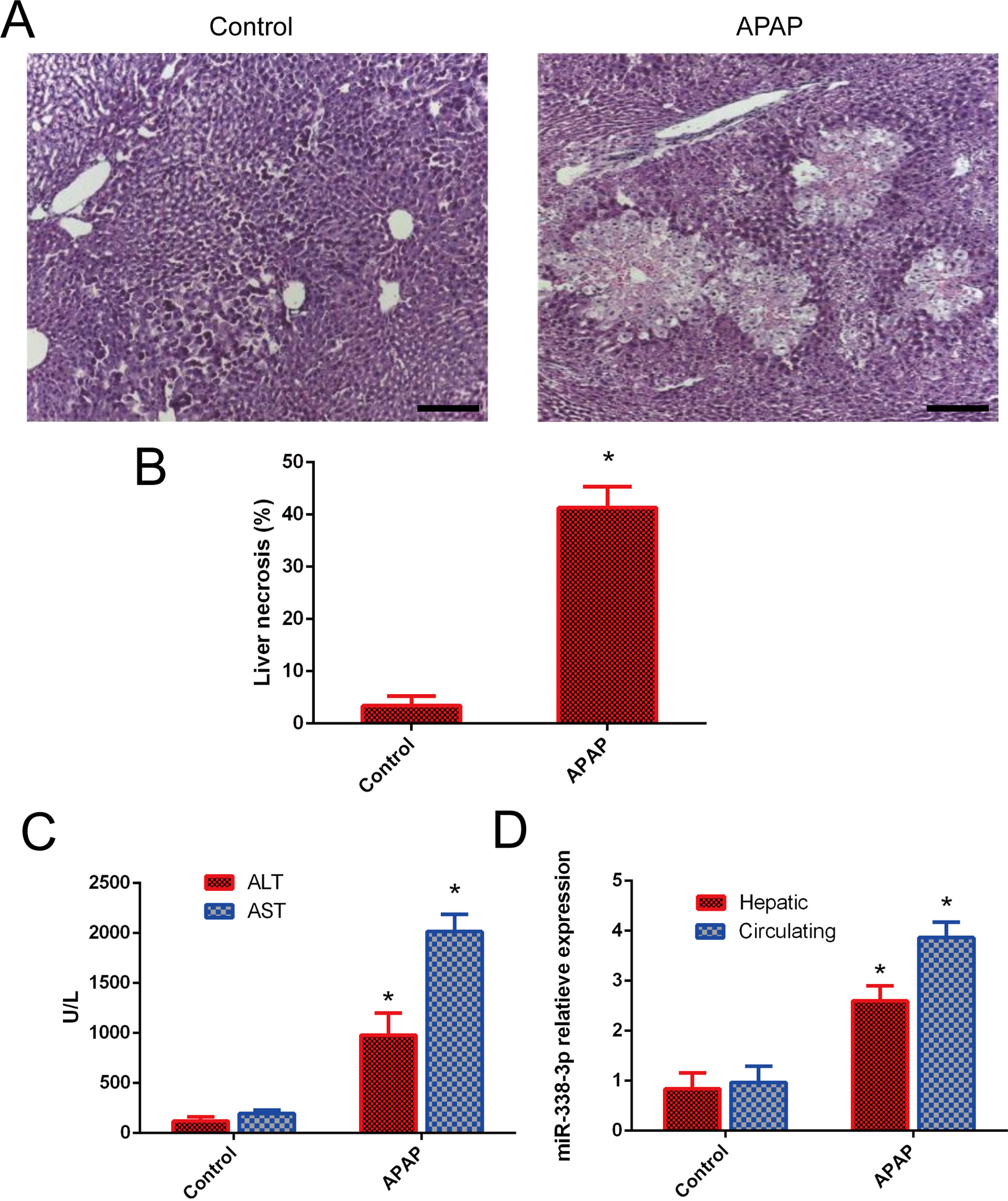
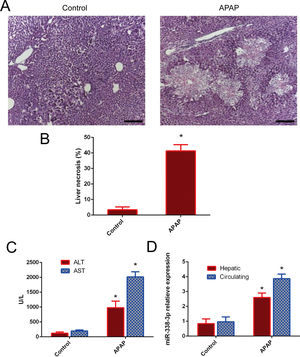
3Results3.1miR-338-3p is highly induced following APAP treatment.The degree of necrosis was analyzed histologically 24h after APAP treatment, as this is considered to be the peak of cytotoxicity in the mouse model [17]. H&E staining showed that a single dose of APAP resulted in severe damage in the liver parenchyma (Fig. 1A and B). The induction of liver injury was further confirmed by significant elevation of serum ALT and AST. ALT and AST levels were significantly increased in the APAP-treated group following injury compared with the control group (Fig. 1C). The level of hepatic miR-338-3p expression was investigated in order to determine the potential association of miR-338-3p with APAP-induced liver injury. Liver from mice in the APAP-treated group was characterized by significantly increased miR-338-3p levels 24h post-APAP injection compared with the control group (Fig. 1D). Furthermore, the level of circulating miR-338-3p in the APAP-treated group was increased 3-fold compared with the PBS control group (Fig. 1D).
miR-338-3p expression levels in APAP-induced liver injury. (A) Representative images of H&E-stained liver tissue (magnification, ×200. (B) Quantification of the necrotic area (%) by evaluating six independent fields for each section. (C) The levels of serum AST and ALT from APAP and PBS-treated mice. (D) The liver and serum expression levels of miR-338-3p in mice in the APAP- and PBS-treated groups. Histogram shown as means±standard deviation of values from three independent experiments. *Significant compared with APAP group, P<0.05.
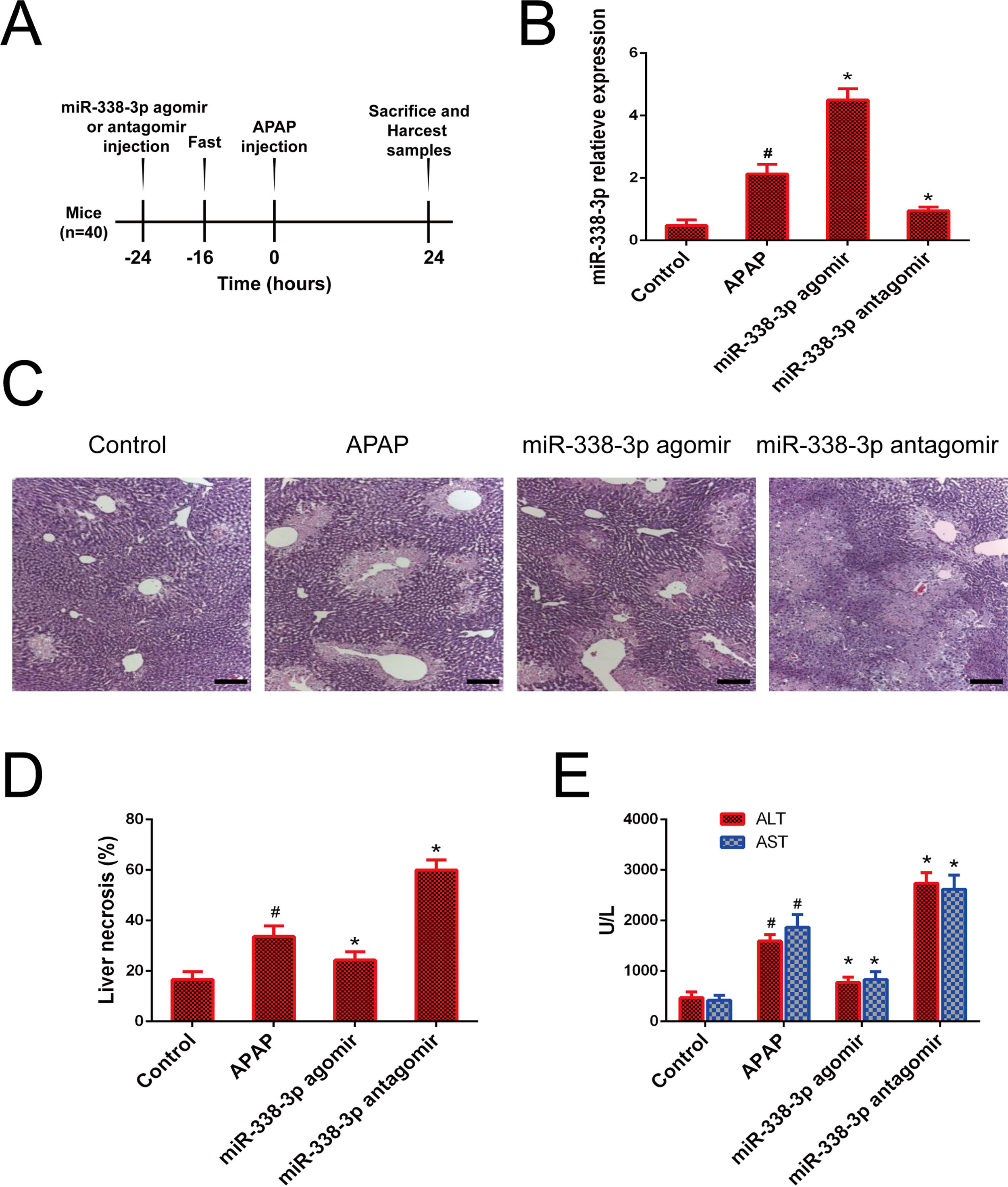
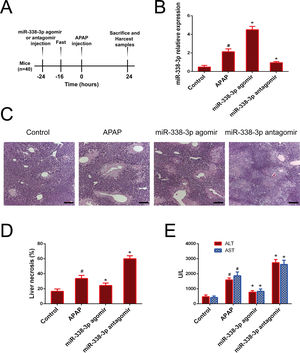
Agomirs and antagomirs are classes of chemically synthesized oligonucleotides that can easily penetrate cell membranes and efficiently modify the expression pattern of target miRNAs [18]. The processing procedure of miR-338-3p-specific agomir and antagomir was shown in Fig. 2A. Treatment with the miR-338-3p agomir effectively increased the expression level of miR-338-3p in the liver to >100-fold compared with the control group (Fig. 2B). Notably, mice that received the miR-338-3p agomir exhibited less hepatocyte necrosis (Fig. 2C and D) and reduced serum ALT and AST levels (Fig. 2E), while, miR-338-3p antagomirs and agomir had the opposite results (Fig. B–E). Taken together, these data demonstrated that in vivo overexpression of miR-338-3p alleviated acute liver injury caused by APAP overdose, while silencing of miR-338-3p had a detrimental effect.
Role of the miR-338-3p antagomir and agomir in APAP-induced liver injury. (A) Intervention flow chart: the mice were intravenously injected with miR-338-3p antagomir or agomir at a dose of 1000nmol/kg 24h prior to APAP injection. Mice liver and serum samples were collected one day following APAP administration. (B) The expression of miR-338-3p in the liver tissues. (C) Representative images of H&E-stained liver tissue. (D) Quantification of the necrotic area (%) by evaluating 6 independent fields for each liver section (magnification, ×200). (E) Serum AST and ALT levels. Histogram shown as means±standard deviation of values from three independent experiments. #Significant compared with control group, P<0.05. *Significant compared with APAP group, P<0.05.
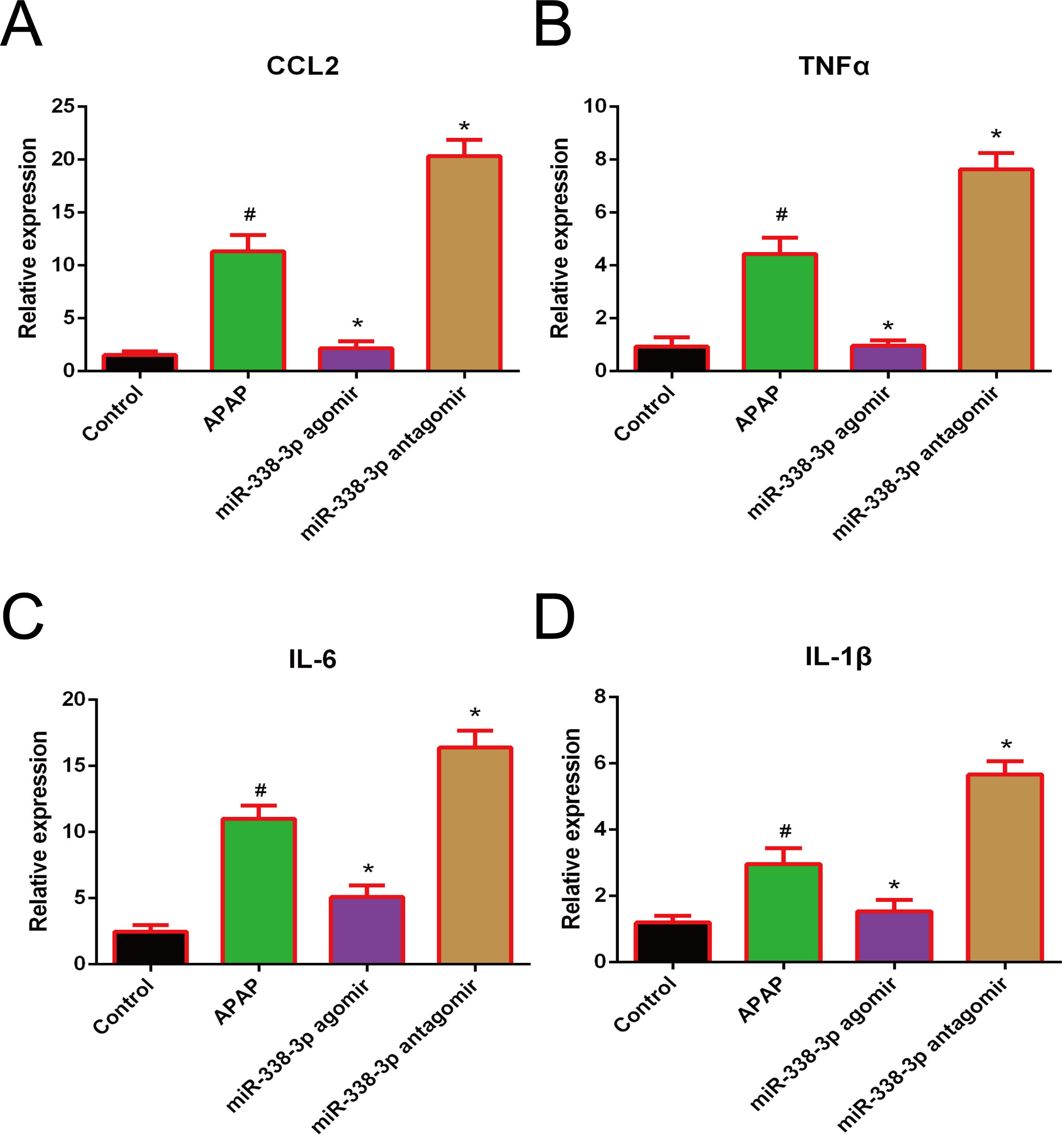
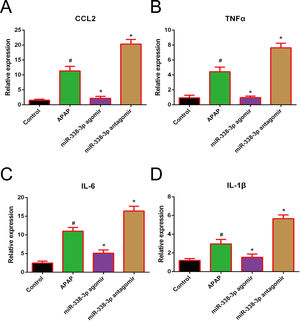
Accumulating evidence has demonstrated that the clinical outcome of APAP-induced acute liver failure is not only critically affected by the initial hepatocyte damage, but also by the subsequent inflammatory response following injury [19,20]. In this studies, the pro-inflammatory cytokines IL-1β, IL-6, TNF-α and CCL2 were highly induced following APAP treatment. Additionally, overexpression of miR-338-3p significantly reduced the expression levels of these factors, while silencing of miR-338-3p increased their expression (Fig. 3). Therefore, miR-338-3p may protect the liver from APAP-induced injury by inhibiting the expression of various pro-inflammatory mediators.
Effect of miR-338-3p on the expression of pro-inflammatory cytokines in APAP-induced liver injury. The mRNA expression levels of (A) CCL2, (B) TNF-α, (C) IL-1B and (D) IL-6 were quantified by reverse transcription-quantitative PCR. Histogram shown as means±standard deviation of values from three independent experiments. #Significant compared with control group, P<0.05. *Significant compared with APAP group, P<0.05.
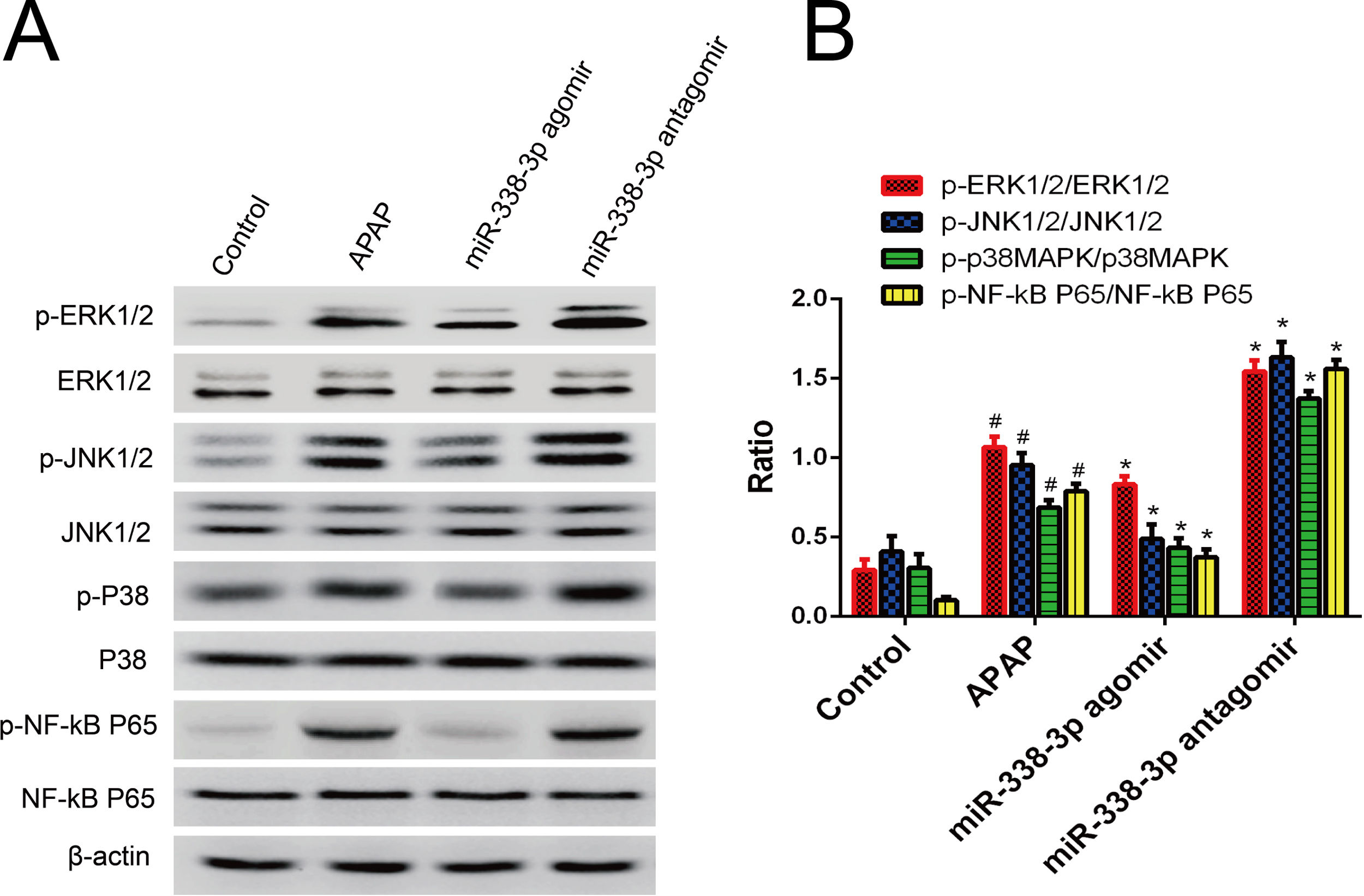
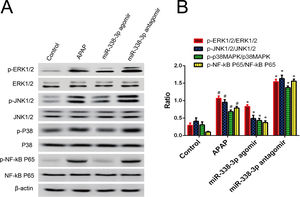
To acquire a comprehensive understanding of how miR-338-3p regulates the inflammatory response, the effect of miR-338-3p on the activation of inflammation-related signaling pathways was investigated. Western blot revealed that overexpression of miR-338-3p significantly decreased the phosphorylation levels of extracellular regulated protein kinases (ERKs), c-Jun N-terminal kinases (JNKs), p38 MAPK and NF-κB (Fig. 4A), while miR-338-3p knockdown increased their phosphorylation (Fig. 4B). These data demonstrated that miR-338-3p inhibited inflammation by negatively regulating the NF-κB/MAPK signaling pathway.
miR-338-3p suppressed the NF-κB/MAPK signaling pathway. (A, B) Mice were intravenously injected with the miR-338-3p antagomir or agomir at a dose of 1000nmol/kg 24h prior to APAP injection. Mice livers were collected one day following APAP administration for protein extraction. Total and phosphorylated levels of NF-kB, p65, ERK1/2, JNK1/2 and p38 MAPK were quantified by western blot. Histogram shown as means±standard deviation of values from three independent experiments. #Significant compared with control group, P<0.05. *Significant compared with APAP group, P<0.05.
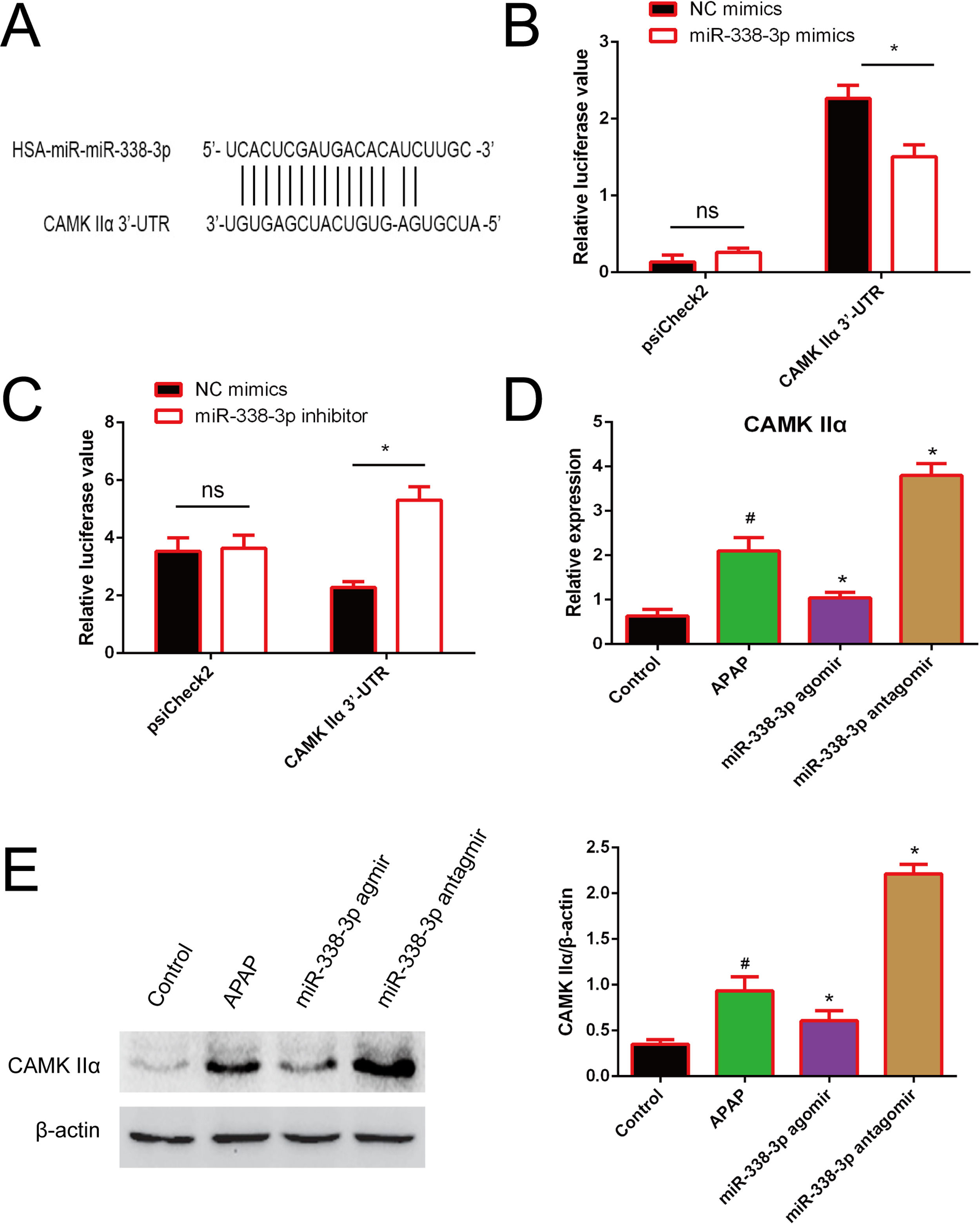
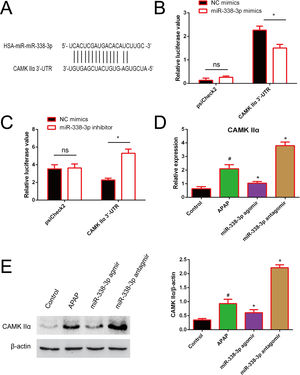
In order to investigate how miR-338-3p negatively regulates the NF-κB/MAPK signaling pathway, the web-based tools TargetScan, miRBase and miRWalk were used to predict miR-338-3p targets. CAMKIIα was identified as a potential miR-338-3p target. As illustrated in Fig. 5A, the CAMK IIα-encoded mRNA contains a 3′-UTR element that is partially complementary to miR-338-3p “seed sequence”, suggesting that miR-338-3p would directly target this site. To validate the miRNA-CAMKIIα interaction, the 3′ UTR sequence of CAMKIIα was cloned into the psiCheck II vector and co-transfected with the miR-338-3p or inhibitor. As shown in Fig. 5B and C, the luciferase reporter assay revealed that the miR-338-3p significantly decreased the luciferase activity in a 3′ UTR-dependent manner and that the miR-338-3p inhibitor increased the luciferase activity compared with the control group.
Identification of CAMKIIα as a direct target of miR-338-3p. (A) The binding site of miR-338-3p and CAMKIIα. (B) 293T cells were transfected with the control construct (psiCheck2), or a construct encoding CAMKIIα-3-UTR, combined the miR-338-3p mimic (B) or miR-338-3p inhibitor (C). At 24h post-transfection, the luciferase activity in the cell lysates was detected by a luminometer. *P<0.05, ns: no significant. (D, E) The mRNA and protein levels of CAMKIIα in liver tissues harvested from APAP-treated mice treated with the miR-338-3p agomir or antagomir were detected by reverse transcription-quantitative PCR and western blotting, respectively. Data are presented as the mean±SEM. #Significant compared with control group, P<0.05. *Significant compared with APAP group, P<0.05.
Furthermore, RT-qPCR results revealed that miR-338-3p agomir treatment resulted in the lower levels of CAMKIIα transcription, while the miR-338-3p antagomir increased CAMKIIα expression (Fig. 5D). Western blotting revealed similar results (Fig. 5E). The above results showed that miR-338-3p directly targets CAMKIIα.
4DiscussionThe roles of miRNAs in drug- or chemical-induced liver damage have been reported in a number of studies [21]; however, the molecular mechanisms by which miRNAs mediate hepatocellular damage and recovery remain unknown. Increasing evidence suggests that miRNAs serve a bidirectional role in liver injury [22]. Hepatic miRNA profiling studies demonstrated that while certain miRNAs may be beneficial and facilitate hepatocyte proliferation, others are implicated in increasing liver cell necrosis [23–25].
There is currently significant interest in the in vivo manipulation of miRNA expression for therapeutic purposes, as well as considering the potential utility of circulating miRNAs as biomarkers for the diagnosis and prognosis of liver injury [26–28]. The levels of certain serum miRNAs are generally more sensitive and specific than traditional markers such as serum ALT and AST [29]. For instance, a previous study reported that the level of circulating miR-122 increased in a relatively early-presenting paracetamol-induced liver injury case, while changes in other clinical markers were not yet evident [30]. Therefore, miRNAs represent a vast reservoir for biomarker discovery regarding the detection and monitoring of liver injury. Currently, multiple circulating miRNAs have been identified to reflect the severity of APAP-induced liver injury [31–33].
To the best of our knowledge, the present study was the first to investigate the expression and function of miR-338-3p in an APAP-induced liver injury model. The present study revealed that silencing of miR-338-3p using an miR-338-3p antagomir exacerbated APAP-induced liver injury by increasing the expression of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α. Additionally, pharmacological augmentation of miR-338-3p using an miR-338-3p agomir reduced the extent of hepatocyte necrosis in liver injury by targeting the aforementioned pro-inflammatory cytokines and the upstream NF-κB/mitogen-activated protein kinase (MAPK) signaling pathway.
NF-κB and MAPK are important signaling components that regulate pro-inflammatory cytokine and chemokine expression [34]. In addition, it is known that intracellular Ca2+ can affect cell functions by activating CaMKII and TNF-α may induce Ca2+ influx and activate CaMKII [35]. Meanwhile, TNF is closely related to NF-κB/MAPK signaling pathway [36]. Our present study revealed that miR-338-3p may mediate the NF-κB/MAPK signaling pathway by directly binding to CAMKIIα. Understanding the involvement of miR-338-3p in drug-induced liver injury may aid the development of targeted molecular therapeutics based on miRNA agomirs and antagomirs.
In conclusion, the present study revealed that miR-338-3p plays a protective role in APAP-induced acute liver injury, and provided a novel insight into the molecular mechanisms underlying drug-induced liver injury. Targeting miR-338-3p via an agomir may serve as novel therapeutic strategy for modulating the cellular response to APAP overdose.AbbreviationsCAMKIIα calcium/calmodulin-dependent protein kinase IIα acetaminophen or N-acetyl-p-aminophenol alanine aminotransaminase aspartate transaminase mitogen-activated protein kinase nuclear factor kappa-B extracellular regulated protein kinases c-Jun N-terminal kinases
Dr. Rong Fang provided the idea of this article. Miss. Li Kang screened the target, analyzed data and wrote this article. Mr. Chen Zhang and Mr. Hai-hui Zhu performed this experiment. Ms. Jing Li analyzed data and organized the discussion of this article.
Conflicts of interestThe authors have declared that there is no conflict of interest.
This study was supported by grants from Natural Science Basic Research Plan in Shaanxi Province (Grant No. 2012JQ4016).