The aim of the present study was to investigate the significance of serum HBsAg levels in treatment cessation of nucleoside analogues (NAs) in patients with chronic hepatitis B (CHB) infection.
MethodsIn 158 CHB patients with long-term NAs treatment, 74 patients were in HBeAg negative and had a HBsAg level <1500IU/mL, 36 of whom were informed and consented to cease NAs. HBsAg, HBV DNA and liver function were examined in the 1st, 3rd, 6th, 9th and 12th month after treatment cessation.
ResultsThe sustained response rate was 88.89% (32/36) within one year after NAs cessation. Sub-group analysis was based on HBsAg levels of patients with NAs cessation, there was no relapse case in 11 patients whose HBsAg <50IU/mL, and the negative predictive value (NPV) was 100%. Seroconversion of HBsAg occurred in 3 patients. 2 patients from 21 cases whose HBsAg was between 50IU/mL and 1000IU/mL relapsed. 2 of 4 patients whose in HBsAg >1000IU/mL relapsed. HBsAg of patients with a sustained response decreased slowly. In contrast, HBsAg levels increased gradually in relapsed patients, and the increase of HBsAg was precedent to relapses of HBV DNA and ALT. Multivariate analysis suggested that only HBsAg level showed a close correlation with HBV DNA relapses. ROC curve analysis suggested that the increase of HBsAg level in the 3rd and 6th month after NAs cessation had a great predictive value for relapses.
ConclusionMonitoring of base line HBsAg level can predict outcomes of NAs cessation in HBeAg-negative chronic hepatitis B. HBsAg <50IU/mL has higher predictive values of better sustained responses in HBeAg-negative CHB patients.
Globally, rates of hepatitis B virus (HBV) infection in patients with cirrhosis and hepatocellular carcinoma (HCC) are approximately 30% and 45% respectively [1,2]. Among Chinese, HBV infection rate in these two groups is even higher, approaching 60% and 80%, respectively [3]. Nucleoside analogues (NAs) can effectively inhibit the replication of HBV DNA, restore liver function and reduce the risk of end-stage liver diseases. However, even in chronic hepatitis B (CHB) patients who have successfully achieved a virological response, patients often relapse after the cessation of the NAs treatment [4–7]. CHB patients require long-term or life-long treatment with NAs. For hepatitis B e-antigen (HBeAg)-negative CHB patients, the American Association for the Study of Liver Diseases (AASLD) [8] and the European Association for the Study of the Liver (EASL) [9] recommend long-term treatment with NAs until the patient is HBsAg negative. Although HBsAg negative conversion is an ideal target, it occurs only in a very small number of patients (<1%). The Asia-Pacific Association for the Study of Liver (APASL) maintain different criteria for the cessation of nucleoside analogue treatment [10]: (1) the therapy course lasts for at least 2 years; (2) HBV DNA monitoring has 3 or more negative results and interval time between two tests was at least 6 months; (3) if these conditions are met, even though HBsAg is not negatively converted, the treatment can still be ceased. Many scholars believe that quantitative detection of serum HBsAg can indirectly reflect the liver cccDNA levels, especially in CHB patients who are HBeAg positive [11–14]. The potential of serum HBsAg quantitation in guiding the cessation of NAs has become a hot topic in the field. Hadziyannis et al. and Jeng et al. have shown HBsAg quantitation is of great significance in the guidance of NAs cessation [15,16].
Previously studies showed HBsAg ≥1000IU/mL and HBV DNA ≥200IU/mL could be used to identify patients with high risk of reactivation [17], beside the baseline HBsAg level, the degree of HBsAg level decrease during treatment is also related to the outcome of NAs cessation [18,19]. In general, after long-term NAs therapy, HBV DNA can be suppressed below the limit of detection, and relapses of HBV DNA may occur after the treatment of NAs cessation. HBsAg quantification is considered as a method to predict relapses of HBV DNA. In view of these issues, the primary objective of this study was to investigate the sustained response of patients after the cessation of NAs by regular HBsAg quantitative detection. The secondary objective was to monitor the relationship between the dynamic changes of HBsAg and the HBV DNA and alanine aminotransferase (ALT) relapses after cessation of NAs treatment.
2Methods2.1Patients and study designThis retrospective study included 158 patients who were admitted to the Department of Infectious Diseases of the Second Affiliated Hospital of Chongqing Medical University between July 2014 and December 2015. These patients received long-term treatment of NAs and had good compliance. For patients whose HBeAg was positive at commencement of NAs treatment, their HBeAg should sero-converted, HBV DNA level should below detection limit and ALT should normalize for more than 1 year after NAs therapy. For patients whose HBeAg was negative, they had sustained responses after another 24-month treatment after HBV DNA levels were below the lower limit of detection and ALT levels recovered. There were 74 patients who met the cessation criteria of the Chronic hepatitis B Prevention and Treatment Guideline 2010 version [20] and had HBsAg levels <1500IU/mL. Of these, 36 patients were informed and consented to cease the NAs treatment. HBsAg quantitation, liver function, CHB markers and HBV DNA quantitation were tested in the 1st, 3rd, 6th, 9th and 12th month after treatment cessation. Another 38 patients were reluctant to stop NAs treatment for fear of biological relapses. Exclusion criteria included superinfection or co-infection with hepatitis A, C, E, HIV or other non-hepatotropic viruses, and complication with autoimmune liver disease, alcoholic liver disease, drug-induced liver disease, metabolic liver disease, liver cirrhosis and cancer. Sustained response was defined as HBV DNA <200IU/mL, HBeAb-positive and ALT
The quantitative detection of serum HBsAg was performed by using Roche Modular E170 electrochemiluminescence system. The lower limit of detection was <0.05IU/mL. Real-time fluorescence quantitative polymerase chain reaction (PCR) was used for HBV DNA quantitation (Shanghai Fuxing Bioengineering Co., Ltd., Shanghai, China) with a lower limit of detection <200IU/mL. The Roche Cobas E601 electrochemiluminescence platform was used to detect serum HBsAg, HBeAg and anti-HBe.
2.3Statistical analysisSPSS 20.0 (IBM Corp., Armonk, NY, USA) was used for data processing and statistical analysis. The rank sum test was used for inter-group comparison. Receiver-operating characteristic (ROC) curves were used to predict HBV DNA relapses using HBsAg quantitation. Cumulative relapse rate was calculated using the Kaplan–Meier method and the Log-Rank method was used for testing significance. The risk factors for relapses were analyzed by logistical regression. Since the minimum detection limit of HBsAg was less than 0.05IU/mL, it was denoted as 0.05 was below this value. Similarly, the lower limit of detection for HBV DNA was less than 200IU/mL, it was denoted as 200 when below this value. Statistical significance was defined as P<0.05.
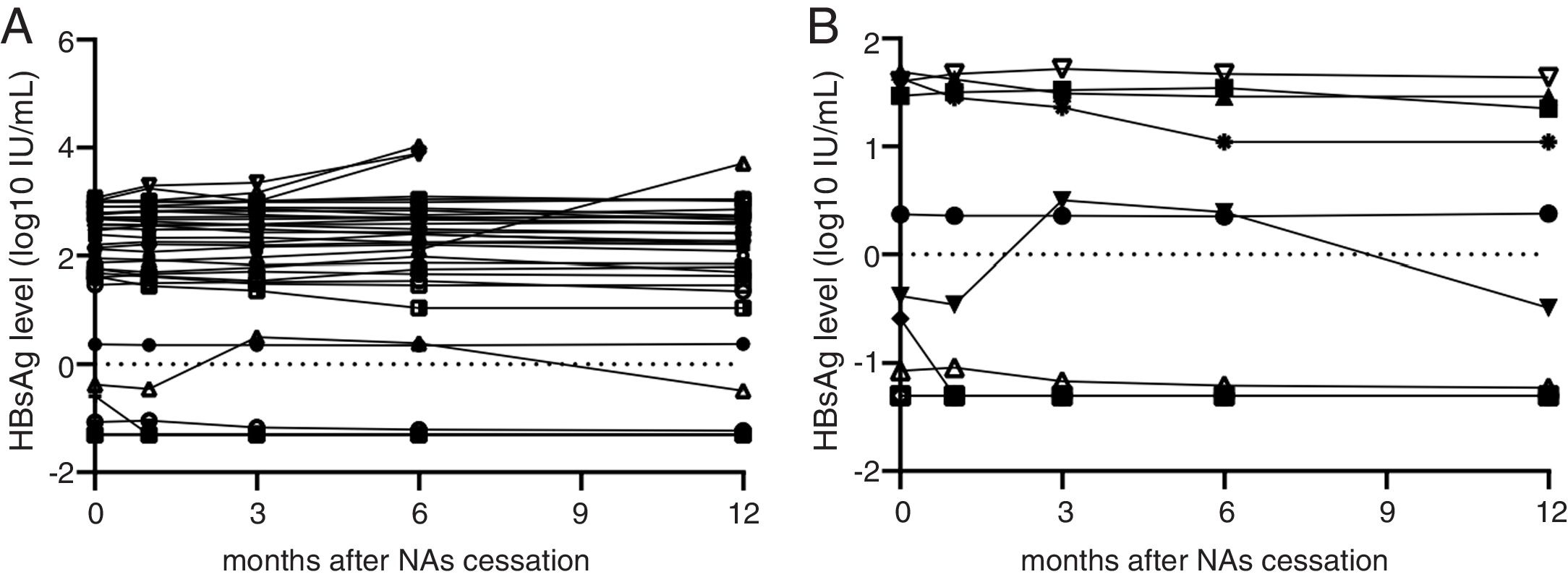
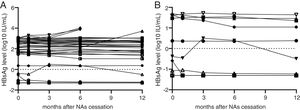
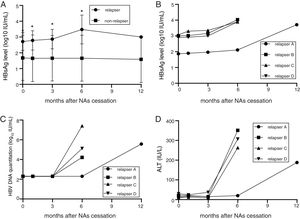
3Results3.1General information about the CHB patients158 CHB patients met the cessation criteria. These patients received long-term NAs treatment, were serum HBV DNA-negative, serum HBeAb-positive, ALT-normal and displayed no signs of cirrhosis when enrolled. 74 patients had HBsAg <1500IU/mL, among them, 36 patients (26 males and 10 females) consented to cease the administration of NAs. 26 patients were in HBeAg positive and 10 patients were in HBeAg negative status at the beginning of treatment. The age range was 17–67 years old and the average age was 41 years old. The numbers of the patients who took lamivudine (LAM), adefovir (ADV), telbivudine (LdT) and entecavir (ETV) were 7, 20, 4 and 5, respectively (one patient had LAM resistance history). The duration of the administration lasted 24–120 months and the average duration was 42 months. In the 12-month follow-up period after the NAs cessation, 32 patients (88.89%) achieved sustained responses. HBsAg seroconversion took place in 3 patients (3/36, 2 males and 1 female; age: 48–67 years old; 2 cases were spontaneous and one was immunized with HBV vaccine, 2 patients took ADV and another took LAM, with treatments lasting for 72–91 months) whose HBsAg levels were all below 1IU/mL at the time of cessation. Four patients experienced virological and clinical relapses within 6 months of NAs cessation (4/36, three males and one female, age: 23–51 years old, 1 HBeAg positive and 3 HBeAg negative at the beginning of treatment), two of which received ADV and the other two patients received ETV treatment (Table 1 and Fig. 1A). 3 of the 4 patients suffering relapses had elevated ALT levels greater than 5× ULN (251, 207, 263), 1 patient showed elevated ALT greater than 4× ULN (188). All these 4 patients achieved good virological responses after retreatment with ETV or TDF for 3 months, without any serious adverse events reported. In patients who achieved sustained responses after the cessation of NAs, the HBsAg levels gradually decreased during the follow-up period. However, in relapsing patients, HBsAg levels showed a significantly increasing trend. The minimum increase was 2.5-fold from the time of NAs cessation to relapse, and the maximum increase was 66.6-fold.
General characteristics of 36 CHB patients with discontinued NAs therapy.
| Variables | The HBsAg level at the time of cessation (IU/mL) | ||||
|---|---|---|---|---|---|
| <50 | 50–100 | 100–500 | 500–1000 | 1000–1500 | |
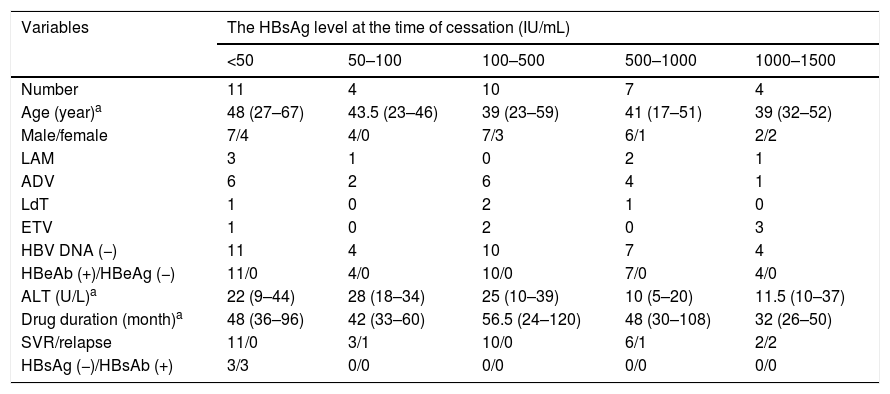
| Number | 11 | 4 | 10 | 7 | 4 |
| Age (year)a | 48 (27–67) | 43.5 (23–46) | 39 (23–59) | 41 (17–51) | 39 (32–52) |
| Male/female | 7/4 | 4/0 | 7/3 | 6/1 | 2/2 |
| LAM | 3 | 1 | 0 | 2 | 1 |
| ADV | 6 | 2 | 6 | 4 | 1 |
| LdT | 1 | 0 | 2 | 1 | 0 |
| ETV | 1 | 0 | 2 | 0 | 3 |
| HBV DNA (−) | 11 | 4 | 10 | 7 | 4 |
| HBeAb (+)/HBeAg (−) | 11/0 | 4/0 | 10/0 | 7/0 | 4/0 |
| ALT (U/L)a | 22 (9–44) | 28 (18–34) | 25 (10–39) | 10 (5–20) | 11.5 (10–37) |
| Drug duration (month)a | 48 (36–96) | 42 (33–60) | 56.5 (24–120) | 48 (30–108) | 32 (26–50) |
| SVR/relapse | 11/0 | 3/1 | 10/0 | 6/1 | 2/2 |
| HBsAg (−)/HBsAb (+) | 3/3 | 0/0 | 0/0 | 0/0 | 0/0 |
LAM: lamivudine, ADV: adefovir dipivoxil, LdT: telbivudine, ETV: entecavir, ALT: alanine aminotransferase, SVR: sustained virological response.
HBsAg levels of patients who agreed to NAs cessation. (A) HBsAg levels of 36 patients who consent to discontinued NAs therapy from cessation point to 12th month. Four patients’ HBsAg levels greatly increased at 3rd, 6th and 12th month. Other patients’ HBsAg levels were stable or decreased gradually. The triangular polyline represents overlap in 3 patients whose HBsAg <0.05IU/mL. (B) HBsAg variations of 11 patients whose HBsAg level less than 50IU/mL. HBsAg levels of this cohort were all stable or decreased gradually. The triangular polyline represents overlap in 3 patients whose HBsAg <0.05IU/mL.
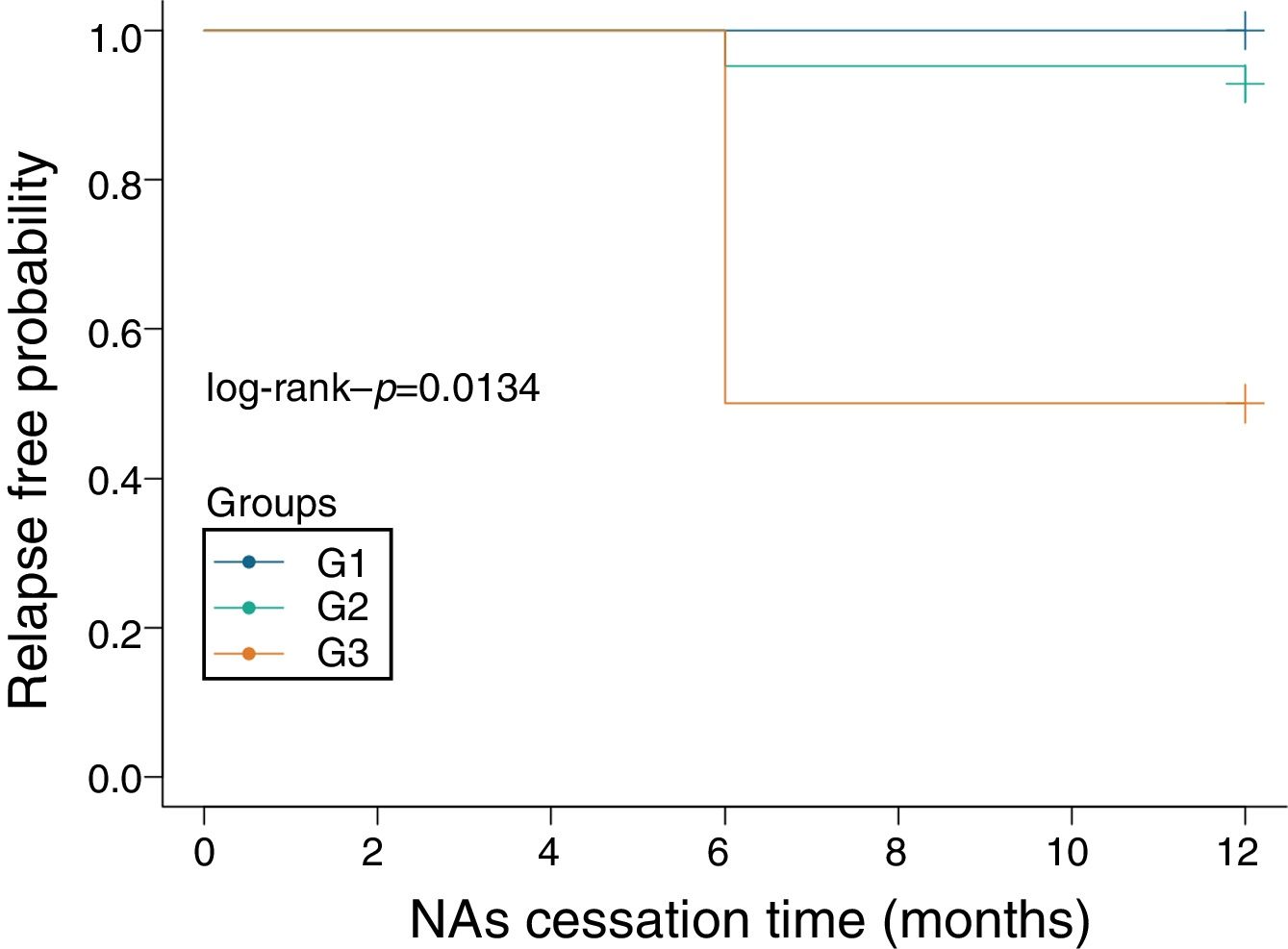
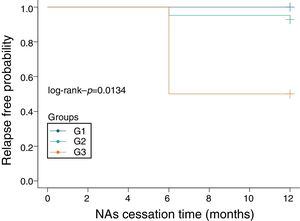
The patients were divided into three sub-groups according to their HBsAg level at the time of NAs treatment cessation: <50IU/mL, 50–1000IU/mL and 1000–1500IU/mL. In 11 patients with HBsAg <50IU/mL, there was no relapse cases and the negative predictive value of this factor (NPV) was 100%. None of these patients had NA-resistance history, The number of patients were treated with ADV, LAM, LdT and ETV is 6, 3, 1 and 1, respectively, the median treatment duration was 72 months (24–97 months). The median value of serum HBsAg quantitation at the time of cessation was 0.421IU/mL (IQR: 0.065–48.640; converted into logarithm: −1.19–1.54log10IU/mL), while this value at the 12 months after cessation was 0.322 (IQR: 0.050–15.840; converted into logarithm: −1.30–1.20log10IU/mL), which meant HBsAg levels showed a slow decrease after treatment cessation (Fig. 1B). In 21 patients whose HBsAg level was between 50IU/mL and 1000IU/mL, there were 2 relapse cases (HBsAg level of one case was 73.59IU/mL, another was 786IU/mL), giving a cumulative relapse rate of 9.52%. In 4 patients whose HBsAg level was between 1000IU/mL and 1500IU/mL, there were 2 relapse cases (HBsAg level of one case was 1031IU/mL, another was 1161IU/mL), giving a cumulative relapse rate of 50%. Cumulative relapse rate of patients with HBsAg level <50IU/mL was obviously lower that other two groups (P=0.013, Fig. 2). Univariate and multivariate analyses suggested that age, sex, drug type, treatment duration and ALT levels were not correlated with relapses. Only HBsAg level at the time of cessation showed close correlation with relapses (Table 2).
Predictors of virological relapses based on multivariate analyses in the patients with discontinuation of nucleoside analogues.
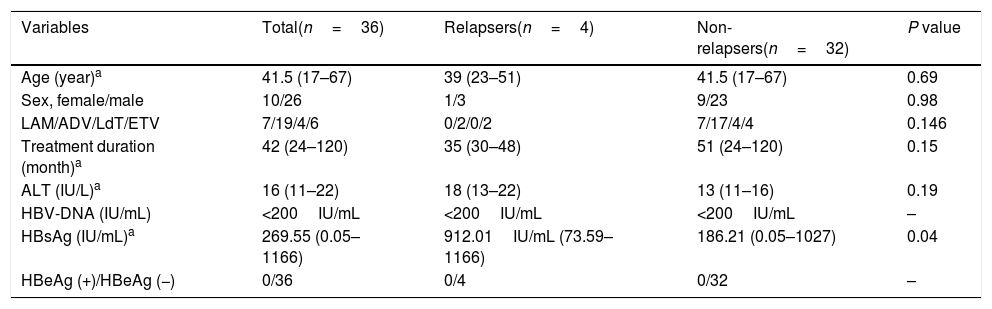
| Variables | Total(n=36) | Relapsers(n=4) | Non-relapsers(n=32) | P value |
|---|---|---|---|---|
| Age (year)a | 41.5 (17–67) | 39 (23–51) | 41.5 (17–67) | 0.69 |
| Sex, female/male | 10/26 | 1/3 | 9/23 | 0.98 |
| LAM/ADV/LdT/ETV | 7/19/4/6 | 0/2/0/2 | 7/17/4/4 | 0.146 |
| Treatment duration (month)a | 42 (24–120) | 35 (30–48) | 51 (24–120) | 0.15 |
| ALT (IU/L)a | 16 (11–22) | 18 (13–22) | 13 (11–16) | 0.19 |
| HBV-DNA (IU/mL) | <200IU/mL | <200IU/mL | <200IU/mL | – |
| HBsAg (IU/mL)a | 269.55 (0.05–1166) | 912.01IU/mL (73.59–1166) | 186.21 (0.05–1027) | 0.04 |
| HBeAg (+)/HBeAg (−) | 0/36 | 0/4 | 0/32 | – |
LAM: lamivudine, ADV: adefovir dipivoxil, LdT: telbivudine, ETV: entecavir, ALT: alanine aminotransferase, ULN: upper limit of normal.
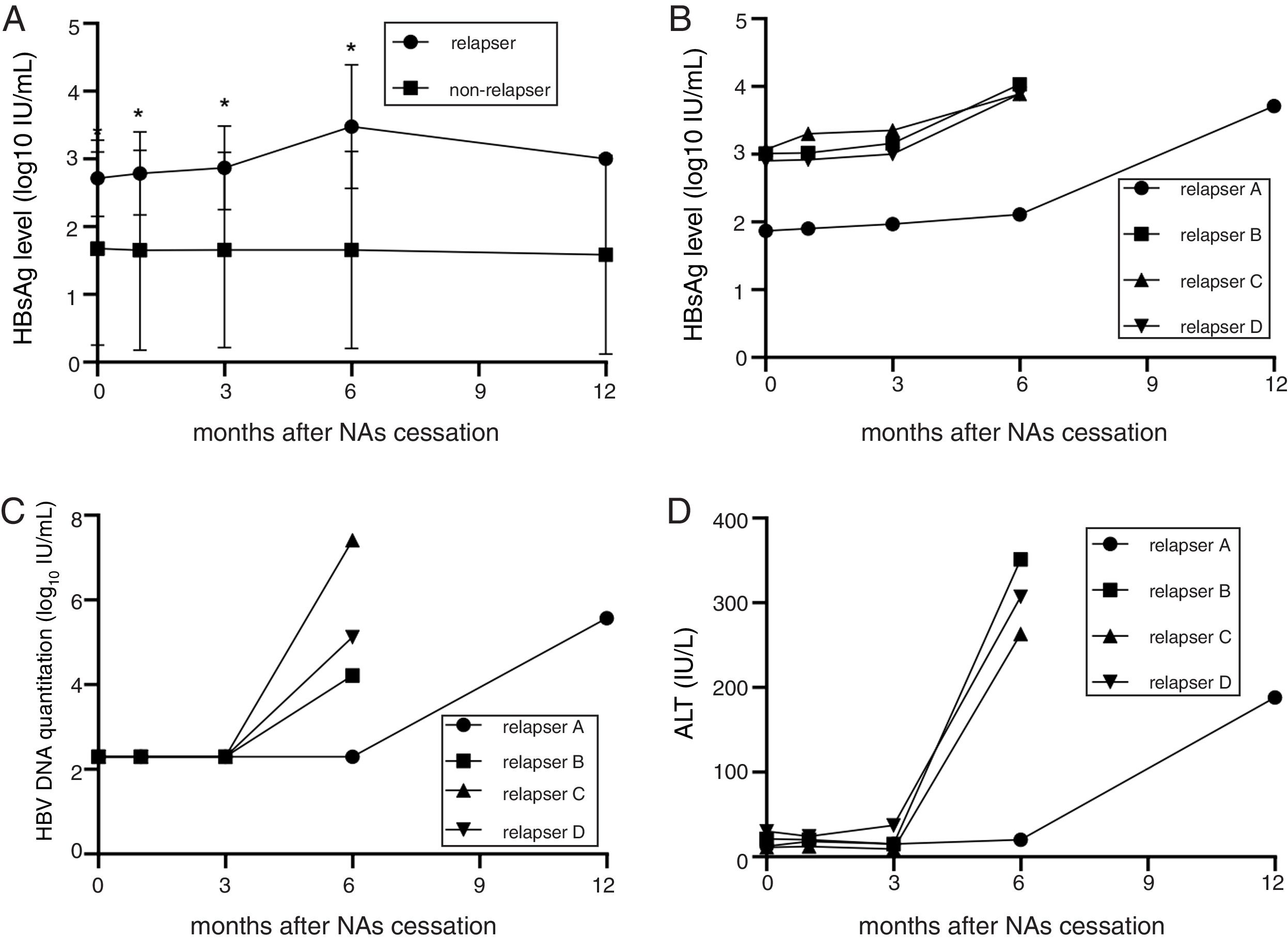
Thirty-six patients were divided into the relapse group (n=4) and the non-relapse group (n=32). The HBsAg levels of the patients in the relapse group were higher than in the non-relapse group (median value: 912.010IU/mL vs 186.210IU/mL; converted into logarithm: 2.27log10IU/mL vs 2.96log10IU/mL, P<0.05). During the follow-up, the HBsAg levels of the patients in the non-relapse group showed a slow decrease. Meanwhile, the level of HBsAg in the relapse group began to increase at 1st month after drug cessation, increased significantly at 3rd month (2.5–66.6 folds). HBV DNA and ALT displayed apparent increase at 3 months after drug cessation, that were obviously slower than the elevation of HBsAg (Fig. 3A–D). HBsAg flare was precedent to the virological and clinical relapses.
HBsAg level of non-relapse group and relapse group. (A) The HBsAg level of the patients in the relapses group was higher than non-relapses group at each monitoring point (P<0.05). During the follow-up period, the HBsAg level of the patients in the non-relapses group showed a slow decrease trend which decreased by 1% during 12-month follow-up period; on the contrary, the HBsAg level of the patients in the relapses group began to increase one month late after cessation and significantly increased from the 3rd month. The minimum increase fold was 2.5 folds and the maximum was 66.6 folds. (B) A line chart of the HBsAg quantification of relapsers at each monitoring point after NAs cessation. (C) A line chart of the HBV DNA quantification of relapsers at each monitoring point after NAs cessation. (D) A line chart of the ALT levels of relapsers at each monitoring point after NAs cessation. The ALT changes of relapsers after NAs cessation. Asterisk represented P<0.05.
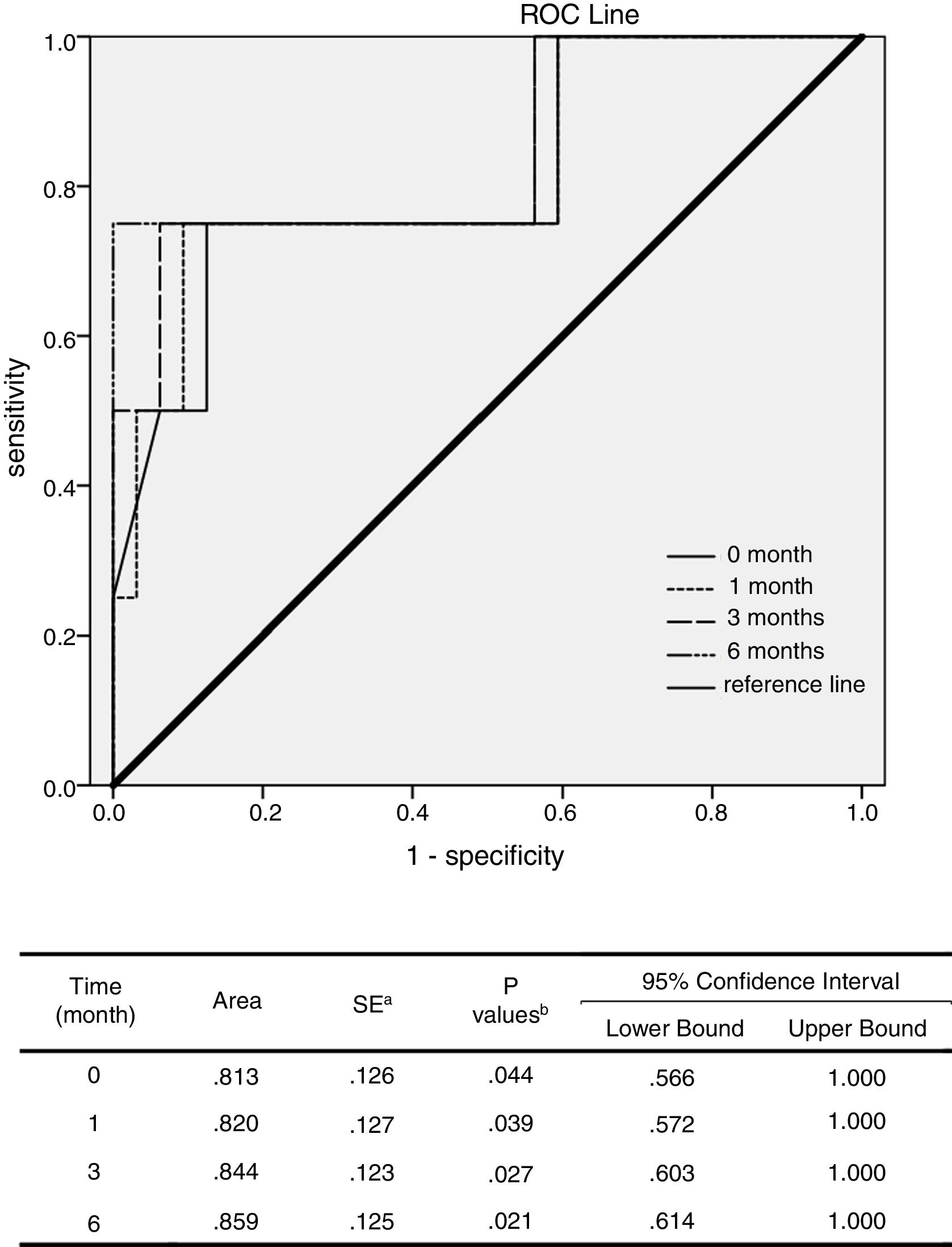
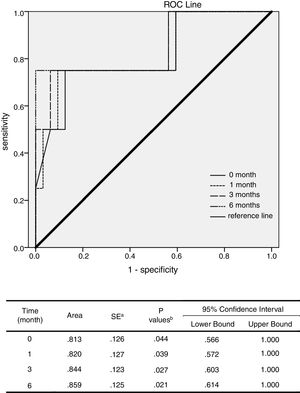
ROC analysis was performed to evaluate the potential relapse prediction role of serum HBsAg levels in the 1st, 3rd, 6th and 12th month after NAs cessation. The results showed that the AUCs of the 3rd and 6th month after the cessation were larger than the time of cessation or the 1st month (P<0.05). Thereby, the increase of serum HBsAg at 3rd and 6th month after treatment cessation probable had more important predictive value for relapses than 1st month (P<0.05, Fig. 4).
ROC analysis of potential relapse prediction role of serum HBsAg levels. ROC analysis was performed to evaluate the potential of serum HBsAg levels in the 1st, 3rd, 6th and 12th month after the cessation of NAs to predict recurrence. The results showed that the AUCs of the 3rd an 6th month after the cessation were significantly larger than the time of cessation as well as the 1st month (P<0.05). It was thereby supposed that the increase of serum HBsAg levels three and six months late after treatment cessation have important predictive value for recurrence.
NAs have a convenient dosing schedule and few side effects. However, even though patients have already achieved sustained virological responses (SVR), cccDNA is still present in the nuclei of infected liver cells. CHB patients have a high relapse rate after the cessation of NAs treatment [4–7]. HBsAg was validated as a tool for monitoring hepatitis B disease activity and determining hepatitis B disease phase [21], and was also considered as an important serum maker in immune evasion and disease progression [22]. In recent years, there have been studies investigating the value of HBsAg quantitation in guiding the cessation of NA treatment. For example, Hadziyannis et al. [15] and Jeng et al. [16] have shown that not all CHB patients require long-term oral antiviral treatment with NAs, and that HBsAg quantitation has great value in guiding the cessation of the treatment of NAs. Chan et al. [23] showed that, compared with HBV DNA, serum HBsAg levels in CHB patients were more likely to reflect SVR 12 months after the cessation of the treatment with LAM. Moreover, the HBsAg level at the time of cessation can also predict the seroconversion of HBsAg after cessation. In the present study, 32 of 36 CHB patients, who took NAs for average 42 months and had HBsAg level <1500IU/mL, achieved an sustained response after NAs cessation (12 months), also proved HBsAg level at NAs cessation point is a good indicator. There were also some different opinions regarding serum HBsAg in guiding the cessation of NAs. Some studies [17,18] suggest that the baseline HBsAg level, the degree of the decease of HBsAg during the treatment, and the HBsAg level at the time of cessation cannot effectively predict virological relapses one year after cessation. However, a recent review [24] indicates many studies show that the HBsAg level at the cessation of NAs is significantly correlated with relapses after cessation. Lower HBsAg levels, especially less than 100–300IU/mL, correlated with lower relapse rates after stopping NAs therapy and higher clearance rates of HBsAg [19,23,25,26], and were associated with the degree of HBsAg decline [23]. Our study showed, in 11 patients with a <50IU/mL HBsAg level at the time of cessation, there was no relapse cases and the NPV was 100%. Meanwhile, three of 11 patients achieved HBsAg seroconversion which represented clinical cure. In 21 patients with 50–1000IU/mL HBsAg levels at the time of cessation, 2 patients experienced clinical relapses. There were significant differences in the cumulative relapse rates among the three sub-groups (P=0.022). In 21 patients whose HBsAg level was between 50 and 1000IU/mL, 2 patients experienced clinical relapses, the relapse rate of this group is lower than previously study [27], which may attribute to the younger age of this subgroup (41.16±2.25 years). Lower serum HBsAg levels at the time point of cessation yielded lower risks for relapse. The serum HBsAg levels of 4 relapsed patients at the time of cessation were 73.59IU/mL, 786IU/mL, 1031IU/mL and 1166IU/mL. Chen et al. [26] found the relapse rate in CHB patients with LAM-resistance history was significantly higher than in patients without such substitution, similar with that, clinical relapses occurred in one patient with a history of LAM-resistance despite having a lower HBsAg level (73.59IU/mL) in our study. The quantitative analysis of HBsAg partly reflects the replication of HBV DNA, which, combined with HBV DNA, can better judge the relapse after NAs cessation.
A recent study [28] investigated the outcomes of CHB patients who ceased the ETV treatment, suggested that serum HBsAg levels at the 6th month after cessation were closely correlated with late relapses of the patients. In our study, HBsAg levels of the patients who did not relapse after NAs cessation showed a gradual decreasing trend, while the HBsAg levels of the relapsed patients increased significantly, the minimum increase fold was 2.5-fold. The increase of HBsAg levels at 3rd months and 6th months after treatment cessation had important predictive value for relapse after treatment cessation (P<0.05).
Beside the HBsAg, HBV core related antigen (HBcrAg), was considered as a novel serological marker of HBV replication. HBcrAg consists of 3 species of related proteins, including hepatitis B core antigen (HBcAg), hepatitis B e antigen (HBeAg), and truncated 22kDa precore protein (p22cr) [29]. Previously study reported serum HBcrAg correlated with HBV cccDNA transcriptional activity in CHB patients [30]. Furthermore, compare with HBV RNA and HBsAg, there was study reported serum HbcrAg correlates with cccDNA levels better than HBV RNA and HBsAg, irrespective of HBeAg status [31]. For controversy, in Caucasian patients with HBeAg negative CHB, HBcrAg was not superior to HBV DNA and qHBsAg in predicting response during PEG-IFN treatment [32]. In addition, HBcrAg quantitation is an enzyme immunoassay (EIA) [33], which is not certified by CFDA till now, limited its clinical application. HBsAg quantitation is still an important assay to reflect HBV replication and monitor other clinical responses [34,35].
We just observed clinical character of NAs cessation in 1 year, which may not fully describe the final ending of NAs cessation. With the help of colleague of informatics department, we checked whether there was any laboratory result update of those 32 sustain response patients. Luckily, 20 of these 32 patients had visit records after 1 year observation windows, 8 patients belong group1 (baseline HBsAg<50IU/mL), 11 patients belongs to group 2 (HBsAg>50IU/mL<1000IU/mL) and 1 patient belong to group 3 (HBsAg>1000IU/mL). We extracted their clinical laboratory results in the 2nd year, and found 1 patient belonging to group 1 and 1 patient belonging to group 2 showed HBV DNA relapse, 1 patients belonging to group 2 showed clinical relapse.
There are some limitations in the present study. Firstly, this study was a retrospective study, patients came from a single centre, the sample size was small, without genotype data and just included Chinese CHB patients. Additionally, we just observed dynamic characters of NAs cessation in 1 year, extended follow up-times would benefit the conclusion of the present study. Another limitation of this study is the number of relapsed patients is not comparable with the number of sustained patients (4 vs 32). Finally, in our study, majority patients achieved satisfactory virological and biochemical responses by using LAM or ADV, both of them were not recommended for first-line treatment, anyway, information from this study still provide reference value for NAs treatment cessation.
From our study, serum HBsAg <50IU/mL is a high value predictor for safe NAs cessation, we also observed the increasement of HBsAg after NAs cessation indicated higher risk of virological and biochemical relapses.AbbreviationsHBV
hepatitis B virus
NAsnucleoside analogues
CHBchronic B virus
HBsAghepatitis B surface antigen
HBeAghepatitis B e antigen
HBcrAghepatitis B core related antigen
NPVnegative predictive value
ROCreceiver operating characteristic
HCChepatocellular carcinoma
ALTalanine transaminase
LAMlamivudine
ADVadefovir
LdTtelbivudine
ETVentecavir
ULNupper limits of normal
Conflict of interestNone.
Informed consentAll included patients in the study signed written informed consent forms about the NAs cessation prior to their inclusion in the study. The study conformed to the Helsinki Declaration of 1975 and its later amendments.
This work was sponsored by a grant from the National Natural Science Foundation of China (No. 81270503), and had no financial and personal relationships with other people or organizations.