Tenofovir disoproxil fumarate (TDF) is a nucleotide reverse transcriptase inhibitor indicated for treatment of patients with chronic hepatitis B virus (CHB) and human immunodeficiency virus (HIV) infections. Despite the good safety profile of the drug, Fanconi syndrome is a possible adverse reaction of TDF treatment, especially in HIV-infected patients. Only a few cases have been reported in patients with CHB-monoinfections. This report presents a case of a 58-year-old man with mild HBeAg-negative CHB who was exposed to TDF and developed drug-induced Fanconi syndrome. Renal dysfunction reverted after TDF discontinuation and a switch to entecavir, and viral replication remained suppressed. A literature review yielded six additional cases of TDF-induced Fanconi syndrome, all with risk factors for renal dysfunction despite the patients having normal glomerular filtration rates. We discuss the overall risk for Fanconi syndrome in CHB-monoinfected patients exposed to TDF and the importance of careful monitoring of glomerular and tubular functions even when pre-existing kidney disease is not present.
Tenofovir disoproxil fumarate (TDF) is commonly used as an effective antiviral agent in the treatment of chronic hepatitis B (CHB) or human immunodeficiency virus (HIV) infections and has favorable efficacy, safety and tolerability profiles.1–5 However, in HIV patients, renal proximal tubular dysfunction (PTD), including the development of Fanconi syndrome, is an uncommon adverse reaction of TDF.4–7 To date, only a few cases in CHB-monoinfected patients have been reported in the literature.8–11 Fanconi syndrome is characterized by impaired reabsorption of bicarbonate, phosphate, glucose, uric acid and amino acids with increased glycosuria, hypouricemia and/or aminoaciduria. Clinical manifestations include polyuria, polydipsia, dehydration, and osteomalacia.7
Here, we report cases of Fanconi syndrome after TDF exposure in CHB patients who had viral replication suppressed upon switching to entecavir (ETV).
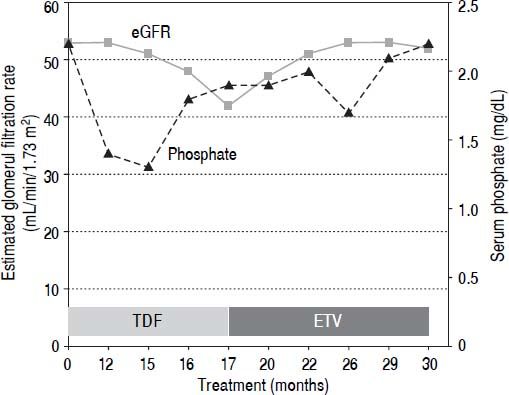
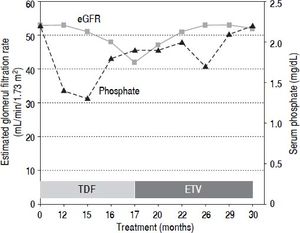
Case ReportA 58-year-old Caucasian man with mild HBeAg-negative CHB started TDF at 245 mg/day in August 2012. His medical history excluded the presence of diabetes or chronic kidney disease. He had a high body mass index (29.8 kg/m2) and was on beta-blocker treatment for arterial hypertension. At the first observation, in 1990, HBV serovirological status was HBeAg-negative and HBeAb-positive (e-minus CHB), HBV-DNA 27 pg/mL and alanine aminotransferase above 3 times the upper limit of the normal range. The patient failed treatment with interferon-α and received lamivudine (LAM) at a daily dose of 100 mg starting in 1997. After the development of LAM-resistance (M204I + L180M mutation), adefovir (ADV) at daily dose of 10 mg was added. During the treatment with ADV, his serum inorganic phosphorus and calcium concentrations were normal (range 2.4-3.0 and 8.4-9.5 mg/dL, respectively). In April 2012 (104 months after starting ADV), a slight increase in serum creatinine level (1.39 mg/ dL, normal range: 0.5-1.2 mg/dL) was observed. When he was switched to TDF in accordance with the 2012 European Society for the Study of the Liver (EASL) guide-lines,12 his serum creatinine was 1.46 mg/dL, and his estimated glomerular filtration rate (eGFR) by MDRD was 53 mL/min/1.73 m2 with normal dipstick urine analysis. Twelve months later, he developed severe progressive generalized bone pain and muscle weakness with functional impairment. On physical examination, he had normal muscle strength. Neurologic symptoms were excluded, and an electromyography examination did not identify neuromuscular disorder. Myoglobin and phospho-creatine kinase levels were normal (88 IU/L and 140 ng/mL, respectively), and serum creatinine was stable (1.45 mg/dL). A moderate hypophosphatemia (1.4 mg/dL) was noticed. Laboratory data confirmed alow serum inorganic phosphorus concentration and features of proximal renal tubule dysfunction, including the following: elevated fractional excretion of phosphate (TmPO4/GFR 0.66 mmol/L), proteinuria (960 mg/24 h) and normoglycemic glycosuria (500 mg/dL) without leukocyturia, bacteriuria or crystals. Serum bicarbonate was low (17.1 mEq/L), with metabolic acidosis (pH 7.26). Parathyroid hormone and calcium levels were within a normal range (42 pg/mL and 9.4 mg/mL, respectively); 25-hydroxyvitamin D levels were significantly low (15.6 ng/mL). Ultrasound and Doppler imaging did not detect renal macroscopic parenchymal and/or vascular abnormalities and did not identify changes in blood flow at the microvascular level. An increase of serum creatinine associated with the presence of tubulopathy (expressed by hypophosphatemia, hyperphosphaturia, proteinuria and normoglycemic-glycosuria) in a patient treated with TDF and without other causes of renal disease suggested a diagnosis of acquired Fanconi syndrome associated with TDF therapy. Because creatinine levels remained unchanged during the following months, the patient continued with TDF at a full dose, and vitamin D and calcium supplementation were added. In January 2014 (17 months after starting TDF), the patient’s glomerular function further deteriorated (creatinine 1.76 mg/dL, eGFR 42 mL/min/1.73 m2, phosphate 2.3 mg/dL), and he was managed by switching from TDF to ETV at daily dose of 0.5 mg (Figure 1). After five months, his symptoms resolved, and his laboratory abnormalities improved (creatinine 1.50 mg/dL, eGFR 51 mL/min/1.73 m2, phosphate 2.0 mg/dL). One year after ETV switching, his serum inorganic phosphorus concentration normalized (2.5 mg/dL), and his glomerular function was further improved (creatinine 1.46 mg/dL, eGFR 53 mL/min/1.73 m2), as well as his fractional excretion of phosphate (TmPO4/GFR 0.46 mmol/L). A decline of proteinuria (220 mg/24 h) and glycosuria (30 mg/dL) and a normalization of pH were also observed. Serum HBV-DNA remained persistently undetectable (<10 IU/mL), and serum alanine aminotransferase levels remained persistently within a normal range.
DiscussionWe have reported a case of Fanconi syndrome in a CHB patient taking TDF at a daily dose of 245 mg. In contrast with its high frequency in HIV patients, this complication rarely occurs among CHB patients after exposure to TDF. To date, TDF-induced Fanconi syndrome has been described in only seven CHB-monoinfected patients (Table 1). This difference may be due to the direct action of HIV infection on the kidney or to the combination of using TDF with antiretroviral drugs for the treatment of HIV infection.13 In patients with CHB, TDF-induced renal failure is not frequent or dose-dependent and is often reversible; hypophosphatemia can be secondary to decreased proximal tubular re-absorption induced by TDF exposure and/or vitamin D deficiency. As reported in EASL guidelines,12 in all patients with CHB who undergo treatment with TDF, serum creatinine, estimated glomerular filtration rate and serum phosphate levels may be monitored every 3 months in the first year and every 6 months thereafter, especially in those with a high risk of developing renal dysfunction. Furthermore, during therapy with TDF, the following is required:
- •
Avoid the use of other nephrotoxic drugs.
- •
Adjust drug dose for renal impairment.
- •
Verify the presence of proximal tubulopathy using calculation of TMPO4/GFR; and
- •
Consider discontinuation of TDF if phosphoremia is < 1 mg/dL or if the clearance of creatinine is < 50 mL/min.
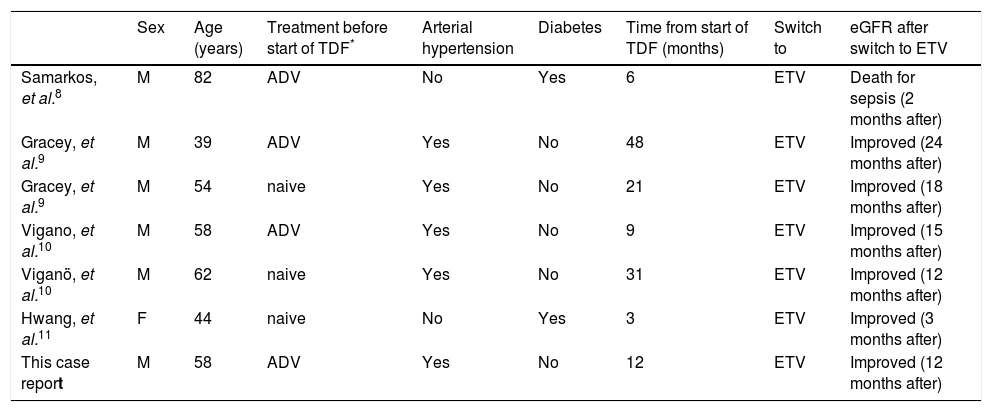
Review of characteristics of patients developed TDF-induced Fanconi syndrome.
| Sex | Age (years) | Treatment before start of TDF* | Arterial hypertension | Diabetes | Time from start of TDF (months) | Switch to | eGFR after switch to ETV | |
|---|---|---|---|---|---|---|---|---|
| Samarkos, et al.8 | M | 82 | ADV | No | Yes | 6 | ETV | Death for sepsis (2 months after) |
| Gracey, et al.9 | M | 39 | ADV | Yes | No | 48 | ETV | Improved (24 months after) |
| Gracey, et al.9 | M | 54 | naive | Yes | No | 21 | ETV | Improved (18 months after) |
| Vigano, et al.10 | M | 58 | ADV | Yes | No | 9 | ETV | Improved (15 months after) |
| Viganö, et al.10 | M | 62 | naive | Yes | No | 31 | ETV | Improved (12 months after) |
| Hwang, et al.11 | F | 44 | naive | No | Yes | 3 | ETV | Improved (3 months after) |
| This case report | M | 58 | ADV | Yes | No | 12 | ETV | Improved (12 months after) |
The characteristics of our patient confirmed previous observations,10 including that the onset of Fanconi syndrome seems to be related to patient age (fifties or sixties), male sex, HBeAg-negative status, prior treatment with ADV and the presence of risk factors for renal impairment (such as arterial hypertension and/or diabetes), which could predispose an individual to develop TDF-associated renal damage.
Except for patients who died after two months, all patients described in the literature fully recovered from Fanconi syndrome after switching to ETV treatment. Among these, none had previously developed LAM resistance. The choice of antiviral treatment in patients who develop LAM resistance and whether TDF should be discontinued is another important topic to be addressed. In fact, ETV is not recommended as a therapeutic regimen in patients with LAM resistance because the rates of genotypic resistance to ETV are much higher in these patients (i.e., there is a 51% 5-year cumulative probability when HBV-DNA is detectable).14 Nevertheless, no data are available about drug-resistance in patients who are switched to ETV when viral load is not detectable. After one year, HBV-DNA in our patient was still suppressed.
ConclusionIn conclusion, our case of Fanconi syndrome during treatment with TDF shows that careful monitoring of glomerular and tubular functions is recommended in patients with CHB who are receiving TDF even in the absence of an overt pre-existing kidney disease. Arterial hypertension or diabetes and/or previous exposure to ADV seem to be risk factors for renal dysfunction even in patients with normal eGFR at the start of TDF. A correct differential diagnosis to identify other possible causes of hypophosphatemia is recommended to avoid a premature and erroneous discontinuation of therapy with TDF.
Abbreviations- •
ADV: adefovir.
- •
CHB: chronic hepatitis B.
- •
EASL: European Society for the Study of the Liver.
- •
eGFR: estimated glomerular filtration rate.
- •
ETV: entecavir.
- •
HIV: human immunodeficiency virus.
- •
LAM: lamivudine.
- •
PTD: proximal tubular dysfunction.
- •
TDF: tenofovir.
- •
TmPO4/GFR: elevated fractional excretion ofphosphate.
The authors have nothing to disclose.