Background. Cirrhotic patients are prone to having bacterial infections due to impaired innate immunity. This nationwide population-based study aimed to identify the effect of bacterial infections on the mortality of the cirrhotic patients with esophageal variceal bleeding (EVB).
Material and methods. The Taiwan National Health Insurance Database was used to collect data about the cirrhotic patients receiving endoscopic procedures for EVB between January 1, 2004 and December 31, 2004. The enrolled patients were followed up individually for one year to identify their 6-week and 1-year mortalities.
Results. Of the 2,053 cirrhotic patients with EVB, 318 (15.5 %) were diagnosed with bacterial infections. Compared to non-infection group, the adjusted hazard rations (HRs) of bacterial infection for 6-week and 1-year mortalities were 2.69 (2.06-3.52) and 1.89 (1.56-2.28), respectively. Compared to non-infection group, the HRs of pneumonia, spontaneous bacterial peritonitis, urinary tract infection, and sepsis without specific focus (SWSF) were 3.54, 1.91, 1.04, and 3.95 for 6-week mortality, and 3.18, 1.52, 1.15, and 2.23 for 1-year mortality of cirrhotic patients with EVB.
Conclusions. In cirrhotic patients with EVB, bacterial infections increase 2.7 folds of 6-week mortality and 1.9 folds of 1-year mortality. Of all infections, pneumonia and SWSF contributed higher risks for mortality.
Esophageal variceal bleeding (EVB) is a frequent and potentially fatal complication in cirrhotic patients with portal hypertension. Early mortality after cessation of initial bleeding is significantly associated with bacterial infection and re-bleeding.1 In a previous meta-analysis, bacterial infections were present in up to 20% of patients hospitalized with gastrointestinal bleeding.1 Bacterial infections, especially hospital-acquired ones, occur more frequently in cirrhotic patients admitted for gastrointestinal bleeding than for other causes.2 In addition, bacterial infections aggravate EVB by increasing sinusoidal pressure, altering hemostasis, and endotoxemia, which are triggers for EVB in cirrhotic patients.3
According to previous studies, infections increase 4-fold of mortality in cirrhotic patients.4 The most common infections were urinary tract infection (UTI), spontaneous bacterial peritonitis (SBP), pneumonia, and sepsis without specific focus (SWSF).4–7 Bacterial infection is proved to be associated with higher mortality and re-bleeding rate in cirrhotic patients with EVB.8 However, there is a lack of population-based data to identify the exact effects of variable infections on the mortality of cirrhotic patients with EVB; therefore, we used Taiwan National Health Insurance Database to collect enough cirrhotic patients with EVB to do this job.
Material and MethodsDatabaseThe database used in this study was Taiwan’s National Health Insurance research database which was established and is maintained by the Taiwan National Health Insurance Bureau and the National Health Research Institute. The Taiwan National Health Insurance program was developed in 1995 to include all citizens residing in Taiwan, and now covers more than 95% of Taiwan’s population. The privacy of health care providers and patients was protected, and the study protocol was evaluated and approved by the National Health Research Institute (application and agreement number: 100101).
Study sampleThis retrospective study enrolled cases who were discharged with a diagnosis of cirrhosis (International Classification of Diseases, 9th Revision, Clinical Modification codes 571.5, or 571.2 in the database) (ICD-9-CM) between January 1, 2004 and December 31, 2004. In the cases with multiple hospitalizations, only the first episode was included. Patients who were admitted before January 1, 2004 were not included. Patients < 30 years old were not included because of the natural course of liver cirrhosis was less likely. Because of the different mechanisms for liver cirrhosis, we excluded most cases with biliary cirrhosis (ICD-9-CM code 571.6). Of these, patients were enrolled only if they had both esophageal variceal bleeding (EVB) (ICD-9-CM codes 456.0, or 456.20) and received an endoscopic procedure to control bleeding (ICD-9 v3 procedure code 42.33).
A total of 2,053 cirrhotic patients with EVB who had received endoscopic procedures to control bleeding were enrolled. The infectious diseases included in our study were pneumonia (ICD-9-CM codes 481-486, without 484), sepsis (ICD-9-CM codes 038, 020.0, 790.7, or 112.81), urinary tract infection (UTI) (ICD-9-CM codes 590.1, 595.0, 595.9 or 599.0), biliary tract infection (ICD-9-CM code 571.6), empyema (ICD-9-CM code 510), cellulitis (ICD-9-CM code s681 or 682), necrotizing fasciitis (ICD-9-CM code 728.86), central nervous system (CNS) infection (including bacterial meningitis or brain abscess: ICD-9-CM codes 324 or 320), septic arthritis, (ICD-9-CM code 711), infective endocarditis (ICD-9-CM code 421), perianal abscess (ICD-9-CM code 566), liver abscess (ICD-9-CM code 572.0) and spontaneous bacterial peritonitis (SBP). As in previous studies, to be diagnosed with SBP, a patient had to have ICD-9-CM diagnostic codes for both cirrhosis and peritonitis (567.2, 567.8, or 567.9). In order to exclude secondary peritonitis, patients with other causes of peritonitis (such as appendicitis, hollow organ or biliary tract perforation, or ischemic bowel disease), as well as those having an additional procedure code for abdominal surgery, were not included.9–11 We checked other diagnostic codes to identify the focus for sepsis in the patients with diagnostic codes for sepsis (ICD-9-CM codes 038, 020.0, 790.7, or 112.81). When the patients did not have other diagnostic codes for infectious focus, they were considered to have sepsis without specific focus (SWSF).
Statistical analysesThe SPSS statistical package (SPSS System for Windows, version 13.0) was used to perform the analyses in this study. Cox proportional hazard regressions were performed to evaluate mortality as a result of cirrhosis with all infectious diseases compared to cirrhosis with EVB and no infectious disease. Comorbid diseases were considered if the condition was noted at the time of admission. Comorbid medical disorders included alcoholism (ICD-9-CM codes 291, 303, 305.00-305.03, 571.0-571.3), hepatocellular carcinoma (HCC) (ICD-9-CM code 155.0), renal failure (ICD-9-CM codes 582, 585, 586, or 572.4), hepatic encephalopathy (ICD-9-CM code: 572.2), and ascites (ICD-9-CM code 789.5, or procedure code 54.91). Chi square tests were used to compare categorical variables and Student’s t test was used to compare continuous variables. Hazard ratios (HRs) and 95% confidence intervals (CI) using a significance level of 0.05 were also calculated.
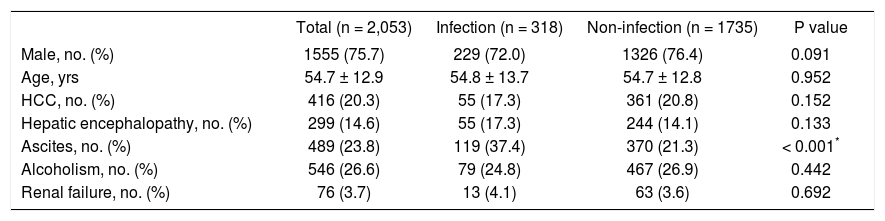
ResultsBetween January 1, 2004 and December 31, 2004, a total of 2,053 cirrhotic patients with EVB received endoscopic procedure to control bleeding. Their mean age was 54.7 ± 12.9 years and 1,555 (75.7%) were male. Of the total, 318 (15.5%) were diagnosed with bacterial infectious diseases. In the group with infection, the mean age was 54.8 ± 13.7 years, and in the group without infection, the mean age was 54.7 ± 12.8 years (P = 0.952). The demographic characteristics of cirrhotic patients with and without infectious diseases are shown in table 1. The overall 6-week and 1-year mortality rates for patients with EVB bleeding were 12.8% (262/2053) and 32.0% (656/2053), respectively. The overall 6-week mortality rates for patients with and without bacterial infections were 25.5% (81/318) and 10.4% (181/ 1735), respectively (P < 0.001). The hazard ratios of the predisposing factors after the Cox regression analysis for 6-week and 1-year mortality are shown in table 2. After adjusting for patients’ gender, age, HCC, hepatic encephalopathy, ascites, alcoholism, and renal failure, the adjusted HR for 6-week mortality with all infectious diseases compared to noninfectious diseases was 2.69 (95% CI, 2.06-3.52; P < 0.001).
Demographic characteristics of patients with esophageal variceal bleeding with and without infectious diseases (n = 2,053).
| Total (n = 2,053) | Infection (n = 318) | Non-infection (n = 1735) | P value | |
|---|---|---|---|---|
| Male, no. (%) | 1555 (75.7) | 229 (72.0) | 1326 (76.4) | 0.091 |
| Age, yrs | 54.7 ± 12.9 | 54.8 ± 13.7 | 54.7 ± 12.8 | 0.952 |
| HCC, no. (%) | 416 (20.3) | 55 (17.3) | 361 (20.8) | 0.152 |
| Hepatic encephalopathy, no. (%) | 299 (14.6) | 55 (17.3) | 244 (14.1) | 0.133 |
| Ascites, no. (%) | 489 (23.8) | 119 (37.4) | 370 (21.3) | < 0.001* |
| Alcoholism, no. (%) | 546 (26.6) | 79 (24.8) | 467 (26.9) | 0.442 |
| Renal failure, no. (%) | 76 (3.7) | 13 (4.1) | 63 (3.6) | 0.692 |
HCC: hepatocellular carcinoma.
Risk factors for 6-week and 1-year mortality in cirrhotic patients with esophageal variceal bleeding.
| 6-w mortality | 1-y mortality | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age | 1.01 (1.00-1.02) | 0.043 | 1.02 (1.01-1.03) | < 0.001* |
| Male | 1.21 (0.89-1.64) | 0.229 | 1.35 (1.11-1.65) | 0.003* |
| HCC | 3.13 (2.42-4.06) | < 0.001* | 3.62 (3.07-4.28) | < 0.001* |
| Hepatic encephalopathy | 2.54 (1.94-3.33) | < 0.001* | 2.10 (1.75-2.53) | < 0.001* |
| Ascites | 1.32 (1.01-1.71) | 0.040* | 1.48 (1.25-1.75) | < 0.001* |
| Alcoholism | 0.76 (0.54-1.09) | 0.137 | 1.00 (0.81-1.23) | 0.974 |
| Renal failure | 3.25 (2.17-4.87) | < 0.001* | 2.89 (2.14-3.91) | < 0.001* |
| Infection | 2.69 (2.06-3.52) | < 0.001* | 1.89 (1.56-2.28) | < 0.001* |
HCC: hepatocellular carcinoma. HR: hazard ratio. CI: confidence interval.
After Cox regression model, the patients with HCC (HR, 3.13; 95% CI, 2.42-4.06; P < 0.001), hepatic encephalopathy (HR, 2.54; 95% CI, 1.94-3.33; P < 0.001), and renal failure (HR, 3.25; 95% CI, 2.17-4.87; P < 0.001) had a greater likelihood of 6-week mortality. The 1-year mortality was increased in patients with HCC (HR, 3.62; 95% CI, 3.07-4.28; P < 0.001), hepatic encephalopathy (HR, 2.10; 95% CI, 1.75-2.53; P < 0.001), renal failure (HR, 2.89; 95% CI, 2.14-3.91; P<0.001), ascites (HR, 1.48; 95% CI, 1.25-1.75; P < 0.001) and male gender (HR, 1.02; 95% CI, 1.01-1.03; P < 0.001).
In a total of 318 cirrhotic patients having EVB with infections, there were 131 (41.2%) patients with SWSF, 59 (18.6%) patients with UTI, 53 (16.7%) patients with SBP, 39 (12.3%) patients with pneumonia, 10 (3.1%) patients with cellulitis, 9 (2.8%) patients with BTI, and the remaining patients with other infectious diseases or dual infections (5.3%). Their 6-week and 1-year mortalities were showed in table 3.
The 6-week and 1-year mortalities of different infections in hospitalized cirrhotic patients with esophageal variceal bleeding.
| Condition | Number (%) | 6-week mortality | 1-year mortality |
|---|---|---|---|
| Non-infection | 1,735 | 10.4 | 29.6 |
| Infection | 318 | 25.5 | 45.0 |
| Pneumonia | 39 (12.3) | 30.8 | 59.0 |
| Antobody UTI | 59 (18.6) | 8.5 | 27.1 |
| Antobody SBP | 53 (16.7) | 30.2 | 56.6 |
| Antobody SWSF | 131 (41.2) | 32.1 | 44.3 |
| Antobody Cellulitis | 10 (3.1) | 10.0 | 30.0 |
| Antobody BTI | 9 (2.8) | 33.3 | 55.6 |
| Antobody Other* | 17 (5.3) | 11.8 | 47.1 |
UTI: urinary tract infection. SBP: spontaneous bacterial peritonitis. SWSF: sepsis without specific focus. BTI: biliary tract infection.
After Cox proportional regression modeling adjusted for age, gender and other confounding factors, including HCC, hepatic encephaopathy, ascites, alcoholism, and renal failure, the results of HRs of different infections on the mortalities of cirrhotic patients with EVB are shown in table 4. The HR of SWSF for 6-week mortality of cirrhotic patients with EVB was the highest, followed by pneumonia and SBP. The HR of pneumonia for 1-year mortality was highest, followed by SWSF and SBP. The effect of UTI for the mortality of cirrhotic patients with EVB was not significant.
Adjusted hazard ratios of variable bacterial infections on 6-week, and 1-year mortalities of cirrhotic patients with esophageal variceal bleeding, compared to those without co-existing infections.
| 6-week mortality HR (95% CI) | P value | 1-year mortality HR (95% CI) | P value | |
|---|---|---|---|---|
| SBP | 1.91 (1.09-3.36) | 0.024 | 1.52 (1.03-2.26) | 0.037 |
| Pneumonia | 3.54 (1.96-6.40) | < 0.001 | 3.18 (3.15-4.55) | < 0.001 |
| UTI | 1.04 (0.42-2.58) | 0.925 | 1.15 (0.70-1.92) | 0.580 |
| SWSF | 3.95 (2.81-5.56) | < 0.001 | 2.23 (1.70-2.94) | < 0.001 |
SBP: spontaneous bacterial peritonitis. SWSF: sepsis without specific focus. UTI: urinary tract infection. BTI: biliary tract infection. HR: hazard ratio. CI: confidence interval.
In the recent studies, the 4-6 week mortalities of cirrhotic patients with EVB are about 10-20% and beta-adrenergic-antagonist therapy is effective in reducing the mortality associated with EVB.1,12–14 In the present study, coexisting bacterial infection was seen in 15.5% of all cirrhotic patients with EVB. Analysis of the data showed that the presence of a bacterial infection, irrespective of gender, age, and comorbid medical disorders was associated with an increased 2.69 folds of their 6-week mortality. Previous studies showed evidence that bacterial infection was an independent prognostic factor for EVB patients.1,15 Our study agrees that bacterial infection is strongly associated with an increased mortality for cirrhotic patients with EVB. In addition, we found SWSF was the most common bacterial infection, followed by UTI, SBP and pneumonia.
In addition to the hemodynamic instability, bacterial infections precipitate the severity of EVB and increase EVB rebleeding rate. During a bacterial infection, there is a release of endotoxin into the systemic circulation. The ability of the reticuloendothelial system to remove the endotoxin is impaired in cirrhotic patients, causing a generalized intravascular activation of inflammatory mediators such as nitric oxide, interleukin-1, tumor necrosis factor, platelet-activating factor and leukotrienes. This results in structural and functional damage to the gastrointestinal tract, which in turn causes platelet dysfunction and activation of coagulation and fibrinolytic systems, as well as vascular congestion and hemorrhage. The release of vasoactive substances following a cytokine cascade leads to an increase in variceal pressure and impairment of primary hemostasis which, in turn, may cause rebleeding.15,16 That is why coexisting bacterial infection can increase mortality of cirrhotic p atients with EVB so much.
In our previous studies, we found that coexisting SWSF or pneumonia contributed higher mortality in cirrhotic patients with ascites or with hepatic encephalopathy than other infections.17,18 In the present study, SWSF and pneumonia played important roles again for the mortality of cirrhotic patients with EVB. Aspiration of gastric content to induce aspiration pneumonia is an important cause of pneumonia in cirrhotic patients. It can induce pulmonary inflam-mation, capillary leakage, and oxidative damage to cause acute lung injury. Hypoxia caused by pneumonia can directly make the perfusion of peripheral tissue poorer during EVB and contribute worse prognosis. Septicemia without specific focus accounts for 26% of bacteremia in cirrhotic patients.19 It is considered as a result of bacterial translocation from intestine and impaired innate immunity in cir-rhotic patients. We considered that poor prognosis of EVB patients coexisting SWSF was attributed to severe circulatory collapse and coagulopathy caused by SWSF.
We analyzed several variables to determine if there were other potential risk factors associated with increased mortality after EVB. In addition to a concomitant bacterial infection, an increased 6-week mortality rate can be seen in patients with HCC, hepatic encephalopathy, and renal failure; each of these factors is consistent with previous studies.5–7,20,21 Renal failure even increased 7-fold of mortality in cir-rhotic patients.20 The second most important prognostic factor was the presence of HCC. These factors were still significant for predicting 1-year mortality.
This was the first nationwide study to identify the effects of variable bacterial infections for the mortalities of cirrhotic patients with EVB. We also determined and evaluated possible risks for the 1-year mortality of these patients. We are aware that previous epidemiological studies may have been biased because patient populations differ in severity in medical centers and local hospitals.22 Due to the nature of our database, all patients hospitalized for EVB in Taiwan were included, thus preventing selection bias. Furthermore, patients were included only if EVB was proven via an endoscopic procedure to control bleeding. This helped to eliminate other causes of gastrointestinal bleeding. We limited our cases to a short designated time frame to prevent heterogeneity of medical management. We also reported an overall mortality for cirrhotic patients with acute EVB, including both in and out-of-hospital mortality. This prevented underestimating the mortality, which is possible in studies which include only in-hospital mortality.
There are limitations to our study. First, although the severity of liver cirrhosis was commonly based on the Child-Pugh score or the MELD score, it was not possible to identify the laboratory data regarding albumin, creatinine, bilirubin, or prothrombin time by ICD-9 coding numbers in the database; however, recently the concept of stages of cirrhosis separated by easily defined clinical criteria has been proposed.23 In this proposal, four clinical stages of cirrhosis have been identified, and each stage has distinct clinical features and a markedly different prognosis. Cirrhotic patients with esophageal variceal bleeding are actually in a decompensated status and are in clinical stage 4. Other complications of cirrhosis, such as hepatic encephalopathy or ascites, HCC, and impaired renal function were considered in this study; therefore, we believe that the lack of such lab data was not a major flaw in this study, because all the patients were in stage 4 by this clinical staging system. Second, the exact etiology of non-alcoholic liver cirrhosis could not be identified. We could divide the cirrhotic patients only into alcohol-related or non-alcohol related groups. We believe that this did not affect our results because the most common cause of cirrhosis in Taiwan has always been hepatitis B infection, but the etiology of non-alcoholic liver cirrhosis (hepatitis B or C) cannot be confirmed as a prognostic factor in determining survival from EVB. Thirdly, the database used in the present study could not provide the microbiologic data. So, we could not know the pathogens of infections in cirrhotic patients with EVB. Fourthly, the database used in the present study could not provide enough data to identify community-acquired or healthcare-associated infection. Fifthly, we cannot collect the major therapeutic methods for bacterial infections from the database. These factors may affect the prognosis of bacterial infections in cirrhotic patients with EVB. Sixthly, we are not sure that there is no incorrect coding in our database, even if the Taiwan National Health Insurance Bureau has an audit to check the correctness of the diagnostic coding of the patients. In addition, the 2004 database used in the present study may overestimate the mortality of cirrhotic patients with EVB today due to medical progress in the recent decade. However, the result still provides reliable data about the effects of variable bacterial infections on the mortality of cirrhotic patients with EVB.
In conclusion, the presence of bacterial infection, especially SWSF and pneumonia much increases the mortality of cirrhotic patients with EVB. Renal failure and HCC are the two most important prognostic factors in these patients.
Conflict of Interest StatementThe authors declare no conflict of interest.
Author ContributionsManuscript preparation and study design: Tsung-Hsing Hung, Chen-Chi Tsai, Zuo-Hua Gan.
Journal review and consultant: Yu-Hsi Hsieh, Kuo-Chih Tseng.
Statistical consultant: Chih-Chun Tsai.
AcknowledgmentsThis study was based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by the National Health Research Institutes (Registered number:100101). The interpretation and conclusions contained herein do not represent those of the Bureau of National Health Insurance, the Department of Health or the National Health Research Institutes.
Compliance With Ethical RequirementsFor studies with human subjects, this study was initiated after approval by the Institutional Review Board of the Buddhist Dalin Tzu Chi General Hospital Taiwan (IRB B1010410). Since all identifying personal information was stripped from the secondary files before analysis, the review board waived requirement for written informed consent from the patients involved.