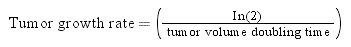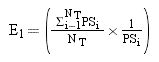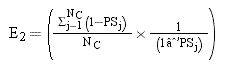Evidence supporting benefit of hepatocellular carcinoma (HCC) surveillance in reducing mortality is not well-established. The effect of HCC surveillance in reducing mortality was assessed by an inverse probability of treatment weighting (IPTW)-based analysis controlled for inherent bias and confounders in observational studies.
Material and MethodsThis retrospective cohort study was conducted on 446 patients diagnosed with HCC between 2007 and 2013 at a major referral center. Surveillance was defined as having at least 1 ultrasound test within a year before HCC diagnosis. Primary outcome was survival estimated using the Kaplan-Meier method with lead-time bias adjustment and compared using the log-rank test. Hazard ratio (HR) and 95% confidence interval (CI) were computed using conventional Cox and weighted Cox proportional hazards analysis with IPTW adjustment.
ResultsOf the 446 patients, 103 (23.1%) were diagnosed with HCC through surveillance. The surveillance group had more patients with the Barcelona-Clinic Liver Cancer stage A (80.6% vs. 33.8%, P < 0.0001), more patients eligible for potentially curative treatment (73.8% vs. 44.9%, P < 0.0001), and longer median survival (49.6 vs. 15.9 months, P < 0.0001). By conventional multivar-iate Cox analysis, HR (95% CI) of surveillance was 0.63 (0.45-0.87), P = 0.005. The estimated effect of surveillance remained similar in the IPTW-adjusted Cox analysis (HR: 0.57; 95% CI: 0.43-0.76, P < 0.001).
ConclusionsHCC surveillance by ultrasound is associated with a 37% reduction in mortality. Even though surveillance is recommended in all guidelines, but in practice, it is underutilized. Interventions are needed to increase surveillance rate for improving HCC outcome.
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the second leading cause of cancer death worldwide.1 Approximately 782,000 new cases are diagnosed with HCC annually1 and the global incidence has been increasing. It has been projected that by 2030, this number will increase by 53% or up to over 1.2 million cases.2 Outcomes of patients diagnosed with HCC vary substantially depending on the stage when the disease was detected. Patients diagnosed with an early stage, at which potential curative treatment is feasible, have a 5-year survival rate of 50-70% whereas those diagnosed with an advanced stage have a median survival of only 6 months.3 Therefore it is important to make the diagnosis at an early stage so the outcome of HCC can be significantly improved.
Approximately 80% of HCCs develop in patients with underlying cirrhosis.4 Generally, cirrhosis increases the risk for HCC development by 3-8% annually.3 In addition to cirrhosis, chronic viral hepatitis B infection is a major risk factor for HCC, particularly in East Asian countries, except for Japan where viral hepatitis C infection is more prevalent. Hepatitis B carriers have a 226-fold increased risk for developing HCC compared to non-carriers.5 Accordingly, a number of guidelines universally recommend screening and surveillance for HCC using ultrasonogra-phy and/or serum alpha-fetoprotein (AFP) among at-risk populations.3,6-8 Based on the American Association for the Study of Liver Disease (AASLD) and the European Association Study for the Study of the Liver (EASL) guidelines, active HCC surveillance by ultrasound every 6 months is recommended for individuals at-risk for HCC.3,6
Screening and active surveillance has been shown to enhance rate of early HCC detection, increase potential access to curative treatment and possibly improve survival outcome.9,10 However, it remains inconclusive whether HCC surveillance can decrease the mortality rates, partly due to the inconsistent results from two large, randomized controlled studies.11,12 Furthermore, other evidences drawn from observational studies are prone to biases and confounders. Theoretically, a conclusive evidence of the benefit of surveillance in reducing the mortality of HCC should be drawn from a randomized controlled trial. However, it is not feasible to conduct a randomized controlled study enrolling patients into 2 arms with surveillance and without surveillance, especially if informed consent is needed, because most of the participants would prefer to be in the surveillance arm rather than being randomized to the non-surveillance arm.13 As a result of this, there is currently insufficient evidence that can strongly demonstrate the benefits of HCC surveillance in reducing mortality. Thus, our primary aim was to determine whether HCC surveillance is associated with a reduction in mortality using an inverse probability of treatment weighted (IPTW) analysis controlled for biases and confounders existing in observational studies.
Although it has been suggested that the HCC surveillance may have potential benefits, yet its use in clinical practice remains disappointing. For example, in Europe and the US, the use of HCC surveillance is less than 20%.9,14 The surveillance rate for HCC in Thailand, where chronic HBV is endemic and HCC is the most leading cause of cancer death, is currently unknown. As the secondary aim, we sought to determine the rate of HCC surveillance among at-risk populations in a large referral center in Thailand.
Material and MethodsThis study was approved by the Chulalongkorn University's Institutional Review Board Committee. All patients potentially diagnosed with HCC between 2007 and 2012 at the King Chulalongkorn Memorial Hospital, Bangkok, Thailand, were identified by the ICD-9 code C22.0 (n = 469). Medical records were retrospectively reviewed to verify the diagnosis of HCC. The diagnosis of HCC was ascertained using the AASLD diagnostic criteria as follows: 1) histopathological diagnosis; or 2) focal mass lesion of >1cm in diameter with typical radiological findings including arterial enhancement and rapid wash-out in the portovenous or delayed phase assessed by contrast-enhanced imaging (computed tomography and/or magnetic resonance imaging). Patients with age < 18 years, benign liver masses (n = 4) and other types of liver cancer (5 cholangiocarcinoma, 1 mixed hepatocholangiocarcinoma, and 3 metastatic tumor) were excluded from the study. The final cohort had 446 HCC patients.
Clinical information were retrospectively abstracted from the medical records, including age, sex, co-morbidities, alcohol consumption, etiology of chronic liver diseases (chronic viral hepatitis B or C infection, alcohol, NASH or others), date of HCC diagnosis, Eastern Cooperative Oncology Group (ECOG) performance status at the time of HCC diagnosis, tumor size defined as the maximum radial diameter of the largest tumor, tumor number, severity of liver impairment classified by Model for End-Stage Liver Disease (MELD) score, the Child-Turcotte-Pugh (CTP) classification, HCC stage by the Barcelona Clinic Liver Cancer (BCLC) classification, serum AFP level, treatment received (potentially curative or palliative treatment), last follow-up date, and vital status at the last follow-up date. Vital status was acquired by using the National Death Registration database.
The patients were classified into 2 groups: surveillance group and non-surveillance group. Surveillance was defined as having at least 1 abdominal ultrasound test within a year prior to HCC diagnosis. The non-surveillance group was defined as diagnosis by the presence of symptoms or in other terms, patients diagnosed without HCC surveillance.
Statistical analysisQuantitative data were analyzed using the Student t-test or Wilcoxon Rank Sum test and reported as means ± standard deviation (SD) or median (interquartile range, IQR) as appropriate. Categorical data were analyzed using the χ2 test and reported as a number (%).
Survival was estimated from HCC diagnosis date until the last follow-up date using Kaplan-Meier methods and compared using the Log-rank test. Patients who survived were censored on January 1st, 2015. To account for lead-time bias, defined as a spurious amelioration of survival of surveilled patients due to the diagnosis of HCC at an earlier stage by surveillance rather than by the presence of the symptoms,3 we calculated the lead-time for the surveillance group using the Duffy's formula:15,16
lead time = (1-e-λt)/ λ
Where:
t was survival in days of each patient,
λ was rate of transition from asymptomatic to symptomatic disease.
The λ was equal to: λ=1Sojourn time
St=lnTvdetected by surveillanceTvin the absence of surveillanceTumor growth rate
Where:
St: sojourn time
Tv: Tumor volume
Assuming tumor volume doubling time of 60, 90 and 120 days.
The calculated lead-time was subtracted to the survival for each patient in the surveillance group.
Factors associated with death were identified using the Cox-proportional hazard analysis. Variables with p < 0.05 in the univariate model were included in the multivariable model.
Inverse Probability of Treatment Weighted (IPTW)-Based Survival AnalysisTo account for the selection bias and unavoidable confounding factors, we performed the weighted Cox proportional hazards regression models using IPTW with robust standard errors.17 IPTW creates a pseudo-population which is weighted by the IPTW. In time-to-event analyses, IPTW using the propensity score resulted in estimates with lower mean squared error, suggesting that the estimates calculated using the weighted analyses have a better precision than those calculated with matched analyses.18 For the IPTW survival analysis, first, the weights for chance of “being surveilled” for each patient were calculated using a propensity score. The propensity score, which indicated the predicted probability of receiving HCC surveillance based on all observed covariates except for the treatment variable (i.e. curative or non-curative treatment), was calculated using the multiple logistic regression analysis. Multiple logistic regression model included the following variables: age, sex, etiologies of chronic liver diseases, cirrhosis, ECOG status, MELD score, CTP score, BCLC stage, tumor number (single vs. multiple), tumor size, presence of portal vein thrombosis, regional metastasis, distant metastasis, AFP level (< 200 vs. ≥ 200 ng/mL), and serum albumin level.
In this study, the weights for patients with surveillance were the inverse of the propensity score and for those patients without HCC surveillance, it was the inverse of 1-propensity score. To reduce the variability of the IPTW weights and give individuals with extreme weights less influence, stabilization technique was developed.18 Stabilization is accomplished by multiplying the treatment and comparison weights (separately) by a constant, equal to the expected value of being in the treatment or comparison groups. Equations for stabilizing the treatment and comparison group weights, respectively are:19
Equation 1.
Equation 2.
Where:19
i and j: denote treated person ‘i’ and comparison person ‘j’
NT and NC: total number of treated and comparison individuals, respectively.
PS: propensity score.
This stabilization does not affect the point estimate of the treatment effect as the IPTW weights in each group are multiplied by a constant, but it decreases the variance.19 Therefore, this stabilization will reduce the weights for either treated subjects with low propensity score or untreated subjects with high propensity scores before applying the weight in the analysis model, resulting in a more robust estimation.20
The model discrimination index assessed using c-statistics was 0.886, suggesting an excellent ability of our model to discriminate between those with and without the outcome of interest. The Hosmer-Lemeshow goodness-of-fit test was used to evaluate whether the logistic regression model was well-calibrated so that the probability predictions from our model would reflect the occurrence of the events (χ2 = 9.47, df = 8, p = 0.304). A two-sided p value < 0.05 was considered significant. All statistical analyses were performed using R software 3.1.3. (R Foundation for Statistical Computing [http://www.r-project.org]).
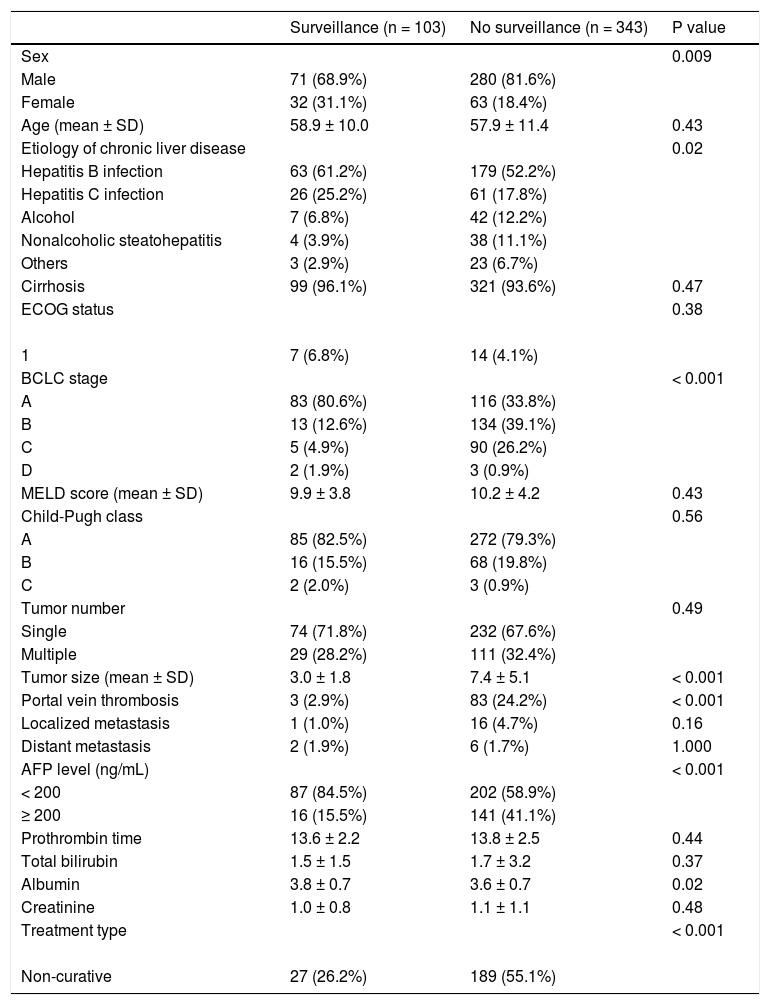
ResultsBaseline characteristicsTable 1 displays the baseline characteristics of the study patients. Of the 446 HCC patients, 351 (78.7%) were males with a mean (SD) age of 58.1 (11.1) years. Chronic HBV infection (n = 242, 54.2%) was the most common underlying chronic liver disease, followed by chronic HCV infection (n = 87, 19.5%), alcohol (n = 49, 11.0%) and non-alcoholic fatty liver disease (n = 42, 9.4%). There were 357 (80.0%), 84 (18.8%) and 5 (1.1%) patients with Child-Pugh class A, B and C, respectively.
Baseline characteristics of study patients.
| Surveillance (n = 103) | No surveillance (n = 343) | P value | |
|---|---|---|---|
| Sex | 0.009 | ||
| Male | 71 (68.9%) | 280 (81.6%) | |
| Female | 32 (31.1%) | 63 (18.4%) | |
| Age (mean ± SD) | 58.9 ± 10.0 | 57.9 ± 11.4 | 0.43 |
| Etiology of chronic liver disease | 0.02 | ||
| Hepatitis B infection | 63 (61.2%) | 179 (52.2%) | |
| Hepatitis C infection | 26 (25.2%) | 61 (17.8%) | |
| Alcohol | 7 (6.8%) | 42 (12.2%) | |
| Nonalcoholic steatohepatitis | 4 (3.9%) | 38 (11.1%) | |
| Others | 3 (2.9%) | 23 (6.7%) | |
| Cirrhosis | 99 (96.1%) | 321 (93.6%) | 0.47 |
| ECOG status | 0.38 | ||
| 1 | 7 (6.8%) | 14 (4.1%) | |
| BCLC stage | < 0.001 | ||
| A | 83 (80.6%) | 116 (33.8%) | |
| B | 13 (12.6%) | 134 (39.1%) | |
| C | 5 (4.9%) | 90 (26.2%) | |
| D | 2 (1.9%) | 3 (0.9%) | |
| MELD score (mean ± SD) | 9.9 ± 3.8 | 10.2 ± 4.2 | 0.43 |
| Child-Pugh class | 0.56 | ||
| A | 85 (82.5%) | 272 (79.3%) | |
| B | 16 (15.5%) | 68 (19.8%) | |
| C | 2 (2.0%) | 3 (0.9%) | |
| Tumor number | 0.49 | ||
| Single | 74 (71.8%) | 232 (67.6%) | |
| Multiple | 29 (28.2%) | 111 (32.4%) | |
| Tumor size (mean ± SD) | 3.0 ± 1.8 | 7.4 ± 5.1 | < 0.001 |
| Portal vein thrombosis | 3 (2.9%) | 83 (24.2%) | < 0.001 |
| Localized metastasis | 1 (1.0%) | 16 (4.7%) | 0.16 |
| Distant metastasis | 2 (1.9%) | 6 (1.7%) | 1.000 |
| AFP level (ng/mL) | < 0.001 | ||
| < 200 | 87 (84.5%) | 202 (58.9%) | |
| ≥ 200 | 16 (15.5%) | 141 (41.1%) | |
| Prothrombin time | 13.6 ± 2.2 | 13.8 ± 2.5 | 0.44 |
| Total bilirubin | 1.5 ± 1.5 | 1.7 ± 3.2 | 0.37 |
| Albumin | 3.8 ± 0.7 | 3.6 ± 0.7 | 0.02 |
| Creatinine | 1.0 ± 0.8 | 1.1 ± 1.1 | 0.48 |
| Treatment type | < 0.001 | ||
| Non-curative | 27 (26.2%) | 189 (55.1%) |
The median (IQR) level of AFP was 42.6 (5.6, 465.8) ng/mL. Out of 446 patients, 199 (44.6%), 147 (33.0%), 95 (21.3%) and 5 (1.1%) were diagnosed with BCLC stage A, B, C, and D, respectively. Regarding treatments, 230 (51.6%) received potentially curative treatment (136 surgical resection, 46 radiofrequency ablation, 39 liver transplantation, and 9 percutaneous ethanol injection) while another 174 (39.0%) patients received palliative treatments, including transarterial chemoembolization (n = 147), targeted therapy sorafenib (n = 13), radiotherapy (n = 11) and systemic chemotherapy (n = 3), and the other 42 (9.4%) patients received supportive treatment.
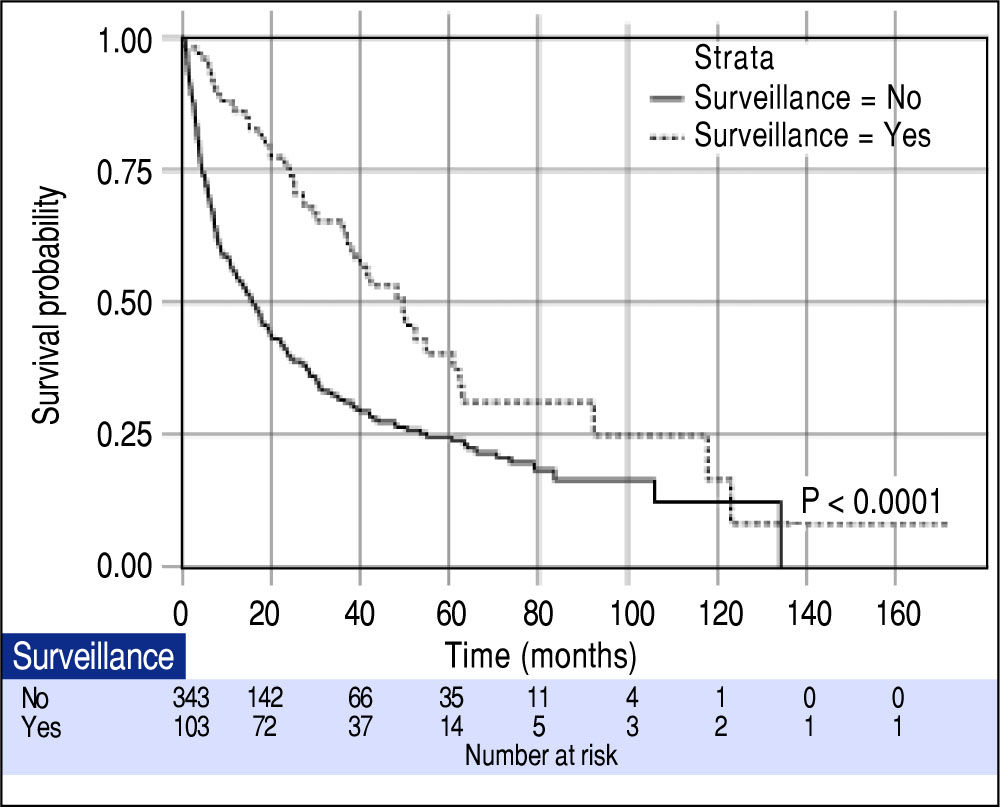
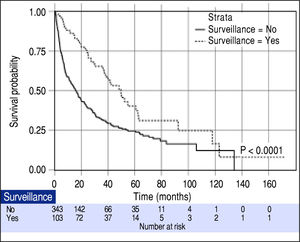
Effect of surveillance on patient survivalOverall median survival of the entire cohort was 19.0 months. When classified by the BCLC stage, the median survivals of patients with BCLC stage A, B, C and D were 47.8, 19.2, 4.2 and 3.0 months, respectively (P < 0.001). From a total of 446 patients, 103 (23.1%) were diagnosed with HCC through the surveillance program (surveillance group). The other 343 (76.9%) patients were not under the surveillance program and diagnosed with HCC because of presentations of symptoms (non-surveillance group). The patients in the surveillance group had more females (31.1% vs. 18.4%, P = 0.009), smaller mean tumor size (3.0 ± 1.8 vs. 7.4 ± 5.1, P < 0.001), fewer incidences of portal vein thrombosis (2.9% vs. 24.2%, P < 0.001) and more patients with AFP < 200 ng/mL (84.5% vs. 58.9%, P < 0.001). Since a significantly higher proportion of patients diagnosed with BCLC stage A were in the surveillance group (80.6% vs. 33.8%, P < 0.0001), hence there were more patients eligible for potentially curative treatment (73.8% vs. 44.9%, P < 0.0001). As expected, the surveillance group had a significantly longer median survival compared to the non-surveillance group (49.6 vs. 15.9 months; P < 0.0001) as shown in figure 1.
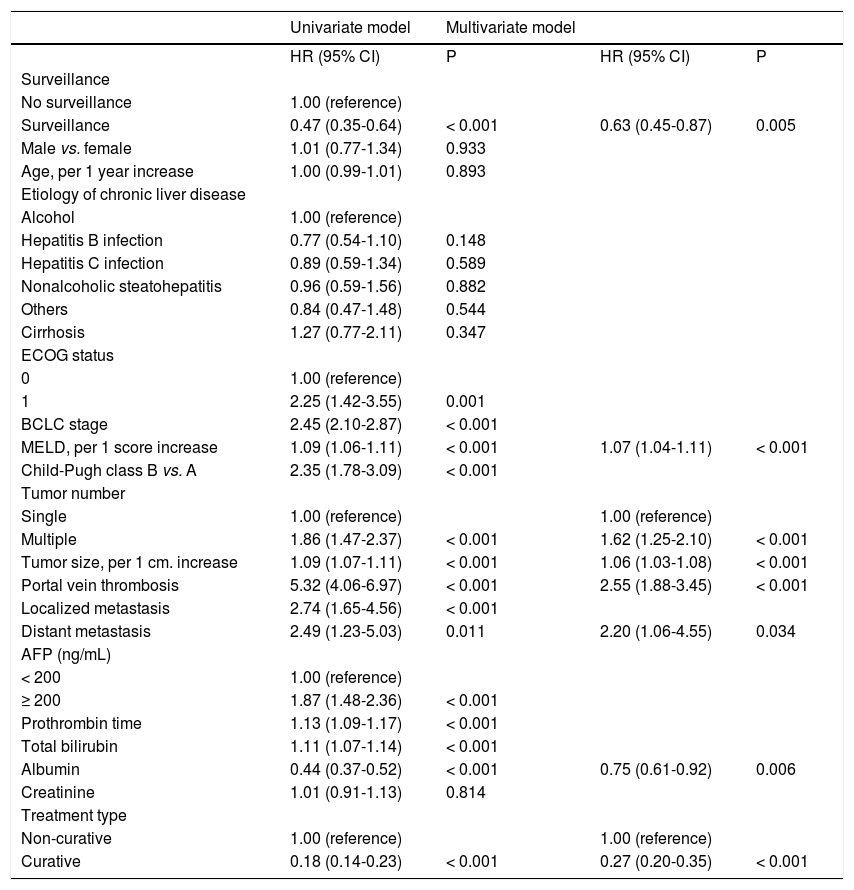
Factors associated with survival of HCC patientsBy conventional Cox univariate analysis, surveillance was associated with improved survival with HR (95% CI) of 0.47 (0.35-0.64), P < 0.001 (Table 2). Other variables associated with survival included ECOG performance status, MELD score, CTP score, BCLC stage, tumor number and size, vascular invasion, local and distant metastasis, prothrombin time, total bilirubin, albumin, and initial treatment type (Table 2). By conventional Cox mul-tivariate analysis, surveillance remained significantly associated with survival with adjusted HR (95% CI) of 0.63 (0.45-0.87), P = 0.005. MELD score, tumor size and number, vascular invasion, distant metastasis, albumin level and initial treatment type were also independent factors associated with survival of HCC patients with HR (95% CI) of 1.07 (1.04-1.11), 1.06 (1.03-1.08), 1.62 (1.25-2.10), 2.55 (1.88-3.45), 2.20 (1.06-4.55), 0.75 (0.61-0.92), and 0.27 (0.20-0.35), respectively, P < 0.05 for all.
COX regression analysis for factors associated with survival of HCC patients.
| Univariate model | Multivariate model | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Surveillance | ||||
| No surveillance | 1.00 (reference) | |||
| Surveillance | 0.47 (0.35-0.64) | < 0.001 | 0.63 (0.45-0.87) | 0.005 |
| Male vs. female | 1.01 (0.77-1.34) | 0.933 | ||
| Age, per 1 year increase | 1.00 (0.99-1.01) | 0.893 | ||
| Etiology of chronic liver disease | ||||
| Alcohol | 1.00 (reference) | |||
| Hepatitis B infection | 0.77 (0.54-1.10) | 0.148 | ||
| Hepatitis C infection | 0.89 (0.59-1.34) | 0.589 | ||
| Nonalcoholic steatohepatitis | 0.96 (0.59-1.56) | 0.882 | ||
| Others | 0.84 (0.47-1.48) | 0.544 | ||
| Cirrhosis | 1.27 (0.77-2.11) | 0.347 | ||
| ECOG status | ||||
| 0 | 1.00 (reference) | |||
| 1 | 2.25 (1.42-3.55) | 0.001 | ||
| BCLC stage | 2.45 (2.10-2.87) | < 0.001 | ||
| MELD, per 1 score increase | 1.09 (1.06-1.11) | < 0.001 | 1.07 (1.04-1.11) | < 0.001 |
| Child-Pugh class B vs. A | 2.35 (1.78-3.09) | < 0.001 | ||
| Tumor number | ||||
| Single | 1.00 (reference) | 1.00 (reference) | ||
| Multiple | 1.86 (1.47-2.37) | < 0.001 | 1.62 (1.25-2.10) | < 0.001 |
| Tumor size, per 1 cm. increase | 1.09 (1.07-1.11) | < 0.001 | 1.06 (1.03-1.08) | < 0.001 |
| Portal vein thrombosis | 5.32 (4.06-6.97) | < 0.001 | 2.55 (1.88-3.45) | < 0.001 |
| Localized metastasis | 2.74 (1.65-4.56) | < 0.001 | ||
| Distant metastasis | 2.49 (1.23-5.03) | 0.011 | 2.20 (1.06-4.55) | 0.034 |
| AFP (ng/mL) | ||||
| < 200 | 1.00 (reference) | |||
| ≥ 200 | 1.87 (1.48-2.36) | < 0.001 | ||
| Prothrombin time | 1.13 (1.09-1.17) | < 0.001 | ||
| Total bilirubin | 1.11 (1.07-1.14) | < 0.001 | ||
| Albumin | 0.44 (0.37-0.52) | < 0.001 | 0.75 (0.61-0.92) | 0.006 |
| Creatinine | 1.01 (0.91-1.13) | 0.814 | ||
| Treatment type | ||||
| Non-curative | 1.00 (reference) | 1.00 (reference) | ||
| Curative | 0.18 (0.14-0.23) | < 0.001 | 0.27 (0.20-0.35) | < 0.001 |
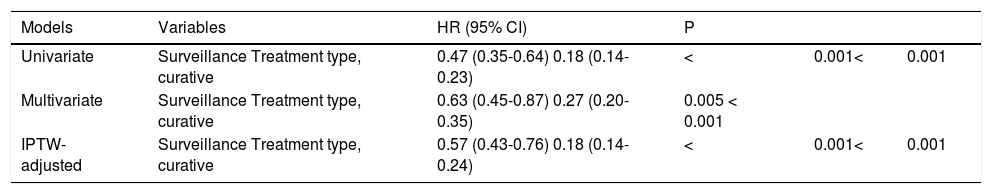
Next, to control for the inherent biases and confound-ers in the conventional Cox analysis, the effect of surveillance was estimated using the IPTW-adjusted Cox analysis. By the IPTW-adjusted Cox analysis, the magnitude of effect of surveillance remained the same, with HR (95% CI) of 0.57 (0.43-0.76), P < 0.001, thus confirming that HCC surveillance by ultrasound significantly reduced mortality of HCC patients (Table 3).
Effects of surveillance by conventional and IPTW-adjusted Cox regression analysis.
| Models | Variables | HR (95% CI) | P | ||
|---|---|---|---|---|---|
| Univariate | Surveillance Treatment type, curative | 0.47 (0.35-0.64) 0.18 (0.14-0.23) | < | 0.001< | 0.001 |
| Multivariate | Surveillance Treatment type, curative | 0.63 (0.45-0.87) 0.27 (0.20-0.35) | 0.005 < 0.001 | ||
| IPTW-adjusted | Surveillance Treatment type, curative | 0.57 (0.43-0.76) 0.18 (0.14-0.24) | < | 0.001< | 0.001 |
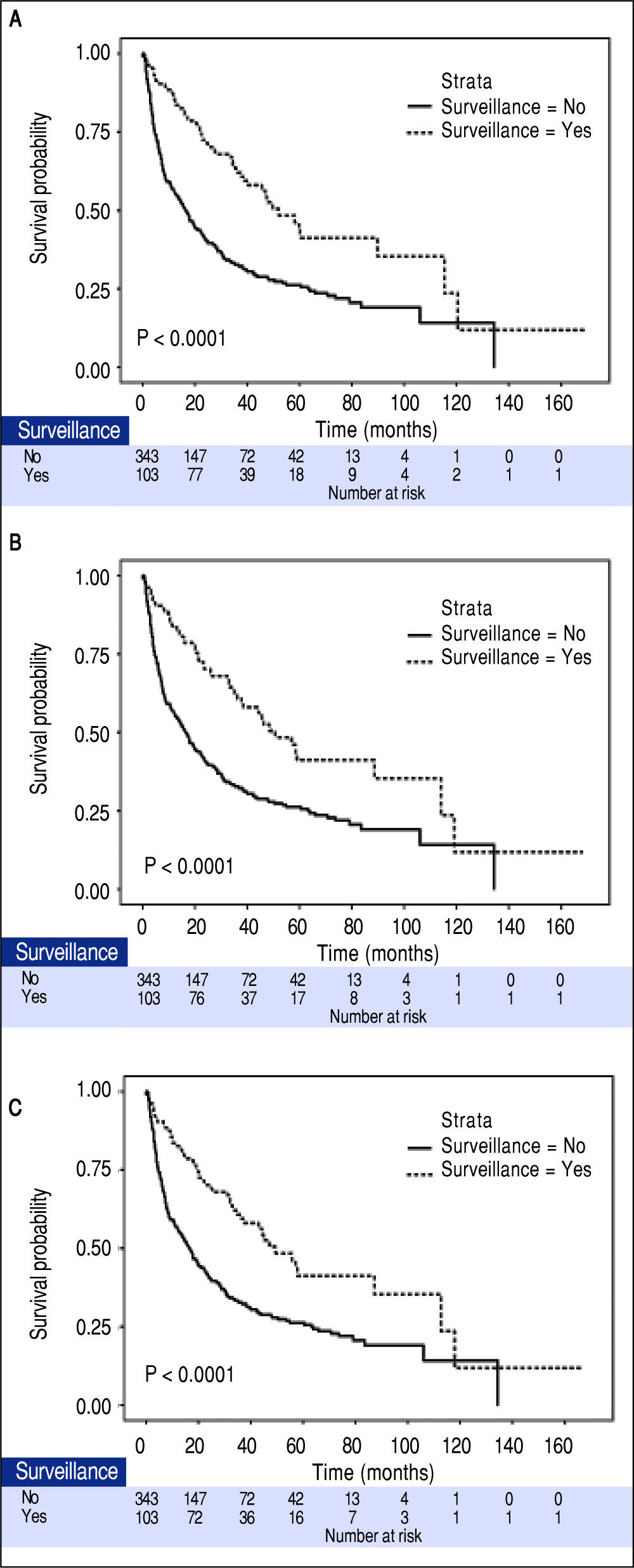
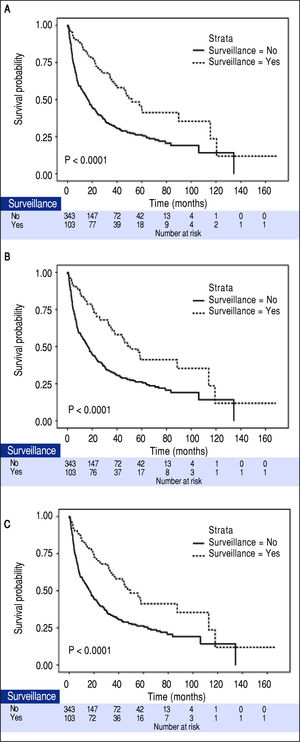
The median tumor size of the surveillance and non-surveillance groups were 2.5 and 6.0 cm, respectively. Based on the reported median tumor volume doubling time of 80-117 days, the tumor volume doubling times of 60, 90, and 120 days were selected for estimating the lead-time in our cohort. After adjusting for the lead-time, the surveillance group had better survival if the assumed tumor volume doubling time was less than 120 days (P = 0.04, Figure 2).
DiscussionThis study determined the impact of surveillance on the mortality of HCC patients by using only ultrasound. We found that the ultrasound surveillance was significantly associated with a 37% reduction in mortality. This finding was confirmed by the IPTW-based analysis. Other factors associated with better survival of HCC patients included serum albumin level and receiving potentially curative treatment. In contrast, factors associated with worse survival were MELD score, number of tumor and size, presence of portal vein thrombosis and distant metastasis. In addition, the rate of surveillance in this cohort was approximately 20%, highlighting the underutilization of the surveillance program for HCC in clinical practice.
Body of evidence has been consistently shown that surveillance is associated with detection of HCC at an early stage and an increased chance of receiving potentially curative treatment at the appropriate time.9,10,21 In the present study, the surveillance group had 2.4 times greater number of patients diagnosed with BCLC stage A. This finding was consistent with that reported in a systematic review showing that patients in the surveillance group were 3 times likely to be diagnosed with operable stage HCC.21 In this study, even though most patients under the surveillance program had HCCs detected at an early stage, yet approximately 20% of the patients under surveillance were diagnosed with HCC at an advanced stage. This could be due to a number of reasons. First, the performance of the ultrasound in detecting early HCC stage is not ideal, with a pooled sensitivity of only 63%.22 Combination of ultrasound and biomarkers, particularly with serial measurements of biomarkers, could potentially enhance the performance of the surveillance tool for detecting early HCC.23,24 Second, the interval of surveillance may affect the rate of early HCC detection as shown in a recent study reporting that the survival outcomes of HCC patients were better when the intervals of the ultrasound screening were shorter.25
We found that patients in the surveillance program had a significantly better survival than those who did not undergo surveillance, after accounting for the lead-time bias. A recent multi-center study from Italy showed that the lead-time bias contributed to a survival benefit of surveillance during the first 3 years after HCC diagnosis.15 After the third year with lead-time bias adjustment, the benefit of surveillance on patient survival was real, thus confirming its advantages.15
To date, only 2 randomized studies of HCC screening, surveillance and mortality were conducted yielding inconsistent results.11,12 A large cluster, randomized, controlled study from China showed that HCC screening with combined ultrasound and AFP every 6 months in patients with hepatitis B infection or history of chronic hepatitis was associated with a 37% reduction in mortality.11 But, the interpretation of the main finding of the Chinese study was limited by the fact that the cluster randomization was not taken into account in the analysis. On the other hand, another large randomized, controlled trial found that HCC surveillance every 6 months was not associated with reducing mortality of HCC. But the problem with the study was that they only used serum AFP as its surveillance tool.
Even though this study is not a randomized clinical trial, but we observed a 37% reduction in HCC mortality when ultrasound was used as a single tool for HCC surveillance as recommended by most widely accepted guidelines.3,6 The results were validated by the IPTW analysis by which the effect of the surveillance was estimated after adjustment for all baseline characteristics, thus the biases and confounders were minimized given the distribution of all baseline variables were equal in all study pa-tients.17,18,20 Our finding therefore strongly suggests that HCC surveillance reduces mortality of HCC patients.
But the problem here is not with the guidelines. As a matter of fact, various guidelines on the management of HCC universally recommend screening and surveillance for HCC in at-risk populations,3,6,7 unfortunately, the surveillance rate remains extremely low, as low as 20%, in most regions of the world,9,14,26-28 suggesting a gap between the recommended guidelines and the pattern of clinical practice. Likewise, in this study, the proportion of patients diagnosed with HCC through the surveillance program was only 23%. Alarmingly enough, a recent study from the U.S. reported that less than 20% of the cirrhotic patients who developed HCC had regular ultrasound surveillance within the 3 years prior to HCC diagnosis.26 Given the benefits of surveillance in improving survival and reducing mortality, it is important to enhance the surveillance rate by identifying barriers to surveillance, and developing strategies and effective intervention to overcome these obstacles.28 Suboptimal knowledge about the guidelines was identified as the most common barrier for HCC surveillance.7 For our cohort, reasons for the low surveillance rate require further investigation. A strategy to enhance surveillance rate is also critically needed, such as a simple clinical reminder system, which was shown to increase the surveillance rate by 50% in cirrhotic patients when used in a primary care setting.29
The strength of this study is that it was able to determine the benefits of ultrasound as a single surveillance tool, as recommended by the AASLD and EASL guidelines, in a real clinical setting. The benefit of ultrasound surveillance on mortality was estimated using the analysis method that minimized inherent biases in observational studies, thus the results are robust. However, there are some limitations in our study. Because the study was conducted in one of the largest referral centers in Thailand, it was subject to referral bias as shown by the number of patients with terminal stage HCC. We believe that most of the patients with terminal stage HCC probably received supportive treatment at their local hospitals so were not transferred to our center, given the abysmal prognosis for these patients. We did not investigate the impact of different intervals of ultrasound surveillance because, due to the retrospective nature of the study, the dates when the ultrasounds were performed were not always available in the medical records.
In conclusion, our study provides another piece of evidence that using ultrasound alone as a surveillance tool was significantly associated with a reduction in HCC mortality. However, the rate of utilization of HCC surveillance in clinical practice remains low. Given the benefits of surveillance in reducing the mortality, it is important to intervene and improve the surveillance rate in at-risk populations.
Abbreviations- •
AFP: alpha-fetoprotein.
- •
CI: Confidence interval.
- •
HCC: Hepatocellular carcinoma.
- •
HR: Hazard ratio.
- •
IPTW: Inverse probability of treatment weighting.
None.
Financial Disclosure And Conflict Of InterestNone.