The introduction of direct-acting antiviral (DAA) agents promises to change dramatically the management of hepatitis C in kidney transplant recipients, a patient group where the treatment of hepatitis C is historically challenging. The purpose of the current study was to assess (in a ‘real-life’ setting) the safety and efficacy of all-oral, interferon-free, direct-acting antiviral agents in kidney transplant recipients with HCV.
Material and MethodsWe performed a single-arm, multi-center study in a cohort (n = 95) of kidney transplant recipients who underwent antiviral therapy with DAAs. The primary end-point was sustained virologic response (SVR) (serum HCV RNA < 15 IU/mL, 12 weeks after treatment ended; SVR12). We recorded data on on-treatment adverse events (AEs), serious AEs, and laboratory abnormalities.
ResultsVarious regimens were adopted at the discretion of the treating physician: elbasvir/grazoprevir (n = 11), paritaprevir/ritonavir/ombitasvir/dasabuvir (PrOD) regimens ± ribavirin (n = 23), and sofosbuvir-based regimens ± ribavirin (n = 61). The SVR12 rate was 93.7% (89/95) (95% CI, 88%; 98%), according to intention-to-treat analysis; three patients without viral response (n = 3) were found. Ribavirin was administered in 8 (8.4%) allograft recipients. The frequency of drop-outs was 4.2% (4/95) (95% CI, 0.2%; 8.2%); these were related to arthralgia/myalgia (n = 2), fatigue (n = 1), and lowered estimated glomerular filtration rate (eGFR) (n = 1). There were no differences with regard to serum creatinine and eGFR before and after antiviral therapy and during follow-up in the whole cohort. The patient who interrupted antiviral treatment due to raised serum creatinine was on sofosbuvir/daclatasvir regimen; one of the four drop-outs obtained SVR.
ConclusionsAll-oral, interferon-free therapy with DAAs for chronic HCV after kidney transplantation was effective and well-tolerated in a ‘real–life’ clinical setting. Identical results have been observed in patients with intact kidneys or advanced chronic kidney disease. Careful evaluation of kidney function over follow-up in kidney transplant recipients who received DAAs regimens is recommended. Clinical trials aimed to assess whether sustained viral response translates into improved patient/graft survival are under way.
It has been recently estimated that around 10% of kidney transplant recipients in developed countries show chronic hepatitis C virus (HCV) infection [1]. Chronic HCV frequently causes cirrhosis, liver failure and hepatocellular carcinoma; kidney transplant recipients with HCV infection have better survival than HCV seropositive patients who remain on the waiting list [2]. Patients with end-stage renal disease and HCV infection who received kidney transplant have lower patient/graft survival in comparison with –negative recipients [3–5]. HCV infection poses a significant risk of developing various post-transplant complications such as diabetes, malignancies, mixed essential cryoglobulinaemia, and glomerular disease. In addition, chronic HCV raises the risk for end stage renal disease and cardiovascular mortality [6,7].
The hypothesis underlying the current study is that direct-acting antiviral (DAA) agents can be effective and safe for treatment of HCV even in the context of kidney transplant. Abundant evidence in the medical literature shows that all oral, interferon-free regimens using combinations of DAAs are effective in patients with intact kidneys and those with immune compromise from uraemia [8]. According to the last update of the AASLD/IDSA guidelines, various combinations with DAAs have been recommended for treatment-naïve and non-DAA-experienced kidney transplant patients (with or without compensated cirrhosis), depending on HCV genotypes- daily fixed-dose combination of glecaprevir/pibrentasvir, daily fixed-dose combination of ledipasvir/sofosbuvir, daily fixed-dose combination of sofosbuvir/velpatasvir, and daily fixed-dose combination of elbasvir/grazoprevir. It has been suggested for DAA-experienced kidney transplant patients (with or without compensated cirrhosis) the daily fixed-dose combination of sofosbuvir/velpatasvir/voxilaprevir with or without ribavirin [8].
Nonetheless, the clinical studies supporting these guidelines have limited size and some reports concerning the treatment of HCV among kidney transplant recipients are industry-funded studies. It is well known that industry-sponsored research is more likely to be reported when results are favourable [9].
We have conducted a multicenter study with the aim to evaluate efficacy and safety of antiviral therapy (all-oral, interferon-free ribavirin-free regimens of DAAs) for chronic HCV after kidney transplantation.
2Material and methods2.1Study design and eligibility of patientsThis was a retrospective, non-interventional, multicentre study of kidney transplant recipients belonging to the Latin American Liver Research, Educational and Awareness Network (LALREAN) and who underwent anti-HCV therapy with DAAs. The study was conducted in a ‘real world’ (or ‘real life’) setting, i.e., data were collected outside of the controlled environment of conventional randomized clinical trials and were part of real world evidence [10]. All patients attended nephrology units included in the study [8 kidney transplant centres from regional hospitals in Latin America [Argentina and Brazil]). The study cohort was enrolled from February 1, 2016 and patients were followed until February 28, 2020. Eligible patients were adults (>18 years old) with chronic HCV infection, with or without cirrhosis, and treatment naïve or treatment experienced (previously treated with conventional or pegylated IFN-based therapy) treated with DAAs and with 12 weeks post-treatment evaluation for the final effectiveness analysis as part of per protocol analysis. Patients co-infected with human immunodeficiency virus and/or hepatitis B virus were also included. Clinical data were recorded in a centralized database, and patients were identified with a code. We recorded demographic, clinical and biochemical data for each patient. We gathered viral data including HCV RNA, HCV genotype, and co-infection and previous treatment for HCV infection. Total bilirubin, serum albumin, serum creatinine, estimated glomerular filtration rate (eGFR), blood cells, and International Normalized Ratio (INR) were collected at baseline (time 0), at the end of antiviral therapy with DAAs (EOT), and 12 weeks after completing anti-HCV therapy (W12). Patients were enrolled irrespective of their liver fibrosis stage, genotype, or prior HCV treatment status. At completion of the study, we assessed any side effects that occurred during anti-HCV therapy with DAAs.
Pre-treatment stage of liver fibrosis was appraised by liver biopsy (Ishak nomenclature was adopted). In the absence of liver biopsy, patients underwent transient (Fibroscan®; Echosens, Paris, France) or ARFI (acoustic radiation force impulse) elastography. Liver fibrosis stage (F1–F4) originated from the liver stiffness values in kilopascals (kPa) obtained by elastography according to the indications provided by the manufacturer.
2.2Antiviral regimenDAA regimens were administered at the discretion of the treating physician depending on the genotype, presence of cirrhosis, prior treatment experience, and drug availability at time of treatment. Various antiviral regimens were prescribed: elbasvir/grazoprevir (daily fixed-dose combination of elbasvir, 50 mg/grazoprevir 100 mg); ledipasvir/sofosbuvir (ledipasvir was prescribed at 90 mg administered orally once daily in co-formulation with sofosbuvir 400 mg [fixed dose]); PrOD regimen (paritaprevir/ritonavir/ ombitasvir/dasabuvir) administered at standard doses with or without ribavirin (OBV, 25 mg once daily/paritaprevir, 150 mg once daily/ritonavir, 100 mg once daily/dasabuvir, 250 mg twice daily); sofosbuvir and daclatasvir ± ribavirin (daclatasvir was prescribed at 60 mg administered orally once daily in co-formulation with sofosbuvir 400 mg); sofosbuvir and velpatasvir (daily fixed-dose combination of sofosbuvir, 400 mg/velpatasvir, 100 mg); sofosbuvir, velpatasvir and voxilaprevir (daily fixed-dose combination of sofosbuvir, 400 mg/velpatasvir, 100 mg/ voxilaprevir, 100 mg), with or without ribavirin; pibrentasvir and glecaprevir (daily fixed-dose combination of glecaprevir, 300 mg/ pibrentasvir, 120 mg).
Sofosbuvir administration to patients with GFR < 30 mL/min per 1.72 m2 was considered to be off-label use of the drug and patients gave consent to the off-label therapy before its initiation according to the local law. Sustained virological response was defined on the basis of AASLD/ISA criteria as an undetectable HCV RNA 12 weeks after antiviral therapy ended [8].
2.3Laboratory assessmentsSerum HCV RNA was estimated using the quantitative COBAS AMPLICOR HCV (Roche) Monitor Assay (limit of detection, 15 log IU/mL). HCV genotyping was determined at baseline by the SIEMENS Versant HCV Genotype 2.0 Assay (LiPA) (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA). Kidney function was measured by estimated glomerular filtration rate (eGFR) using the CKD-EPI equation [11].
2.4Safety evaluationThe patients were evaluated on a regular basis for treatment efficacy and side-effects. The patients’ visits were scheduled as follows: treatment initiation, treatment weeks 4, at the end of therapy, and at 12 weeks after treatment discontinuation. Each visit consisted of a query on medical history and side effects, check of concomitant medication, physical examination, laboratory analyses, and drug delivery. Laboratory analyses included blood chemistry, blood count, prothrombin time, and HCV RNA. The structure of recordings of adverse events (AEs) was as follows: any AE or SAE, including any event which required interruption of antiviral treatment, life-threatening event, or death.
2.5Statistical analysisThis is a retrospective series and calculation for sample size was not performed. Results were calculated in an intent-to-treat analysis. Descriptive statistics were adopted- continuous parameters were reported as means and standard deviations or medians with respective ranges, as appropriate. Descriptive parameters were reported as frequencies and percentages. For quantitative variables, group differences were analyzed with Student’s t test. For all comparisons, statistical significance throughout the study was defined by the occurrence of a two-sided P value <0.05. All analyses were made with Stata, version 9.0 (StataCorp, College Station, TX).
2.6Ethical standardThe study was conducted in accordance with the International Council for Harmonization (ICH) Good Clinical Practice (GCP) Guidelines and ethical principles reported in the 1996 version of the Declaration of Helsinki. Institutional and national ethical standards were followed in all procedures. The requirement for informed consent was waived because of the retrospective design of the study and use of data from which the patients’ identification had been deleted. Ethical Committee approval of the study protocol was obtained from participating centres. All authors had access to the study data and reviewed and approved the final manuscript before journal submission. The STROBE (STrengthening the Reporting of OBservational Studies in Epidemiology) initiative was adopted [12]. We reported the checklist of 22 items that it is considered essential for good reporting of cohort observational studies (Supplemental File n. 1). The patients consented to the off-label use of sofosbuvir, as demanded by local law.
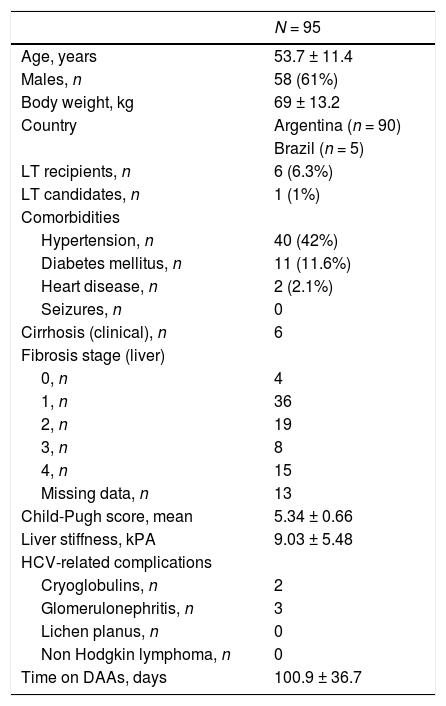
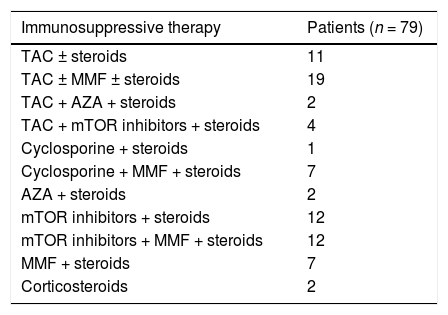
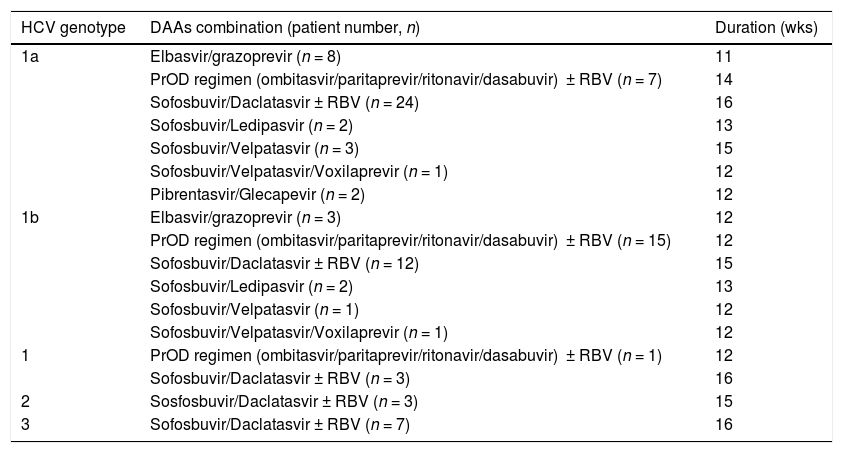
3Results3.1Baseline patient demographicsA total of ninety-seven patients was considered for the study-two patients were not referred as they refused antiviral therapy with DAAs and 95 were enrolled. The baseline demographic, clinical and biochemical characteristics of patients included in the study group are shown in Tables 1–2. Many patients were male (61%); the most frequent genotype was HCV 1 (n = 86). Percutaneous liver biopsy was made in nine (9.5%) patients; 67 (70.5%) and 6 (6.3%) patients underwent transient (Fibroscan) and ARFI (acoustic radiation force impulse) elastography, respectively. Some patients were treatment-experienced (n = 20), and advanced liver fibrosis was found in 23 renal transplant (RT) recipients. Immunosuppressive regimens adopted after kidney transplant are reported in Table 3. Antiviral therapy was scheduled with several combinations of DAAs [Table 4]; treatment duration ranged between 6 and 26 weeks.
Demographic and clinical patients’ characteristics at baseline: kidney transplant recipients (LALREAN).
| N = 95 | |
|---|---|
| Age, years | 53.7 ± 11.4 |
| Males, n | 58 (61%) |
| Body weight, kg | 69 ± 13.2 |
| Country | Argentina (n = 90) |
| Brazil (n = 5) | |
| LT recipients, n | 6 (6.3%) |
| LT candidates, n | 1 (1%) |
| Comorbidities | |
| Hypertension, n | 40 (42%) |
| Diabetes mellitus, n | 11 (11.6%) |
| Heart disease, n | 2 (2.1%) |
| Seizures, n | 0 |
| Cirrhosis (clinical), n | 6 |
| Fibrosis stage (liver) | |
| 0, n | 4 |
| 1, n | 36 |
| 2, n | 19 |
| 3, n | 8 |
| 4, n | 15 |
| Missing data, n | 13 |
| Child-Pugh score, mean | 5.34 ± 0.66 |
| Liver stiffness, kPA | 9.03 ± 5.48 |
| HCV-related complications | |
| Cryoglobulins, n | 2 |
| Glomerulonephritis, n | 3 |
| Lichen planus, n | 0 |
| Non Hodgkin lymphoma, n | 0 |
| Time on DAAs, days | 100.9 ± 36.7 |
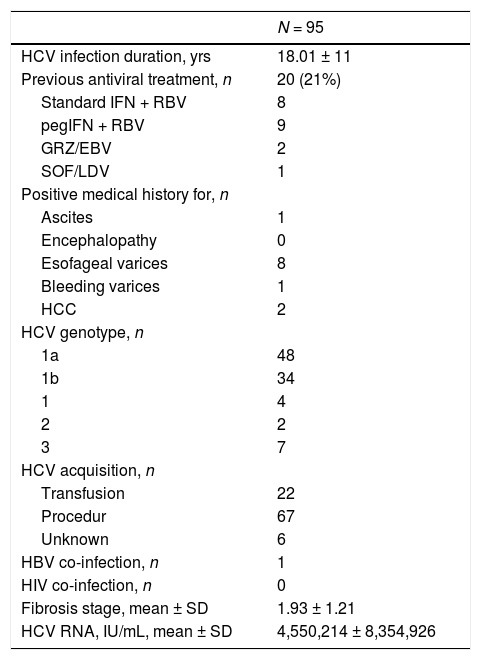
Clinical and virologic characteristics of kidney transplant recipients at baseline (LALREAN).
| N = 95 | |
|---|---|
| HCV infection duration, yrs | 18.01 ± 11 |
| Previous antiviral treatment, n | 20 (21%) |
| Standard IFN + RBV | 8 |
| pegIFN + RBV | 9 |
| GRZ/EBV | 2 |
| SOF/LDV | 1 |
| Positive medical history for, n | |
| Ascites | 1 |
| Encephalopathy | 0 |
| Esofageal varices | 8 |
| Bleeding varices | 1 |
| HCC | 2 |
| HCV genotype, n | |
| 1a | 48 |
| 1b | 34 |
| 1 | 4 |
| 2 | 2 |
| 3 | 7 |
| HCV acquisition, n | |
| Transfusion | 22 |
| Procedur | 67 |
| Unknown | 6 |
| HBV co-infection, n | 1 |
| HIV co-infection, n | 0 |
| Fibrosis stage, mean ± SD | 1.93 ± 1.21 |
| HCV RNA, IU/mL, mean ± SD | 4,550,214 ± 8,354,926 |
Immunosuppressive therapy in kidney transplant recipients (LALREAN).
| Immunosuppressive therapy | Patients (n = 79) |
|---|---|
| TAC ± steroids | 11 |
| TAC ± MMF ± steroids | 19 |
| TAC + AZA + steroids | 2 |
| TAC + mTOR inhibitors + steroids | 4 |
| Cyclosporine + steroids | 1 |
| Cyclosporine + MMF + steroids | 7 |
| AZA + steroids | 2 |
| mTOR inhibitors + steroids | 12 |
| mTOR inhibitors + MMF + steroids | 12 |
| MMF + steroids | 7 |
| Corticosteroids | 2 |
Direct-acting antiviral agents and HCV genotypes in kidney transplant recipients (LALREAN).
| HCV genotype | DAAs combination (patient number, n) | Duration (wks) |
|---|---|---|
| 1a | Elbasvir/grazoprevir (n = 8) | 11 |
| PrOD regimen (ombitasvir/paritaprevir/ritonavir/dasabuvir) ± RBV (n = 7) | 14 | |
| Sofosbuvir/Daclatasvir ± RBV (n = 24) | 16 | |
| Sofosbuvir/Ledipasvir (n = 2) | 13 | |
| Sofosbuvir/Velpatasvir (n = 3) | 15 | |
| Sofosbuvir/Velpatasvir/Voxilaprevir (n = 1) | 12 | |
| Pibrentasvir/Glecapevir (n = 2) | 12 | |
| 1b | Elbasvir/grazoprevir (n = 3) | 12 |
| PrOD regimen (ombitasvir/paritaprevir/ritonavir/dasabuvir) ± RBV (n = 15) | 12 | |
| Sofosbuvir/Daclatasvir ± RBV (n = 12) | 15 | |
| Sofosbuvir/Ledipasvir (n = 2) | 13 | |
| Sofosbuvir/Velpatasvir (n = 1) | 12 | |
| Sofosbuvir/Velpatasvir/Voxilaprevir (n = 1) | 12 | |
| 1 | PrOD regimen (ombitasvir/paritaprevir/ritonavir/dasabuvir) ± RBV (n = 1) | 12 |
| Sofosbuvir/Daclatasvir ± RBV (n = 3) | 16 | |
| 2 | Sosfosbuvir/Daclatasvir ± RBV (n = 3) | 15 |
| 3 | Sofosbuvir/Daclatasvir ± RBV (n = 7) | 16 |
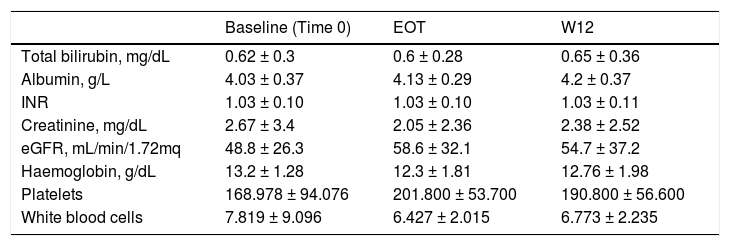
The unadjusted SVR12 rate was 93.7% (89/95) (95% Confidence Interval, 88%; 98%), according to a ITT analysis. There were three viral failures, all were non-responders. The genotypes of the viral failures were: 1a (n = 2), and 1b (n = 1), respectively. There were no differences with regard to total bilirubin, INR, serum albumin and creatinine, eGFR and blood cells at various time points (time 0, end of treatment and 12 weeks after antiviral therapy ended) in the whole cohort (NS) [Table 5].
Biochemical tests at baseline, after antiviral therapy (DAAs) and over follow-up in kidney transplant recipients (LALREAN).
| Baseline (Time 0) | EOT | W12 | |
|---|---|---|---|
| Total bilirubin, mg/dL | 0.62 ± 0.3 | 0.6 ± 0.28 | 0.65 ± 0.36 |
| Albumin, g/L | 4.03 ± 0.37 | 4.13 ± 0.29 | 4.2 ± 0.37 |
| INR | 1.03 ± 0.10 | 1.03 ± 0.10 | 1.03 ± 0.11 |
| Creatinine, mg/dL | 2.67 ± 3.4 | 2.05 ± 2.36 | 2.38 ± 2.52 |
| eGFR, mL/min/1.72mq | 48.8 ± 26.3 | 58.6 ± 32.1 | 54.7 ± 37.2 |
| Haemoglobin, g/dL | 13.2 ± 1.28 | 12.3 ± 1.81 | 12.76 ± 1.98 |
| Platelets | 168.978 ± 94.076 | 201.800 ± 53.700 | 190.800 ± 56.600 |
| White blood cells | 7.819 ± 9.096 | 6.427 ± 2.015 | 6.773 ± 2.235 |
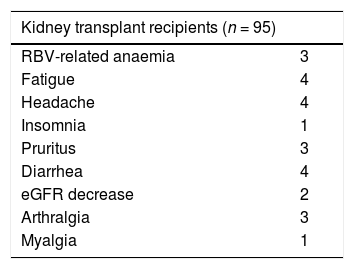
As shown in Table 6, seventeen of 95 kidney transplant patients had at least one AE during treatment with DAAs. The majority of AEs events were mild and were managed clinically without interruption of therapy. Four (4.2%) drop-outs were recorded- the unadjusted drop-out rate was 4.2% (95% CI, 0.2%; 8.2%). Two patients had arthralgia/myalgia, one had fatigue, and one raised creatinine; one of them reached SVR despite intolerance to DAAs. One patient had serum creatinine at baseline of 1.16 mg/dL (eGFR, 75.5 mL/min) and interrupted the treatment (SOF/DCV) when creatinine raised to 2.25 mg/dL. The other patients who discontinued antiviral therapy were on GRZ/EBV (n = 1), and PrOD (n = 2).
The most frequent AEs were fatigue, diarrhea, and headache [Table 6]. Ribavirin-related anaemia was recorded in some (n = 3) patients and was found among those on PrOD (n = 2), and sofosbuvir/daclatasvir combinations (n = 1). Some (n = 4) RT recipients died after the end of antiviral therapy- one had liver disease-related death, the reason for death remained unclear in the others.
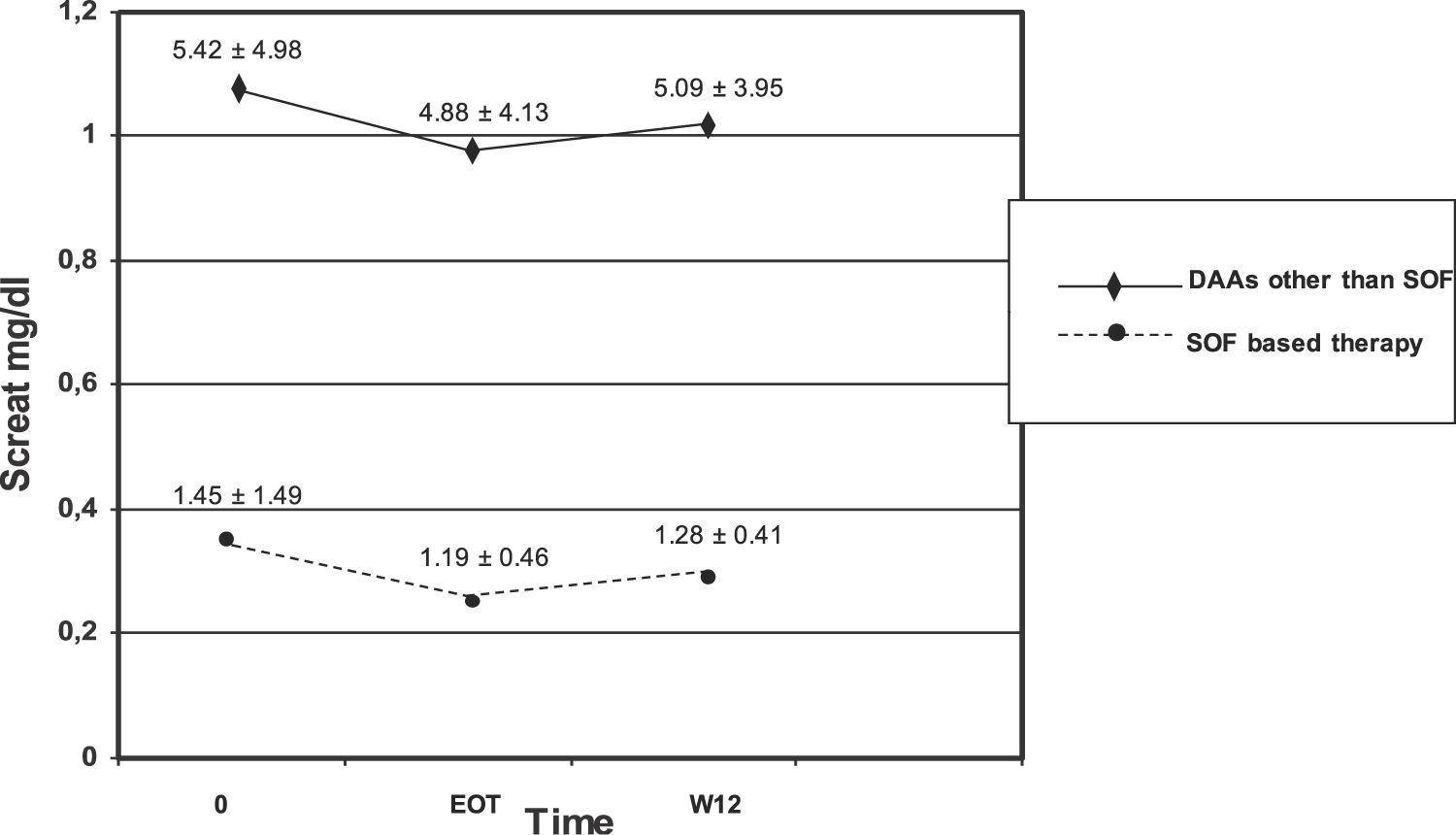
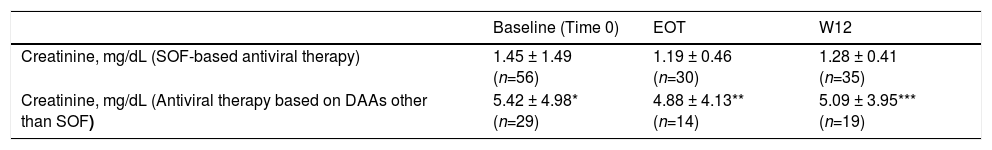
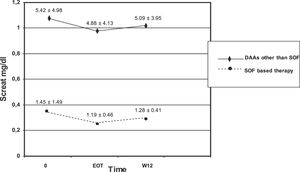
We observed no difference in serum creatinine at various time points (baseline, EOT, and W12) in each subset of patients (those who underwent SOF-based combinations of DAAs and those who did not) [Table 7]. There was significant difference in serum creatinine levels at baseline, EOT, or W12 between individuals who underwent SOF-based therapies and those who received DAAs other than SOF [Table 7, Fig. 1]. As suggested from Fig. 1, there were many RT recipients with raised levels of serum creatinine and some were in the process of returning to dialysis due to severe allograft dysfunction.
Kidney function tests over various time points (baseline, at the end of antiviral therapy, and 12 weeks after antiviral therapy ended) in patients who underwent kidney transplant and received SOF-based therapies or not (LALREAN).
| Baseline (Time 0) | EOT | W12 | |
|---|---|---|---|
| Creatinine, mg/dL (SOF-based antiviral therapy) | 1.45 ± 1.49 (n=56) | 1.19 ± 0.46 (n=30) | 1.28 ± 0.41 (n=35) |
| Creatinine, mg/dL (Antiviral therapy based on DAAs other than SOF) | 5.42 ± 4.98* (n=29) | 4.88 ± 4.13** (n=14) | 5.09 ± 3.95*** (n=19) |
Serum creatinine did not change in each subgroup over various time points.
There was difference in serum creatinine levels between the two subgroups for each time point.
Prior to the availability of all-oral, DAAs for treating HCV, the standard of care was pegylated interferon in combination with ribavirin for 24 or 48 weeks. This combination gave limited cure rates (50%–60%) and was poorly tolerated after kidney transplant due to the increased risk of acute graft rejection which is related to the immunomodulatory properties of IFN [5,6]. IFN-based therapy after RT is suggested only in selected cases, such as post-transplant severe cholestatic liver disease. The introduction in the market of DAAs has profoundly modified the management of HCV infection. However, the evidence addressing the efficacy and safety of HCV treatment with DAAs in kidney allograft recipients is limited and this is one of the largest ‘real-life’ cohorts reported to date.
We found that various regimens of DAAs are highly effective after RT—the SVR rate was 93.7%. These findings are similar to those reported recently [13,14]. Fernandez and colleagues observed that SVR was 98% (101/103) in a large Spanish registry of RT patients who received DAAs [15]. Colombo et al. conducted a European multicentre phase 2 clinical trial (n = 114 RT recipients with HCV genotype 1 or 4 infection) and administered SOF/LDV combination during 12 or 24 weeks; the frequency of SVR was 99%. They did not find difference between 12 or 24 weeks of treatment (SVR rate, 100% vs. 96%) [16]. In France, Kamar and colleagues found a 100% SVR rate in a small cohort (n = 25) of RT patients who received SOF-containing combinations [17]. Additional evidence has been accumulated regarding short treatment duration of DAAs after RT [18], administration of DAAs in recipients with impaired allograft function [19] or in HCV positive with coinfection with HIV [20]. Some investigators have addressed the consequences of HCV eradication on the quality of life after kidney transplantation, even if series with limited size have been addressed [21]. A comparison between RT recipients with HCV who received DAAs and those who did not receive DAAs during the same period is under way [22].
It is noteworthy that the great majority of our patients received all-oral, interferon-free, ribavirin-free therapy with DAAs; in fact, only a minority of patients received ribavirin (only 8 allograft recipients). In other words, most RT recipients at LALREAN received modern combinations of DAAs for chronic HCV and this is in contrast with many studies reported above.
Many RT recipients (n = 61) included in the current survey adopted SOF-based therapies. The adoption of SOF-based regimens in patients with advanced CKD has been hampered by the pharmacokinetics of SOF in patients with kidney dysfunction. Sofosbuvir is a uridine nucleotide analogue whose active metabolism inhibits the NS5B polymerase of HCV. SOF is metabolized to a pharmacologically active nucleotide analogue (GS-461203) by the liver; GS-331007 is an inactive metabolite which is produced by dephosphorylation and is mostly cleared by urine [23,24]. It has been calculated an area under curve (AUC) of 170% for SOF and 450% for GS-331007 in patients with an estimated GFR < 30 mL/min [25]. Previous studies have indicated that SOF-based therapies in patients with baseline eGFR ≤45 mL/min gave a greater risk of worsening kidney function compared with those having eGFR >45 mL/min [26]. Nevertheless, recent reports have shown a great efficacy and satisfactory tolerability of SOF in patients with advanced dysfunction of kidneys. In November 2019 the US Food and Drug Administration authorized the use of SOF-containing regimens for patients on long-term dialysis and those with a eGFR ≤30 mL/min/1.73 m2 [8]. We found no significant difference in serum creatinine levels at various time points in the entire cohort of RT patients and in the subset of those who received SOF-based combinations. Nonetheless, we observed one drop-out related to decreased kidney function while on SOF-based therapy for HCV. The current study did not give the opportunity to explore the safety and efficacy of SOF-containing regimens in severe kidney disease; clinicians belonging to the LALREAN were reluctant to administer SOF in RT recipients with advanced chronic kidney disease, as suggested above [Fig. 1].
The current study shows some shortcomings. It is a multicentre study with retrospective design and the information was collected from an international registry. However, the clinicians engaged at LALREAN followed patients very closely and that assured accuracy of data. A second limitation is that we have not detailed data regarding dose modifications of immunosuppressive therapy and drug-drug interactions during DAAs therapy. Finally, the antiviral regimens were given at the discretion of the clinicians and this precludes the comparison among antiviral combinations.
In summary, this study shows great efficacy and safety of DAAs in a cohort of RT recipients with chronic HCV in a ‘real-life’ experience. The majority of patients enrolled in the current study received all-oral, interferon-free, ribavirin-free combinations with DAAs. One patient developed renal dysfunction while on SOF-based therapy and interrupted the antiviral treatment. Careful monitoring of renal function is indicated. We need to understand the impact of viral response given by DAAs on numerous outcomes after kidney transplant such as quality of life, liver fibrosis, and patient/kidney survival.AbbreviationsAASLD American Association for the Study of Liver Diseases Adverse Events Arterial Hypertension Azathioprine Confidence intervals Chronic kidney disease Chronic kidney disease epidemiology collaboration equation Calcineurin inhibitor Direct-acting antiviral agent Daclatasvir Drug-drug interaction Diabetes mellitus Dasabuvir Estimated glomerular filtration rate End-of-treatment End-stage renal disease Gastrointestinal Glomerulonephritis Hepatitis B virus Hepatitis C virus Haemodialysis Infectious Disease Society of America Interferon Intention-to-treat Ledipasvir Liver transplant Mycophenolate mofetil Mammalian target of rapamycin Not available Ombitasvir Odds ratio Polymerase chain reaction Pegylated interferon Paritaprevir/ritonavir/ombitasvir/dasabuvir Paritaprevir Ritonavir Ribavirin Renal transplant Renal replacement therapy Serious adverse event Sofosbuvir Sustained Virological Response Tacrolimus 12 weeks after antiviral therapy ended
No sources of funding were used for the preparation of this manuscript.
Conflict of interest statementNone.