Background & aim: Alcohol consumption and viral infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) are the first causes of chronic hepatopathy in Mexico. Medical personnel are at high risk of developing HBV and HCV infection because both viruses are transmitted parenteraly. The aim of this study was to determine the prevalence of HCV and HBV infection as well as risk factors in nurses working at Medica Sur Clinic and Foundation. Methods: The complete nurse staff personal from our hospital was included; a questionnaire of risk factors for HCV and HBV infection was assessed. HBV and HCV infection (anti-HCV anti-HBc, and HBsAg) was determined to all of them. In anti-HCV positive persons HCV genotype and viral load was assessed. Results: Three hundred seventy six nurses where studied, Anti-HBc was positive in 1.6% of all participants, none were positive for HBsAg. 0.8% of all studied population was positive for anti-HCV. Major risk factors for HBV infection where tattooing and having more than 4 sexual partners previously, and for HCV infection transfusions before 1992 and age. Only one person was anti-HCV positive with a viral charge of 5 X 106 copies, genotype 2b. Conclusions: HCV seropositivity in people with high risk was lower than general population. None was positive for HBV infection.
List of abbreviations:
HBV, hepatitis B virus
HCV, hepatitis C virus
HCC, hepatocellular carcinoma
RNA, ribonucleic acid
PCR, polymerase chain reaction
HBsAg, hepatitis B surface antigen
HBeAg, hepatitis B e antigen
ALT, alanine aminotransferase
BackgroundChronic liver diseases represent the fourth leading cause of death in the Mexican population and the second among productive-aged population;1 based on mortality trends analysis, chronic liver disease-related mortality will continue to rise beyond the year 2050.2 Along with alcohol, chronic viral infections, like hepatitis B (HBV) and C (HCV) viruses represent the leading causes of liver cirrhosis in our country.3 HCV is a member of flavivirus, with 6 major genotypes and more than 50 subtypes.4 According to blood banks reports, in our country 1.2% of the general population is infected.5,6 In a previous study, we found a 2% prevalence among asymptomatic population in a check-up unit (confirmed though HCV-RNA studies).2
Risk factors for HCV transmission are blood transfusions and surgeries before 1992, intravenous drug use, contaminated parenteral drugs and other invasive non-medical procedures (tattooing, piercing).7 Based on these risk factors, high-risk groups are: multitransfused patients (i.e. hemophiliacs), health personnel, intravenous drug users, inmates, individuals with high-risk sexual behaviors (early sex life, high number of sexual partners and prostitution).8 Other populations at risk are patients on extracorporeal circulation system, such as end-stage kidney disease patients on hemodialysis programs.9
Twenty percent of HCV chronically infected patients develop liver cirrhosis after 20 years, while 1-4% will develop hepatocellular carcinoma (HCC), even in the absence of cirrhosis.10 The relation between HCV and HCC has risen especially in developed countries like Japan, Spain, France and Italy, where the proportion of HCV-related HCC is 50-70%.11 Antiviral therapy based on pegylated alfa-2b or alfa-2a interferon and ribavirin results in sustained viral response in 50% of patients with genotype 1 (which correspond to 70% of infected Mexican population) and 80% in patients with genotype 2 or 3; however, in more advanced stages of the disease, there is a lessen response to therapy, emphasizing early detection as a major tool in controlling HCV infection.12
Four hundred million people in the world are infected with HBV; in high-prevalence areas, such as sub-Saharan Africa, the most important form of dissemination is vertical transmission and in low-prevalence areas, such as Mexico (0.2% of the general population) the most important form of dissemination is sexual transmission, intravenous drug use, tattooing and piercing.13,14 After primary infection, 510% of patients will develop chronic hepatitis and depending on viral antigen e status, 8-30% (positive HBeAg) or 38% (negative HBeAg) will develop HCC annually.15
HBV and HCV infections exhibit parenteral transmission. Health personnel frequent contact with organic material and disposal places this population at high-risk of infection. According to an interview performed by our team in our hospital in 2003, only 10% of nursing personnel is vaccinated against HBV. The aim of this study is to determine the prevalence of risk factors and prevalence of hepatitis virus B and C serum markers among nurses in a tertiary-care hospital in Mexico City.
MethodsDesign and sampleWe performed a descriptive study for which the entire Medica Sur Clinic & Foundation nursing personnel were invited to participate. This hospital provides medical care for middle or high income individuals living in Mexico City and the surroundings. The study was approved by the Human Subjects Committee of the Medica Sur Clinic and Foundation, conforming to the ethical guidelines of the 1983 Declaration of Helsinki, and written informed consent was obtained from all participants before entry into the study. Every participant filled a risk factor questionnaire which included blood transfusions, surgeries or dental procedures before 1992, tattooing, piercing, manicure or pedicure in non-personal equipment, history of more than 4 sexual partners, family history of HBV or HCV infection, acupuncture and intravenous drug use.
Physical examBody weight was measured, in light clothing and without shoes, to the nearest 0.10 kg. Height was measured to the nearest 0.5 cm. Body mass index was calculated as weight (kg) divided by height squared (m2). Waist circumference to the nearest 0.1 cm was measured at the midpoint between the lower border of the rib cage and the iliac crest, and hip circumference was similarly obtained at the widest point between hip and buttock. Body fat percentage was measured by bioelectrical impedance (Omron body fat analyzer model HBF-306INT). Blood pressure was assessed using a mercury sphygmomanometer according to the American Heart Association guidelines.16
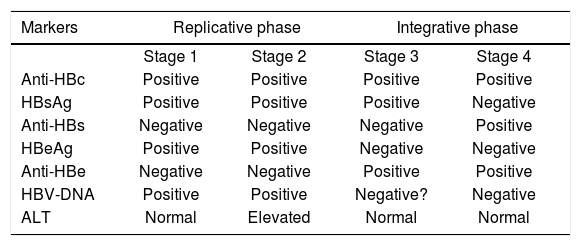
Analytical proceduresLiver function tests was performed in every sample, and included aspartate aminotransferase, alanine ami-notransferase (ALT), alkaline phosphatase, gamma-glutamyl transpeptidase, total, direct and indirect bilirubin, total protein, albumin, globulin and prothrombin time. Serums were analyzed for detection of IgG antibodies anti-VHC with Axsyum HCV system version 3.0 (Abbott Laboratories Ltd., Wiesbaden, Germany). In positive cases, qualitative and quantitative viral load and genotype were assessed through polymerase chain reaction (PCR) for RNA-VHC. HBV surface antigen (HBsAg) and IgG antibodies anti-HB core antigen (anti-HBc) were assessed using Vitros Eci Device (Johnson and Johnson), following the diagnostic criteria shown in Table I. In positive cases, HB antigen e (HBeAg), qualitative and quantitative viral load and genotype were assessed through polymerase chain reaction (PCR) for RNA-VHC.
Interpretation of HBV serum tests.
| Markers | Replicative phase | Integrative phase | ||
|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | |
| Anti-HBc | Positive | Positive | Positive | Positive |
| HBsAg | Positive | Positive | Positive | Negative |
| Anti-HBs | Negative | Negative | Negative | Positive |
| HBeAg | Positive | Positive | Negative | Negative |
| Anti-HBe | Negative | Negative | Positive | Positive |
| HBV-DNA | Positive | Positive | Negative? | Negative |
| ALT | Normal | Elevated | Normal | Normal |
To identify statistically significant risk factors to be a HBV or HCV positive case, we performed a statistical analysis using Fisher exact test (two-tailed) for continuous variables and Mann-Whitney U-test for categorical variables. A logistic regression analysis was performed to study the independent impact of significant risk factors in univariate analysis, controlling for confusing variables. Odds ratios (OR) were derived for the exponential of the regression coefficient, and 95% CI were calculated. All statistical analyses were carried out with the statistics program, SPSS/PC v12.0 (Chicago, IL).
ResultsFrom 485 nurses, 379 agreed to participate. We eliminated three subjects because of insufficient serum material, with a total of 376 subjects, 368 females and 8 males, with a mean age of 30.8 ± 7 years.
Anti-HBc was detected in 1.6% (n = 6) of participants and there were no HBsAg positive cases; for this reason, we classified all these patients as late integrative phase HBV without viral activity, therefore no further testing was performed. In Table II, the results from the risk factor questionnaire is shown, from which the most important are tattooing and more than 4 sexual partners (p < 0.05 y p < 0.1, respectively).
Prevalence of risk factors for HBV and HCV in our population.
| Anti-HBc | Anti-HCV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk factor | Positive | Negative | Positive | Negative | ||||||
| (N = 6) | (n = 370) | n = 3 | (n = 373) | |||||||
| n | % | n | % | p* | n | % | n | % | p* | |
| Female | 6 | 100 | 362 | 97.8 | 1.00 | 3 | 100 | 365 | 97.9 | 1.00 |
| Surgery prior to 1992 | 2 | 33.3 | 80 | 21.6 | 0.62 | 2 | 66.7 | 80 | 21.4 | 0.12 |
| Family history of HBV | 0 | 0 | 6 | 1.6 | 1.00 | 0 | 0 | 6 | 1.6 | 1.00 |
| Transfusion prior to 1992 | 0 | 0 | 9 | 2.4 | 1.00 | 1 | 33.3 | 8 | 2.1 | 0.07 |
| Tattooing | 2 | 33.3 | 18 | 4.9 | 0.04 | 0 | 0 | 20 | 5.4 | 1.00 |
| Manicure/pedicure | 3 | 50 | 188 | 50.8 | 1.00 | 2 | 66.7 | 189 | 50.7 | 1.00 |
| Dental procedure prior to 1992 | 0 | 0 | 97 | 26.3 | 0.35 | 0 | 0 | 97 | 26.1 | 0.57 |
| Piercing | 3 | 50 | 114 | 30.8 | 0.38 | 0 | 0 | 117 | 31.4 | 0.56 |
| Acupuncture | 0 | 0 | 48 | 13 | 1.00 | 0 | 0 | 48 | 12.9 | 1.00 |
| > 4 sexual partners | 2 | 33.3 | 30 | 8.1 | 0.09 | 0 | 0 | 32 | 8.6 | 1.00 |
| IV drug use | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
HCV serological analysis showed that 0.8% (n = 3) of the population studied were positive to IgG anti-HCV. From these three participants, only one showed positive viral load (5 x 106 copies) of HCV genotype 2b and normal ALT levels. In Table II, the results from the risk factor questionnaire is shown, from which the most important is transfusion prior to 1992 with borderline statistical significance (p = 0.07).
In the multivariate study controlled for confusing factors, tattooing (OR = 10, p < 0.01) and a history of more than 4 sexual partners (OR = 5.8, p = 0.06) remained statistically significant to be a positive case of anti-HBc. Anti-HCV was associated with blood transfusions prior to 1992 (OR = 29.6, p < 0.05) and age (OR = 1.15, p < 0.05).
DiscussionIn endemical areas, seroprevalence of HBV infection is as high as 8%, while in low-prevalent areas such as North America, it is 2% or less.14 In Mexico, the seroprevalence of HBsAg is just 0.23%.17 In a study performed in Nepal, the prevalence of HBV infection was studied in several categories of health personnel, 19.2% of nurses studied had evidence of previous or current HBV infection.18 It is estimated that 6-30% of subjects suffering a laboral accident with HBV contaminated material acquire the infection. In the present study, only 1.6% of subjects were positive to anti-HBc and non-to HBsAg, a fact that was interpreted as immunity to the virus due to previous contact but with no viral activity. Even though, the author believe that it is important to remark the fact that only 10% of nurse staff is self-reported as vaccinated against HBV.
In North America, the main risk factors for HBV infection are IV drug use, sexual transmission, nosocomial infection and immigration to endemic areas.15 In this study, the more common risk factors to be anti-HBc positive were tattooing and having a history of more than 4 sexual partners.
The estimated prevalence of HCV infection varies from 1% in Europe, 1.7% in America, and up to 5.3% in Africa.7 The projected number of HCV infected persons in 2020 is 2,861,556.6 Jindal et al. determinated an anti-HCV seroprevalence of 4% in a health-related working population in New Delhi. In our study, anti-VHC seroprevalence was 0.8%, a slightly minor prevalence than reported in the general population in our country, despite being a high-risk group.
In 1994, the health authorities in our country dictated that all blood bank samples must be valuated for anti-HCV, a fact that reduced the risk of transmission in blood transfusion to 1:1,600,000.19 In the present study, we considered blood transfusions prior to 1992 as a risk factor, because effective technology for HCV detection was available since that year in our country, despite not being mandatory.
There is enough evidence to show that percutaneous exposure to blood is the main transmission bias of HCV infection among the health personnel. In the United States, health workers represent almost 2% of the total incidence of new cases of HCV; however, the prevalence of anti-VHC in this population is similar to the general population. The probability of transmission through a contaminated needle-stick is 3-10%.20
Rischitelli et al. evaluated the risk of HCV occupational infection in a 30-year career. The estimated annual needle-stick accidents per capita are 0.12 (IC 95% 0.026-2.0), with a HCV prevalence of 1.8% in their population (IC 95% 1.2-2.3) and an annual risk of seroconversion of 0.01%.21 It is estimated that 3-10% of the exposed to these accidents will acquire HCV infection.22
Other potential HCV transmission biases in health workers have been proposed. HCV-RNA has been detected in saliva and gingival crevicular fluid; there is even a case report that sustains HCV transmission through a human bite.23,24 HCV transmission through a blood splash into conjunctiva in a nurse has also been reported.25 In Table III, differences among risk factors prevalence in the nurses studied in this report and an asymptomatic population in a check-up unit studied in a previous report are compared.26 Blood transfusion and/ or surgery prior to 1992 is the main risk factor for HCV in nurses (33.3% in HCV positive KS 2% in HCV negative), while in the asymptomatic population tattooing (16.6% in HCV positive KS 6.4% in HCV negative) and manicure/pedicure with non-personal instruments (50% in HCV positive KS 39.7% in HCV negative) are the main risk factors.
Risk factors for HCV in nursing personnel and an asymptomatic population in a check-up unit.
| Anti-HCV | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive nurses (n = 3 (0.8%) | Negative nurses (n = 373 (99.2%) | Positive asymptomatic (n = 6 (2%) | Negative asymptomatic | |||||||
| Risk factor | n | % | n | % | p* | n | % | n | % | p* |
| Surgery prior to 1992 | 2 | 66.7 | 80 | 21.4 | 0.12 | 2 | 33.3 | 154 | 52.3 | 0.43 |
| Transfusion prior to 1992 | 1 | 33.3 | 8 | 2.1 | 0.07 | 1 | 16.6 | 36 | 12.2 | 0.55 |
| Tattooing | 0 | 0 | 20 | 5.4 | 1 | 1 | 16.6 | 19 | 6.4 | 0.34 |
| Manicure/pedicure | 2 | 66.7 | 189 | 50.7 | 1 | 3 | 50.0 | 117 | 39.7 | 0.69 |
| Dental procedure prior to 1992 | 0 | 0 | 97 | 26.1 | 0.57 | 1 | 16.6 | 128 | 43.5 | 0.24 |
| Piercing | 0 | 0 | 117 | 31.4 | 0.56 | 0 | 0.0 | 12 | 4.0 | 1 |
| Acupuncture | 0 | 0 | 48 | 12.9 | 1 | 2 | 33.3 | 71 | 24.1 | 0.64 |
| > 4 sexual partners | 0 | 0 | 32 | 8.6 | 1 | 3 | 50.0 | 113 | 38.4 | 0.68 |
| IV drug use | 0 | 0 | 0 | 0 | - | 0 | 0.0 | 0 | 0.0 | - |
Fisher exact test (2-tailed)
In the current report, we found that age was an independent risk factor to be a case of HCV, which suggests that the longer the exposure to occupational hazards the greater the probability to be acquire HCV. A possible explanation for the low HCV prevalence in this report is the mean age of the population studied, mainly fourth decade female with a decade or less of laboral experience.
In conclusion, our results show that the prevalence of HCV in our nurse staff is lower than the general population, despite being a high-risk group. We did not identify a case of HBV chronic infection; however, we detected the need to develop massive vaccination campaigns in our nursing staff.
Acknowledgements: This study was supported partly by Laboratorios Roche de Mexico and a Grant from Medica Sur Clinic and Foundation.