The incidence of gallstone-related disease steadily increased in the last few years. Here, we aimed to investigate the effect of tauroursodeoxycholic acid11
TUDCA: tauroursodeoxycholic acid
(TUDCA) on preventing cholesterol gallstones formation in high-fat fed (HFD) mice. Material and MethodsSpecific pathogen-free male C57Bl/6 mice were fed a lithogenic diet22
LD: lithogenic diet
(LD group) alone or in combination with TUDCA (5g/kg diet) for 8 weeks. Upon sacrifice, serum, gallbladder, liver and small intestine were collected and the formation of gallstones or crystals in the gallbladder was analyzed. Additionally, the intestinal microbiota, and bile acid composition, serum lipids and hepatic lipids were studied. ResultsCholesterol gallstones with cholesterol crystals formed in mice of the LD-fed group (15/15, 100%). However, only cholesterol crystals were found in three mice without the presence of any gallstone in the TUDCA-treated group. Both serum and hepatic total cholesterol levels in the TUDCA group were significantly decreased compared with the LD group. Concomitantly, mRNA expression of Abcg5 and Abcg8 was significantly lower in the liver of the TUDCA group whilst mRNA transcripts for Abcb11, Acat2, and Cyp27 were significantly increased compared with the LD group. Additionally, the gallbladder cholesterol saturation index (1.06±0.15) in the TUDCA group was significantly decreased compared with the LD group. Interestingly, the ratio of Firmicutes/Bacteroides in the TUDCA group was increased 3x fold.
ConclusionsTUDCA can inhibit the absorption and synthesis of lipids in the small intestine by improving the intestinal microbiota in HFD-fed mice, thus reducing gallstone formation.
Gallstone disease is one of the most common digestive diseases worldwide and the incidence rate has steadily increased in recent years. Gallstones can be classified into cholesterol stones, pigment stones and mixed stones according to the type. More than 90% of gallstones are composed of cholesterol and formed in the gallbladder. Studies have shown that high-fat diet3 (HFD) can affect the gut microbiota in mice, while changes in the structure of the gut microbiota might impact serum and hepatic lipid [1]. In the intestine, the complex metabolism of primary bile acids is mainly regulated by the gut microbiota [2]. Certain specific gut microbiota composition may cause significant changes in the bile acids pool [3]. The decrease in the number of bacteroidetes leads to the down-regulation of the expression of farnesoid X receptor (FXR) in the intestine, promotes the expression of CYP7A1 and CYP8B1, and regulates the increase of bile acids synthesis in the liver [4]. As a new treatment for gallstone disease, TUDCA can effectively reduce the cholesterol content of gallbladder bile, reduce the stone formation rate of gallstones [5] and increase the bile acid content in gallbladder bile, thus preventing the formation of cholesterol gallstones. However, the mechanistic behind the reduction of gallstones by TUDCA remains unknown. Most of the studies pointed out that the reduction of stone formation rate by TUDCA is caused by increasing the amount of bile acid and changing the metabolism of liver cholesterol [6].While some studies pointed out that TUDCA activates FXR from the mouse [7]. The activation of the FXR leads to reduced bile acid synthesis in the liver. In the present study, we used stereoscopic microscopic observation to understand the effect of TUDCA in gallstone formation in HFD-fed mice. In addition, gut microbiota was analyzed and changes in genes involved in lipid and bile acid metabolism.
2Material and Methods2.1Animal studiesThirty specific-pathogen free (SPF) healthy male C57bl/6 mice, aged 4 weeks, were purchased from Shanghai Model Organisms Center, Inc (Shanghai). Mice were bred in a SPF barrier environment at the Animal Care Facility of the East Hospital, Shanghai Tongji University School of Medicine. After one week of normal food feeding, mice were randomly divided into two groups (15 mice/group, 5 mice/cage): mice fed with a lithogenic diet (LD), containing 98.25% common food+1.25% cholesterol+0.5% cholic acid, and mice treated with TUDCA (TUDCA group), containing 5g of TUDCA per kilogram of LD. Animals were grown under laboratory standard conditions (light 12h, dark 12h, 21 ∼ 24C, humidity 50% ∼ 55%), 20g of food per cage per day, changed on a daily bases for 8 weeks. After 8 weeks, mice were euthanized by exsanguination after anesthetized using an i.p. injection of 4% chloral hydrate (300mg/kg body weight). Gallbladder, liver, small intestine and coecum were collected from each mouse and frozen in a -80C freezer until analysis.
2.2Total RNA extraction50mg of mouse liver and small intestine tissue were taken in a cryotube, and 500μL Trizol added. Tissues were thoroughly homogenized and transferred into new tubes. 500μL Trizol solution and 200μL chloroform were then added, and tubes shaken, mixed and centrifuged at 4C, 12000rpm, 5min. The upper aqueous phase after the centrifugation was then transferred to a new tubed, 500μL of isopropanol was added, mixed and centrifuged at 4C, 12000rpm, 10min. The supernatant was discarded and the precipitate was washed with 1mL of 75% ethanol formulated with absolute ethanol and DEPC water. The precipitate dissolved in DEPC water was adjusted to a concentration of 200ng/μL as the total RNA used for reverse transcription.
2.3Gene expression in liver and small intestineUsing qPCR method, GAPDH was used as an internal reference. The expression levels of Abcg5, Abcg8, Abcb11, Acat2, Cyp27, Tnfa in the liver and expression levels of Hmgcr, Npc1L1 and Fgf15 mRNA in small intestine of each group were detected. 2μg of total RNA was extracted and reverse-transcribed according to the ThermoFisher reverse transcription kit. The primer sequences of all the tested genes are shown in Table 1.
Primer sequences.
| Primer | Forward primer | Reverse primer |
|---|---|---|
| Gapdh | TGT GTC CGT CGT GGA TCT GA | CCT GCT TCA CCA CCT TCT TGA T |
| Abcg5 | AAT GCT GTG AAT CTG TTT CCC A | CCA CTT ATG ATA CAG GCC ATC CT |
| Abcg8 | TGC CCA CCT TCC ACA TGT C | ATG AAG CCG GCA GTA AGG TAG A |
| Abcb11 | CAA TAG ACA GGC AAC CCG TCA | GTG GAA CTC AAT TTC GCC CTT |
| Acat2 | TGT CCC TGT CTT TGC CAA CA | CAT GCA AGA TGG AGA GCA GC |
| Cyp27 | GCC TTG CAC AAG GAA GTG ACT | CGC AGG GTC TCC TTA ATC ACA |
| Tnfα | GACCCTCACACTCAGATCATCTTCT | TTGTCTTTGAGATCCATGCCATT |
| Hmgcr | TGA TTG GAG TTG GCA CCA T | TGG CCA ACA CTG ACA TGC |
| Fgf15 | GGT CGC TCT GAA GAC GAT TG | CGC GCT CAT GCA GAG GTA |
| Npc1L1 | ATC CTC ATC CTG GGC TTT GC | GCA AGG TGA TCA GGA GGT TGA |
A total of 30 fecal samples of mice were collected at the 8th week of treatment, and the total DNA of the microorganisms in the fecal samples was extracted. The unique primers were synthesized for the V4 region of the 16S rRNA, then PCR amplification was performed. FW primer was 515 F: 5′-GTG CCA GCM GCC GCG G-3′, and the RW was 907R: 5′-CCG TCA ATT CMT TTR AGT TT-3′. Sequencing was performed by the MiSeq platform (Illumina MiSeq personal Sequencing Platform, Illumina, Inc, CA, USA). The sequencing data was processed by using the Quiime software. The obtained original sequence was filtered, the chimera was removed, and the high quality sequence was clustered by similarity 97%, and finally the OTUs (operational taxonomic unit) was obtained, and then compared with the RDP (Ribosomal Database Project) database for species annotation of OTUs.
2.5Hepatic lipid extraction50mg of liver was chopped and placed in a disposable tube. Each tube was filled with 3mL of Folch solution (chloroform: methanol; 2:1) at 4C overnight. Then the 3mL of Folch solution was transferred to a new tube. An extra mL of Folch solution was used to wash the liver. The resulting 4mL Folch solution was concentrated into one tube. Different amounts of liquid (total cholesterol: 100μL, triglyceride: 50μL) were taken into a new tube and dried at 57C. Cholesterol and triglyceride were measured following the manufacturer's instructions of the detection kits (Roche Diagnostics GmbH, Mannheim, Germany).
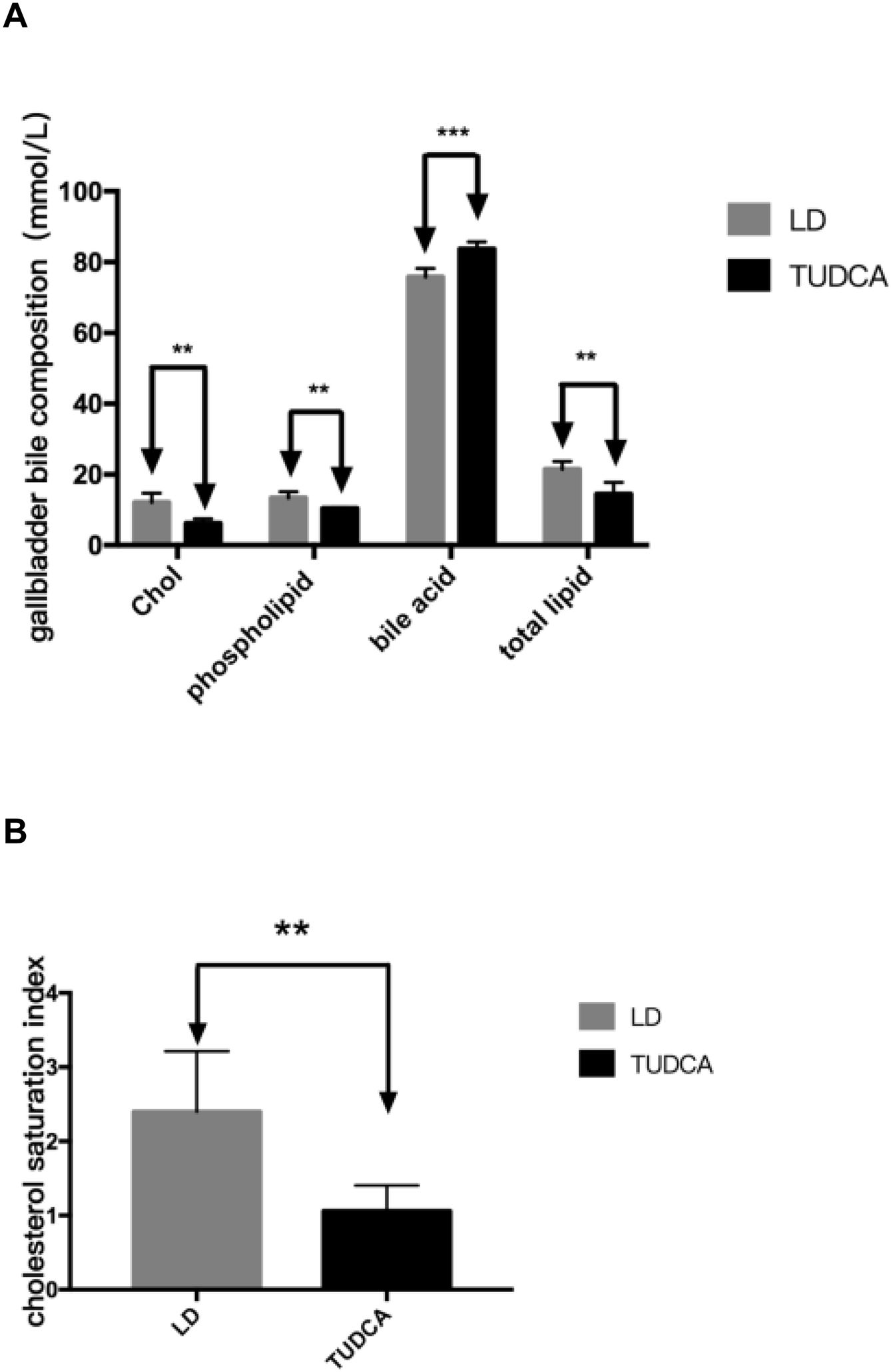
2.6Detection of bile acids and phospholipidsBile was withdrawn from the gallbladder and dripped into a clean tube. Bile was diluted with methanol and assayed using a bile acid and a phospholipid detection kit (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan). The cholesterol saturation index4 (CSI) in the gallbladder bile was calculated from the Carey table.
2.7General ReagentsTotal RNA extraction reagent (ThermoFisher Scientific, Vilnius, Lithuania); Reverse transcription kit (ThermoFisher Scientific); Cholesterol test kit (Roche Diagnostics GmbH, Mannheim, Germany); Triglyceride test kit (Roche Diagnostics GmbH); Phospholipid test kit (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan); Bile acid test kit (Randox Laboratories Ltd, Ardmore, UK).
2.8StatisticsStatistical analysis was performed using SPSS v22.0 software. All data are expressed as mean±standard deviation. Statistical significance was assessed using t-test and defined as p<0.05.
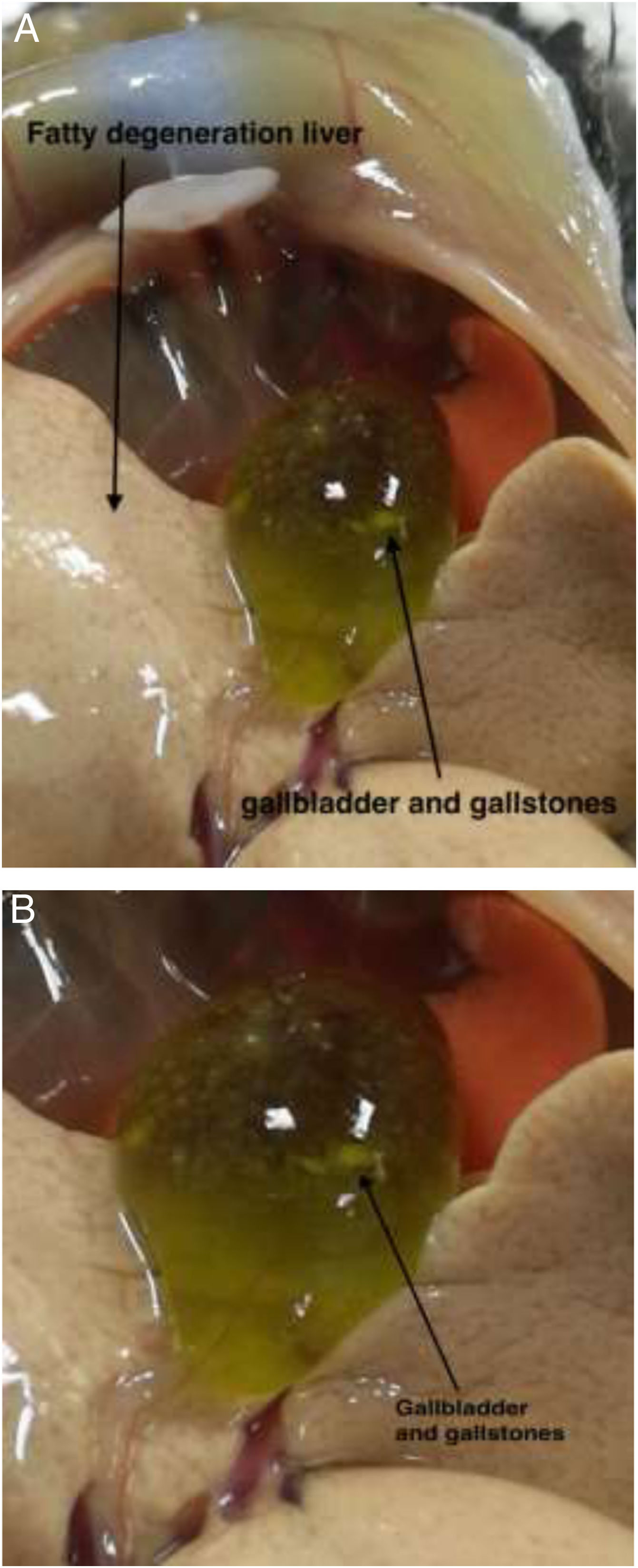
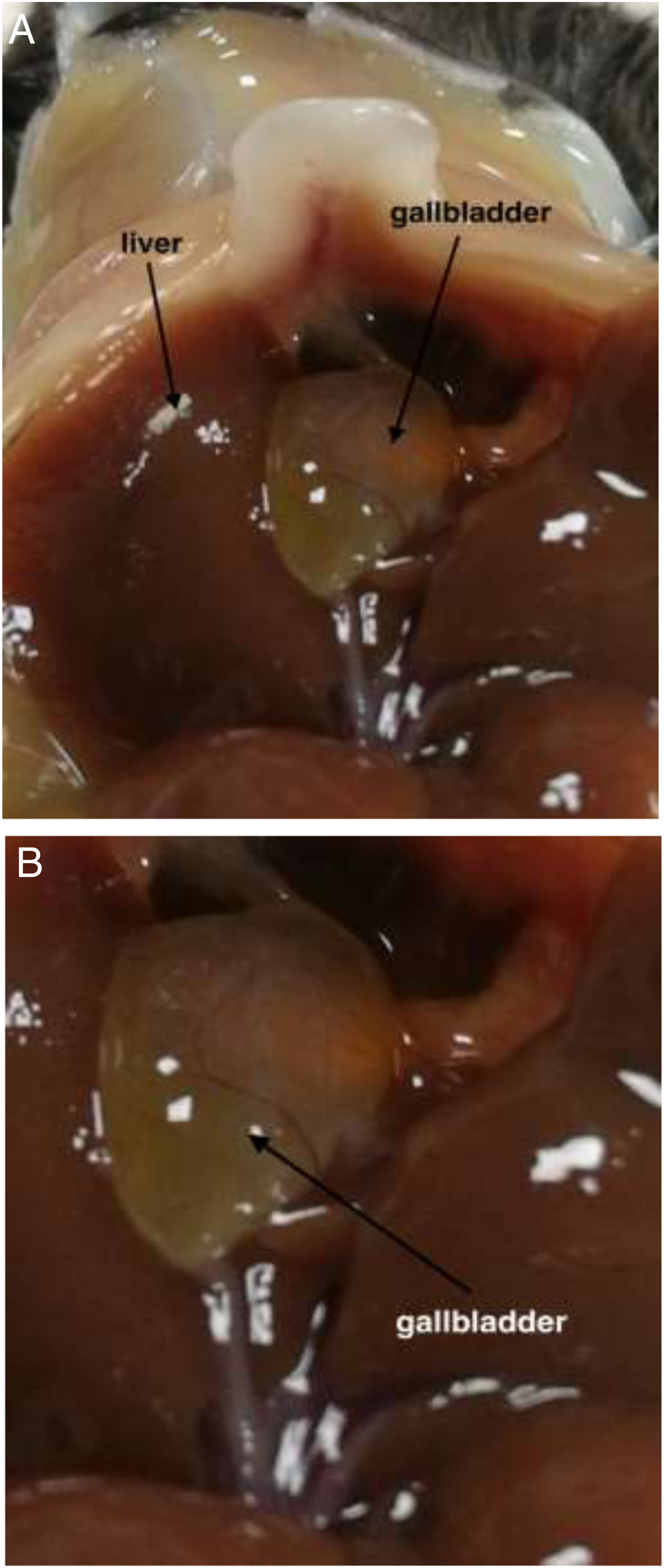
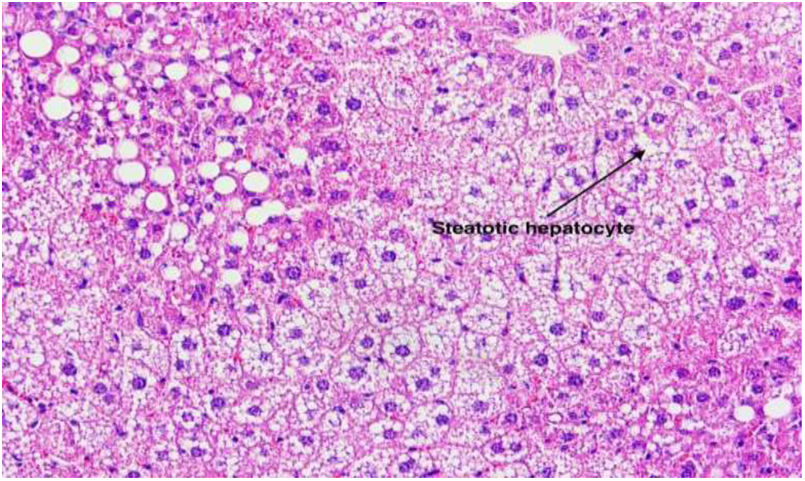
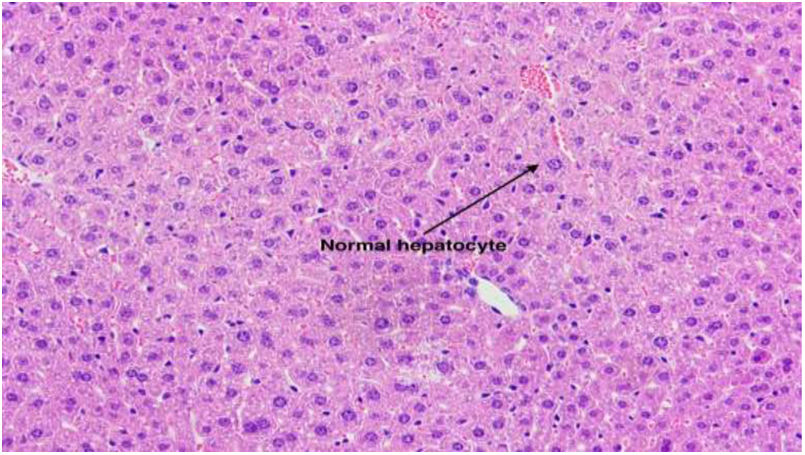
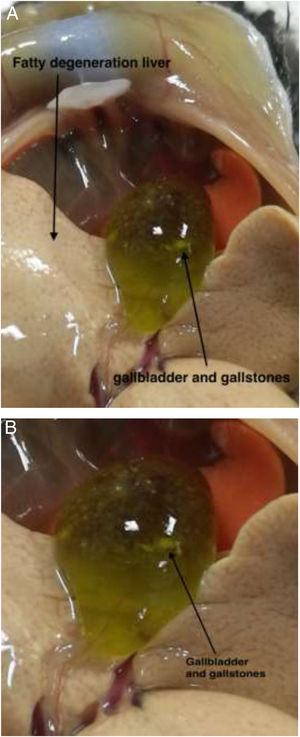
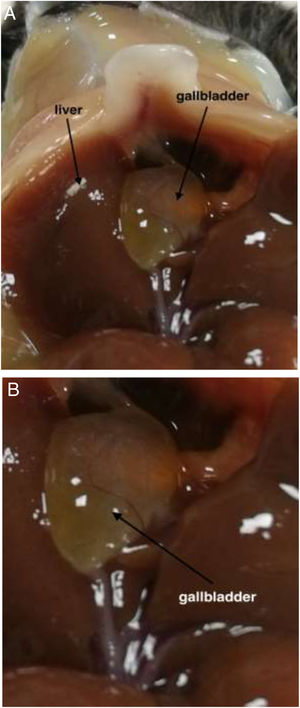
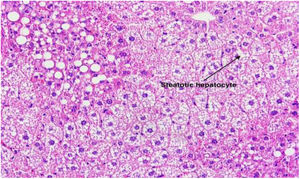
3Results3.1Effect of TUDCA administration in high-fat fed mice: Stone formation rate and liver pathological changesIn the litocholic diet (LD) group, cholesterol stones were observed in the gallbladder accompanied by a large amount of cholesterol crystals (Fig. 1A, B). The gallbladder of the TUDCA group showed clear and translucent appearance (Fig. 2A, B) and no formed cholesterol stones were found. Only a small amount of scattered crystals were found in the gallbladder of three mice (Table 2). Hepatic pathological sections stained with H&E showed severe fatty formation in the liver of mice in the LD group (Fig. 3), while liver cells in the TUDCA group were well-formed (Fig. 4).
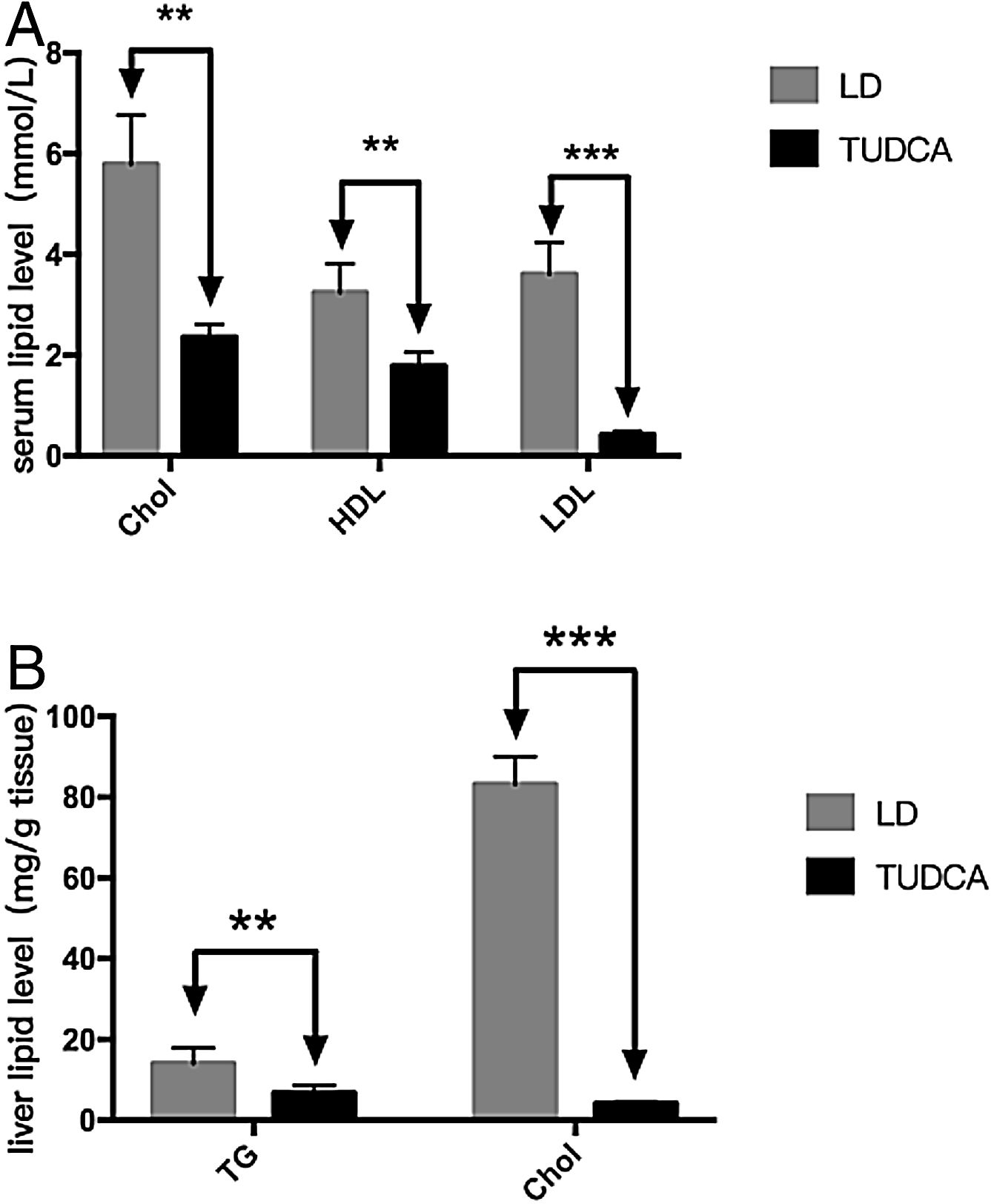
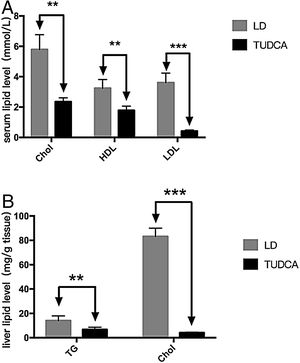
To further analyze the effect of TUDCA on cholesterol absorption, hepatic and serum lipids of each group of mice were analyzed (Fig. 5A). Serum cholesterol (total cholesterol), HDL5 (high-density lipoprotein) and LDL6 (low-density lipoprotein) were significantly decreased in the TUDCA group. At the same time, hepatic tryglicerides7 (TG) and cholesterol of TUDCA-treated mice were significantly decreased compared with the LD group (Tables 3 and 4).
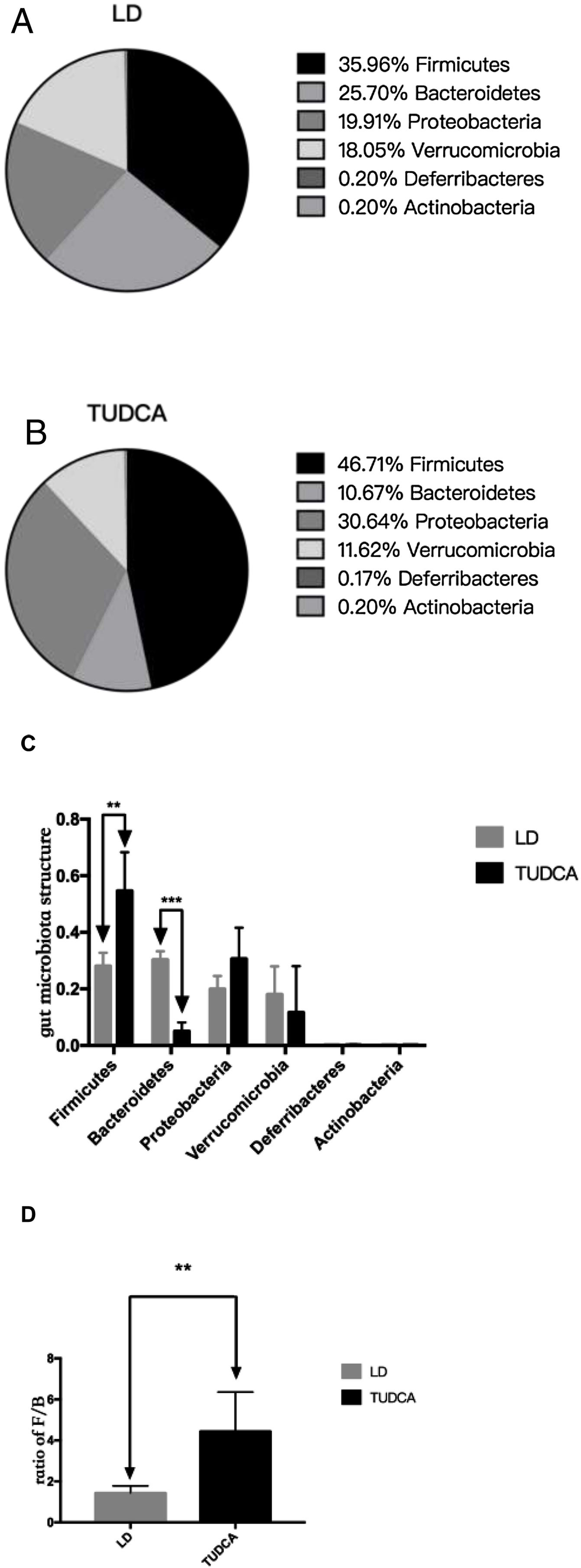
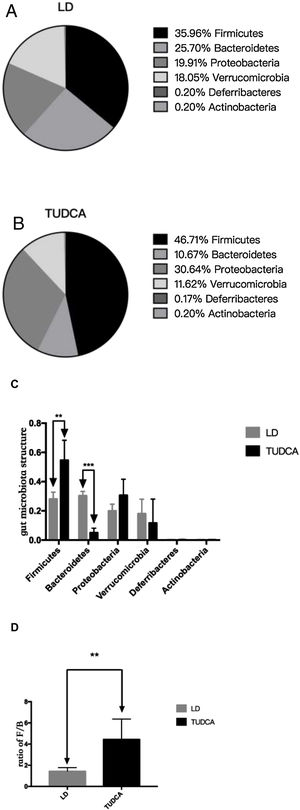
Sequencing of the coecal contents showed that the abundance of intestinal Firmicutes in the TUDCA group was higher than that in the LD group, while the abundance of the Bacteroidetes was decreased (Fig. 6A, B, C). The ratio of (Firmicutes/Bacteroidetes) F/B increased by 3.13 times (Fig. 6D), and the differences in gut microbiota structure between the two groups were statistically significant (P<0.05) (Table 5).
Changes in gut microbiota in LD group and TUDCA group *; P<0.05; **; P<0.01; ***; P<0.001. (A) Gut microbiota structure in LD group; (B) Gut microbiota structure in TUDCA group; (C) Changes in gut microbiota in LD group and TUDCA group; (D) LD group and TUDCA group changes in the ratio of F/B.
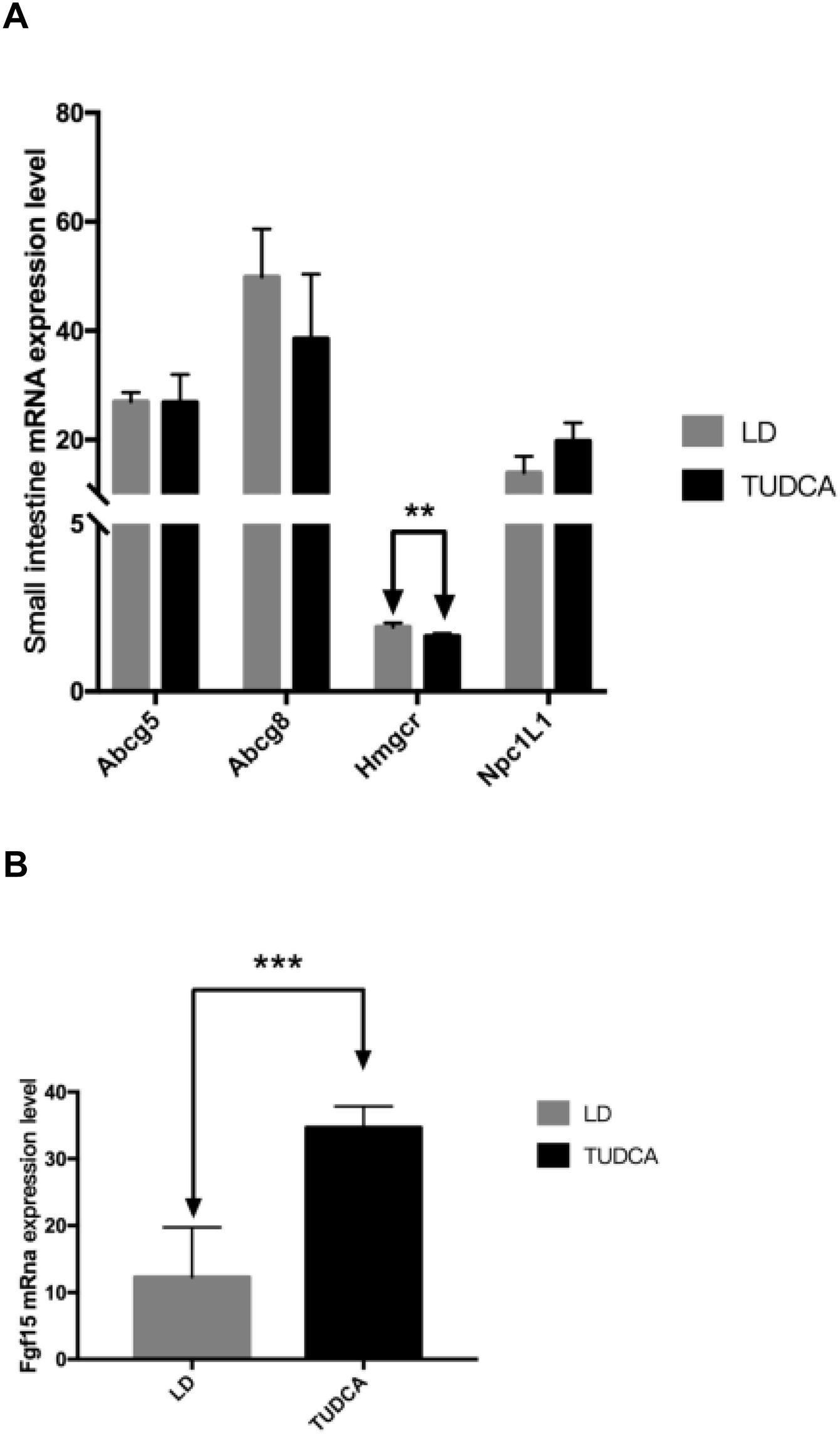
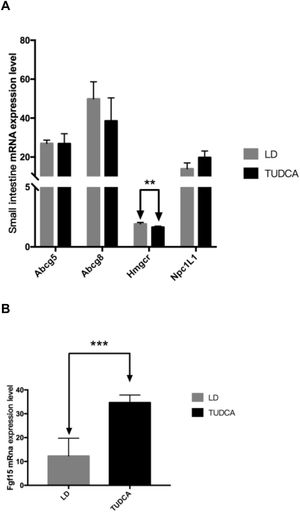
The mRNA expression levels of key genes including Npc1L1, Abcg5 and Abcg8 related to cholesterol transport in the small intestine were not significantly different between animals fed a LD alone or in combination with TUDCA. However, compared with the LD group, the expression of Hmgcr in the small intestine in the TUDCA group was significantly decreased (Fig. 7A), whilst the expression of Fgf15 increased significantly in the TUDCA-treated mice (Fig. 7B).
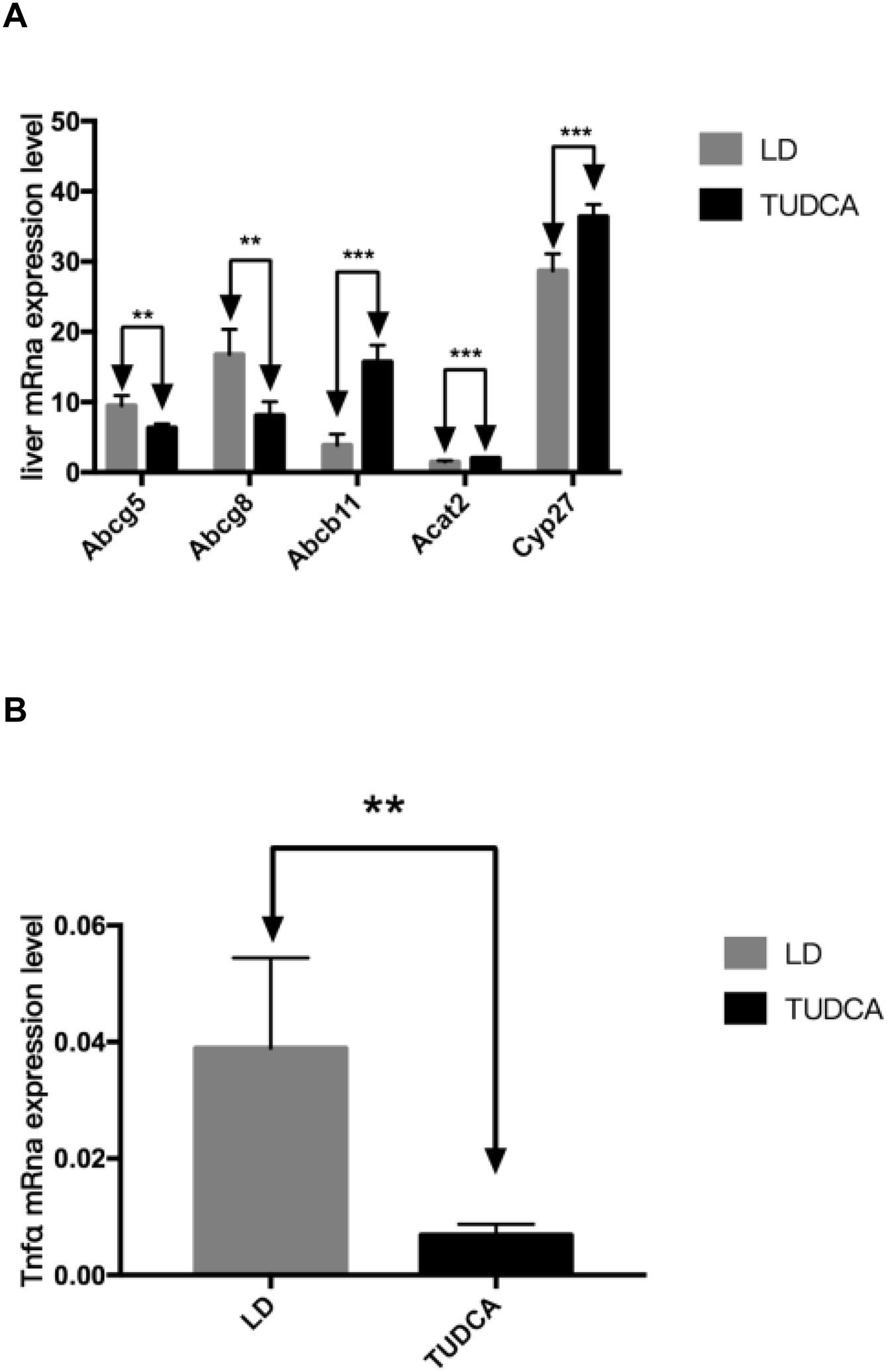
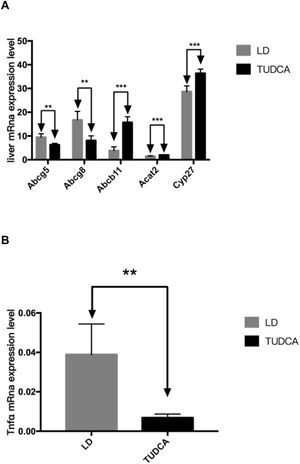
3.5Lipid and bile acid metabolism in the liverHepatic mRNA transcripts of Abcg5 and Abcg8 in the TUDCA group were significantly decreased compared with LD-fed mice, while the expression of Abcb11 significantly increased in TUDCA-fed animals. Additionally, Acat2 expression was significantly elevated in the TUDCA experimental group. Cyp27 is a rate-limiting enzyme of bile acid synthesis, which was significantly enhanced in TUDCA-fed mice compared with the LD experimental group. The ALT, AST and ALP levels are significantly decreased in TUDCA-fed animals. Finally, the expression of Tnfα, a well-known proinflammatory cytokine, was significantly decreased in the TUDCA experimental group, compared with LD-treated mice (Fig. 8A-C; Table 6).
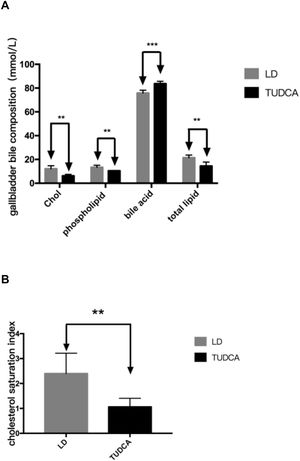
Cholesterol, phospholipids, total lipids and the cholesterol saturation index (CSI) in the gallbladder bile of TUDCA group were significantly decreased, compared with the LD-fed group (Fig. 9A, B; Table 7). Concomitantly, bile acid content in the gallbladder bile increased significantly in the TUDCA group.
The formation of cholesterol gallstones in the gallbladder is associated with the formation of cholesterol crystals due to the precipitation of cholesterol [8]. In this study, TUDCA significantly prevented the formation of cholesterol gallstones in the gallbladder of mice, whereas three mice showed cholesterol crystals in the gallbladder bile. This is related to the reduction of gallbladder bile cholesterol saturation index after the use of TUDCA. Clinically, TUDCA treatment showed a significant effect on lowering cholesterol levels [9]. In our study, serum and hepatic cholesterol levels were significantly decreased after TUDCA treatment, and liver fat infiltration was significantly improved, indicating that TUDCA has the effect of lowering overall cholesterol content in mice fed a high-fat diet (HFD).
Bile acid is one of the main components of bile [10]. It is a both a hydrophilic and a hydrophobic amphiphilic molecule. Hydrophilic bile acids stabilize the liver cell membrane and antagonize the cytotoxic effects of hydrophobic bile acids [11]. As a major example, TUDCA, as a representative substance of hydrophilic bile acids, has the effect of reducing the absorption of cholesterol in the small intestine [12], thereby reducing the body's intake of dietary cholesterol, and lowering the body cholesterol content. In addition, Mueller et al.[13] found that short-term use of UDCA can stimulate bile acid synthesis and promote cholesterol conversion. In that study, the authors demonstrated a raise in Cyp27 expression, a key enzyme for bile acid synthesis, thus reflecting the stimulatory effects of TUDCA on bile acid synthesis. Additionally, Abcg5 and Abcg8 are key transporters of the bile duct of the liver to secrete cholesterol into the bile. Klett and colleagues [14] found that after knocking out the Abcg5 and Abcg8 genes in the mice, the cholesterol concentration in the gallbladder of the mice decreased significantly. TUDCA significantly reduced hepatic expression of these transporters, and thus had an important regulatory effect on reducing hepatocyte's cholesterol secretion to bile and lowering the bile cholesterol saturation index (CSI), and closely related to the reduction of gallstone formation in this experimental group. The ATP-binding cassette transporter is a component of the efflux pump in the body and is responsible for the active transport to remove various cytotoxic substances in the body. The bile salt export pump protein ABCB11 on hepatocytes is mainly responsible for regulating the excretion of a variety of combined bile acids from hepatocytes to the bile duct. At present, many studies have shown that the expression defect of Abcb11 gene in hepatocytes will make the bile acids secreted by the liver cannot be excreted into the bile ducts, resulting in intrahepatic cholestasis [15]. In this experiment, it was found that after TUDCA treatment, the Abcb11 gene expression in the liver of mice increased significantly, and the bile acid content in the gallbladder bile also increased. This indicates that TUDCA effectively up-regulates the bile acid excretion from the liver to the gallbladder, increases the proportion of bile acids in the gallbladder of mice, and further reduces the cholesterol saturation index in the gallbladder of mice.
The small intestine is one of the places to absorb and synthesize cholesterol [16]. A high cholesterol diet will affect the abundance and distribution of the gut microbiota, leading to increased absorption of lipids in the intestine and obesity [17]. There are also reports that the structure of the gut microbiota of patients with gallstones is significantly different from that of normal people. Changing the structure of the gut microbiota will increase the susceptibility of gallstones [18,19]. Improving the structure of the gut microbiota helps to convert liver cholesterol into bile acids and inhibits the accumulation of cholesterol in the serum and liver, and further reduces the cholesterol content in the gallbladder bile. Hildebrandt and colleagues [20] found that the number of Proteobacteria in the intestine of mice increased after feeding a high-fat diet (high cholesterol, no bile acid feed). Previously, it was shown that mice fed a high-cholesterol and low-concentration bile acid diet triggered the decrease in the ratio of the Firmicutes/Bacteroidetes in the intestine, contributing to gallstone formation [21]. Islam and collaborators [22] reported that the percentage of Firmicutes in the intestine of mice increased and the percentage of Bacteroidetes decreased when a diet containing high concentrations of bile acid (2g of bile acid/kg of feed) for 10 days was fed. Therapeutic treatment with TUDCA (5g TUDCA/kg LD), caused an increase in the percentage of Firmicutes in the gut microbiota, while the percentage of Bacteroidetes decreased. The Firmicutes/Bacteroidetes ratio increased 3.13 fold. This might explain the difference in gut microbiota structure among high-fat diet, high-fat+low concentration bile acid feed and high-fat+high concentration bile acid diets, that they are maybe due to the strong selectivity of bile acids for gut microbiota. High concentration bile acids inhibit the proliferation of bacteria that are intolerant to bile acids in the gut. Wang et al.[23] found that after treatment with ursodeoxycholic acid8 (UDCA), serum and liver lipid content significantly decreased in mice fed a HFD. We found that after TUDCA treatment the serum and liver lipid content in mice fed a LD also decreased significantly. Reschly EJ and colleagues [7] found that TUDCA activates FXR from the mouse, and the increase of the FXR upregulates the expression of Fgf15. In our study we have the same result about the Fgf15. Degirolamo and colleagues [4] found that the increase in the percentage of Firmicutes in the intestine can activate the synthesis of bile acids in the liver by up-regulating Cyp7 and Cyp27, and the decrease in the number of Bacteroidetes leads to the down-regulation of the expression of FXR in the intestine. As a result TUDCA can inhibit the expression of FXR by change the structure of gut microbiota. Moreover, intestinal mRNA expression levels of cholesterol transporters including Npc1L1, Abcg5 and Abcg8 did not differ between the two experimental groups. This suggests that TUDCA reduces serum and liver lipid levels through a mechanism not occurring in the small intestine. Jia and collaborators [24] reported that the increase in the number of gut microbiota produces a large amount of bile acid hydrolase, which hydrolyzes the bound bile acid into a large amount of secondary bile acid LCA9 (lithocholic acid) and DCA10 (deoxycholic acid), and Tgr5 is activated by the LCA and DCA to inhibit the synthesis of liver bile acids. According to a previous report [25], bile acid hydrolase in the intestine was mainly produced by Bacteroidetes, and this study found that after treatment with TUDCA, the percentage of Bacteroidetes was significantly decreased from 25.70% in the LD group to 10.67% in the treatment group. Hu and colleagues [26] found that high levels of cholesterol in the LD significantly increased intestinal permeability, leading to excessive LPS11 (lipopolysaccharide) into the blood, resulting in increased Tnfα expression, cholesterol accumulation and liver damage. We found significantly decreased expression of Tnfα in the liver of the TUDCA group, and the liver damage was alleviated in the general and RNA levels, indicating that the LPS was reduced in blood. Due to the strong selective inhibition of high concentrations of bile acids on the gut microbiota, the percentage of the Firmicutes in the gut microbiota structure was up-regulated after TUDCA treatment, the percentage of the Bacteroidetes in the gut microbiota structure was lowered, the abundance of the gut microbiota was also decreased, and the amount of the LPS in blood was also reduced. Finally, the TUDCA treatment increased the synthesis of bile acids in the liver and reduced the accumulation of cholesterol in the serum and liver of mice. This is consistent with the results of the increase of the bile acid content in the gallbladder of mice and the significant decrease of serum and liver cholesterol levels.
5ConclusionIn summary, our study shows that TUDCA has the effect of preventing gallstone formation in mice: (i) by increasing the hydrophilicity of bile acids, inhibiting intestinal cholesterol absorption and lowering liver cholesterol levels, and reducing the expression of Abcg5/Abcg8 in the liver, reducing cholesterol secretion into bile, thereby lowering gallbladder cholesterol saturation index; (ii) TUDCA reduces the hydrophobic index of bile acids in bile, thereby increasing the solubility of cholesterol in bile; (iii) In addition, we also found that TUDCA can change the percentage of intestinal Firmicutes, and the reduction of the percentage of Bacteroidetes can promote the synthesis of bile acids and reduce the inflammation of the liver caused by the LPS in blood.
Conflict of interestsThe authors declare that they have no competing interests.