Preliminary experience with clinical hepatocyte transplantation during the past decade has provided proof of concept that cell therapy can be effective for the treatment of some liver diseases. Recent progress in cell biology resulting in the isolation and characterization of hepatic stem cells and progenitor cells further increased the expectation for a new approach to the treatment of genetic and chronic liver disease. Several potential sources have been identified of hepatic stem/progenitor cells exhibiting both differentiation towards the hepatic lineage in vitro and hepatic parenchymal repopulation with liver-specific metabolic activity in liver-injured animal models. However, a few of these results proved to be poorly reproducible in different laboratories, and it was recognized that some initial optimistic conclusions were drawn from incorrect interpretation of experimental data or from insufficient knowledge of the mechanisms involved in tissue regeneration. Moreover, only modest results have emerged so far from ongoing clinical experience involving the use of putative stem cells in liver disease. There is much need for a joined effort to concentrate the resources on a specific cell population, in order to better characterize its function, to assess its safety and to develop better focused clinical trials. In conclusion, while the biological features of stem cells still justify the hope for future clinical applications, hepatic stem cell therapy has still a long way to go from bench to bedside.
Abbreviations: SHPCs: Small Hepatocyte-like Progenitor Cells; HSCs: Hematopoietic Stem Cells; BM: Bone Marrow; UCB: Umbilical Cord Blood. Grants and Financial supports The financial support of Telethon - Italy (grant no. GGP02051), of the Italian Association for the Study of the Liver (AISF) and of the Regione Veneto (Centro Regionale per la Terapia Cellulare delle Malattie Metaboliche and grant no. 03/03/01) is gratefully acknowledged.
Cell therapy can be defined as «the use of living cells to restore, maintain or enhance the function of tissues and organs».1 The use of isolated, viable cells has emerged as an experimental therapeutic tool in the past decade, due to progress in cell biology and particularly in techniques for the isolation and culture of cells derived from several organs and tissues. However, experimental cell therapy has a longer tradition in Hepatology, since it has been known for more than 30 years that isolated hepatocytes infused into the portal vein engraft into the liver cords and express normal cell function. Such a therapeutic strategy was put forward as an alternative to orthotopic liver transplantation (OLT), which requires major surgery and is limited by the availability of donors. Indeed, it was shown that significant clinical results can be obtained with the transplantation of isolated hepatocytes corresponding to as little as 1-5% of the total hepatocyte mass.2-6
The procedure seems relatively safe, provided portal pressure and/or portal flow are monitored during cell infusion in order to prevent vascular thrombosis.7 Hepatocyte transplantation has recently been used as an alternative to OLT in patients with liver-based congenital metabolic disorders, such as Crigler-Najjar disease,8 α-1-antitrypsin deficiency,9 glycogen storage disease type Ia,10 ornithine transcarbamoylase deficiency11,12 and the deficiency of factor VII.13 The role of hepatocyte transplantation in the treatment of acute and chronic liver disease is less clear,9,14 due to difficulty in organizing large-scale clinical trials. Indeed, the main factor limiting the practice of hepatocyte transplantation is again the availability of liver grafts for cell isolation. Moreover, the metabolic effects of cell transplantation seem to be fading with time, a problem which can partially be solved by repeated hepatocyte infusions15 but which probably indicates the progressive loss of the terminally-differentiated exogenous cells. In theory, both problems could be solved by replacing the hepatocyte with stem or precursor cells, provided they can be isolated from a more affordable source.
Indeed, at present, there is growing interest in the therapeutic use of stem cells.16,17 A stem cell has the ability to divide for indefinite periods of time, to self-renew and to give rise to many different cell types. Embryonic stem cells originate from the inner cell mass of the mammalian blastocyst and are totipotent.18 Adult stem cells are more specialized, being committed to give rise to cells with a particular function within their own specific tissue or organ.19 Precursor/progenitor cells are defined as cells rapidly dividing and already partially determined towards a specific differentiation pathways.20 However, experimental evidence suggests that some adult stem cells are able to develop into different types of specialized cells (a process also known as transdifferentiation), depending on the microenvironment where they are homed, including the liver.19,21-30
This review will address a series of major issues on hepatic stem cells, including their origin, their role in liver regeneration and fibrosis, and their possible use in the treatment of liver disease.
What is the origin and what are the possible sources of hepatic stem cells?In mammals, the liver has the unique ability among solid organs to regenerate following parenchymal injury. During fetal development, hepatoblasts give rise both to hepatocytes and to cholangiocytes. The hepatocyte is traditionally considered as the cell responsible for liver regeneration, being able to re-enter the cell cycle, proliferate and differentiate both into hepatocytic and biliary lineage in response to loss of liver mass. However, an intra-hepatic progenitor cell compartment, resident in the canals of Hering and consisting of precursors known as «oval cells» in rodents (or «hepatic progenitor cells» in human), is activated when the replicative capacity of the main epithelial cell compartment is inhibited or exhausted.31-36 These small round cells express phenotypic markers of both fetal hepatocytes and biliary cells35,37-39 and are able to differentiate into hepatocytes, bile ductural cells and intestinal epithelium.40
SHPCs also appear to be involved in the process of hepatic regeneration. SHPCs are highly proliferative and can generate mature differentiated hepatocytes in vitro.41,42 SHPCs express markers such as albumin, alpha-fetoprotein, transferrin, form bile canaliculi and store glycogen.43 SHPCs also appear to be involved in the process of hepatic regeneration after partial hepatectomy (PH) in rats pre-treated with retrorsine, a pirrolyzidine alkaloid which severely impairs the proliferating capacity of mature hepatocytes.41,44,45 In this model, hepatic repopulation takes place mainly by proliferation of SHPCs, exhibiting phenotypic traits in common with fully differentiated hepatocytes, fetal hepatoblast, and oval cells.46 Some reports suggest that SHPCs don’t originate from oval cells,42 but that they derive from a pre-existing population of retrorsine-resistant hepatocytes.47 Indeed, during liver regeneration, the SHPCs lack hepatic cytocrome P450 protein48 that is responsible for metabolizing retrorsine to pyrrolic derivatives49 and are thus resistant to the toxic effect of the drug. Avril et al.47 labeled mature hepatocytes using a recombinant retroviral vector harboring the β-galactosydase “LacZ” gene in the retrorsine/PH rat model. During parenchymal regeneration, a similar (4%) proportion of β-galactosydase-positive SHPCs and of mature hepatocytes was observed, suggesting that mature hepatocytes could be the actual progenitors of SHPCs.47 However, more recently, using 3-dimensional image analysis in the retrorsine/PH model of liver regeneration, Vig et al. observed that oval cells surround and penetrate SHPCs nodules, suggesting that SHPCs nodules can originate from oval cells.43
The origin of hepatic stem or precursor cells is still a matter of debate. Interestingly, oval cells express markers of Hematopoietic Stem Cells (HSCs), such as Thy-1, CD34, CD45, Sca-1, c-Kit and flt-3.22, 50-54 In particular, Thy-1 is a highly conserved protein. It has been found in the brain and in the hematopoietic system of rat, mouse and humans.53 It is also expressed on stem cells of fetal liver and in bone marrow (BM)-derived cells.55 In addition, the normal adult liver contains hematopoietic cells that are phenotypically similar to cells present in the BM.56 These observations originated the hypothesis that liver stem cells may arise from a population resident in the BM.22 Petersen et al.22 followed the fate of syngeneic BM cells transplanted into lethally irradiated rats whose livers were subsequently injured by 2-acetylaminofluorene and CCl4, a regimen known to induce oval cell proliferation. Using the sry gene as a marker for the Y chromosome, male donor cells were visualized in female recipients. In a separate experiment, didpeptidyl peptidase IV (DPP IV)-positive hepatocytes were identified in the liver of DPP IV-deficient rats transplanted with BM from DPP IV-positive animals.
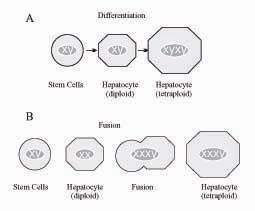
Lagasse et al.,29 transplanted fumarylacetoacetate hydrolase (FAH)-deficient mouse, an animal model of Tyrosinemia type I, with BM cells from a non-affected wildtype animal transgenic for the β-galactosidase “LacZ” gene. The liver of the recipient animals was progressively repopulated with hepatocytes harboring both the β-galactosidase and the fumarylacetoacetate hydrolase enzyme. Thus, intravenous injection of adult BM cells in the FAH-/- mouse rescued the mouse and restored the biochemical function of its liver. It was later shown that the correction of the metabolic disorder was not due to transdifferentiation of HSCs but rather to a fusion process, probably involving macrophages derived from the exogenous hematopoietic cell lineage and the recipient hepatocytes.57,58 This phenomenon can be demonstrated by cytogenetic analysis in sex-discordant transplantation (Figure 1). However, other Authors have later demonstrated in different models that HSCs can convert in hepatocytes without fusion both in vitro and in vivo. By co-culturing HSCs with injured liver tissue, Jang et al.,59 observed production of cells expressing the immunocytochemical and genetic features of hepatocytes, and maintaining the original chromosomal pattern. After two days in culture, about 3% of the cells converted to hepatocytes. A similar finding was observed after transplantation of BM-derived cells from male animals into female animals with liver injury: after 2 days, 8% of hepatocytes incorporated the Y chromosome, while maintaining the original male chromosomal pattern, suggesting differentiation rather than fusion.59 Several investigators have claimed in vitro differentiation of BM-derived cells into hepatocytes, although the results were often difficult to be reproduced in different laboratories. The group of Catherine Verfaille isolated from the BM a population of Multipotent Adult Progenitor Cells (MAPCs) with the ability to differentiate in culture into endothelium, neuroectoderm and endoderm. These cells seem to be indeed pluripotent, a feature of embryonic stem cells. Hepatic differentiation of these cells was obtained after a few days in culture with appropriate growth factors.60 Lee et al.,61 showed that Mesenchymal Stem Cells (MSCs) isolated from human BM are able to differentiate into functional hepatocyte-like cells. Setting up a novel in vitro hepatic differentiation protocol, based on the sequential use of HGF and Oncostatin M, and following cells behavior under hepatogenic conditions at different times, these Authors showed that the MSCs acquired hepatocyte morphology and expressed genes and functions characteristic of liver parenchymal cells. A modified differentiation medium, containing FGF-4, Oncostatin M, HGF and EGF, was used by Shi et al., to induce BM-derived mononuclear cells of C57BL/6 mice to differentiate into hepatocyte-like cells.62 However, in contrast to the above results indicating that oval cells may be derived from the BM, recent work suggests that such cells originate in the liver itself.43, 57, 63
Distinguishing differentiation from fusion by cytogenetic analysis based on the identification of sex chromosomes.
A. Differentiation of BM-derived cells into liver parenchymal cells originates hepatocytes with the same chromosomal pattern as the parent stem cell (XY), and such identity is maintained also in the case of polyploidy, which is common in the liver (e.g. XYXY in tetraploid cells).
B. By contrast, cell fusion between male hematopoietic cell lineage (XY) and female hepatocytes (XX) results in a XXXY pattern, as it was shown in the Lagasse model.58
In summary, experimental evidence suggests that liver parenchymal cells can originate from a specific precursor cell compartment in the liver, from pluripotent stem cells, from transdifferentiation of HSCs or from cell fusion. The occurrence of true transdifferentiation, or reprogramming to the hepatic lineage of an already committed hematopoietic stem cell, is still a matter of debate.30
Additional, potential sources of hepatic stem cells have been identified in the cord blood, in the amniotic fluid and in the placenta. Umbilical Cord Blood (UCB) contains hematopoietic and mesenchymal stem/precursor cells Lee et-al.64,65 The transplantation of UCB has been used for more than 10 years for the treatment of hematologic and genetic diseases.66-71 UCB cells are easily accessible, proliferate in vitro and have longer telomeres compared to adult cells, indicating higher proliferation capacity.72-74 Several Authors have investigated the hepatic potential of UCB-derived cells in vitro and in vivo. Kakinuma et al.,75 showed that human UCB cells cultured in the presence of a particular combination of growth and differentiation factors (i.e. FGF-1, FGF-2, LIF, SCF and HGF) were able to produce albumin and other hepatocytes specific markers in vitro. When inoculated into liver-injured SCID mice, a few functionally differentiated human UCB-derived hepatocytes were found 55 weeks post transplantation. By means of a «two-step» differentiation medium, Lee et al.,65 induced human UCB-derived (CD3-, CD14-, CD19-, CD38-, DC66b-, glycophorin A-) MSCs to differentiate into hepatocyte-like cells. Sharma et al.,76 reported that human UCB-derived mononu-clear cells generate hepatocyte-like cells after transplantation into NOD-SCID mice with severe hepatocellular damage produced by CCl4. Noteworthy, all such cells showed some specific human hepatic markers but not a mature hepatocyte phenotype. Moreover, all the donor-derived hepatic cells expressed human albumin and human hepatocyte-specific antigen Hep Par 1 but also expressed the murine cytokeratine CK18, suggesting the occurrence of fusion between human and mouse cells. Newsome et al.,11 infused an unsorted UCB mononuclear cell preparation into sub-lethally irradiated NOD-SCID mice. These cells were able to engraft into mice livers and differentiate into hepatocytic lineage with no evidence of fusion with mouse hepatocytes.77 Piscaglia et al.,78 transplanted into immunocompetent rats a CD34+/CD45+ and CD133+/CD45+ population from human UBC,79 after inducing liver injury by allyl-alcohol,80 and observed that the human cell population contributed to hepatic regeneration. Kogler et al.,81 isolated a CD45-negative population from human UCB (denominated «Unrestricted Somatic Stem Cells») exhibiting both pluripotency and a high proliferation capacity in vitro. Under appropriate conditions, these cells differentiate into osteoblasts, chondroblasts, adipocytes, hematopoietic and neural cells. When transplanted in the preimmune fetal sheep, they were able to generate albumin-producing human parenchymal liver cells. By analyzing liver parenchymal cells for the coexistence of human and ovine genomes, the Authors conclude that they were the result of differentiation rather than of fusion, although the latter event could not be completely excluded.
Epithelial cells from the amnion express markers of neural progenitors cells,82,83 as well as hepatic specific markers such as albumin and alpha-fetoprotein.84 Furthermore, cells derived from amniotic fluid have a low immunogenicity, are phenotypically similar to MSCs from the BM85 and are able to engraft in different tissues, including the liver.86 Interestingly, amniotic mesenchymal cells appear to induce immunological tolerance, a property that might limit the need for immunosuppression in allogeneic transplantation.86
Do hepatic stem cells participate in the parenchymal regeneration process associated with acute or chronic liver injury?Several studies have addressed the ability of stem/progenitor cells to repopulate a diseased liver. In patients with chronic hepatitis C, Tanja Roskams was able to follow the differentiation of hepatic progenitor cells both into hepatocytes and cholangiocytes, suggesting that this stem cell compartment participates in the parenchymal regeneration associated with chronic viral liver disease.87 Further studies in acute and chronic liver disease of different etiology also demonstrated differentiation of progenitor cells into hepatocytes.88-95 The activation of the stem/precursor cells compartment seems to be correlated with the severity of the disease.96,97 Activation of progenitor cells in chronic liver diseases implies that they form a potential target cell population for hepatocarcinogens,87,98-100 a caveat which should be taken into account if such cells are to be considered as a therapeutic tool.101 The differentiation of the progenitor cells into hepatocytes or biliary cells depends on the type of mature epithelial cell that is damaged35,88,95 and by the remodeling of the surrounding matrix.35,102 However, the factors contributing to the regulation of progenitor cell activation and the components that define the so called «stem cell niche» for adult human liver progenitor cells are poorly characterized.103
The contribution of the BM to liver regeneration is less clear. Several reports in different animal models indicate that the participation of BM-derived hepatocytes to hepatic parenchymal regeneration is insignificant,104-107 with the notable exception of the previously described work by Jang et al.59 Theise et al. infused CD34+ lin- BM cells from male mice into irradiate female mice, and found that only 0.39-1.1% of hepatocytes derived from BM. Following transplantation of BM cells into five irradiate mice, no hepatocytes of BM origin was observed in the liver of recipient animals while in two mice 0.4-2.2% of bile duct cells were positive for the Y chromosome.108 Wagers et al.,104 generated chimeric animals by transplantation of a single green fluorescent protein (GFP)-marked HSC into irradiate mice. Only one hepatocyte out of 70,000 was found to be GFP(+) in the recipient livers. Similarly, Fuji et al.,105 were unable to identify BM-derived hepatocytes following transplantation of GFP(+) BM cells into GFP(-) hepatectomized mice. Kanazawa and Verma106 tested three different animal models of liver injury (CCl4 treatment, albumin-urokinase transgenic mouse and hepatitis B transgenic mouse) and found that only 5/410,000 cells were derived from BM. Finally, Dahlke et al.,101 were unable to demonstrate any contribution of the BM to liver regeneration in a rodent model of CCl4 liver injury associated with retrorsine administration, in order to inhibit the replication of endogenous hepatocytes. In studies on patients with sex-discordant liver or BM transplantation, the contribution of BM to hepatic parenchyma seems also to be absent or minimal.27,28,109-114 The frequency of BM-derived hepatocytes in the different studies was in the range of 0.5-2%,28,112,113 1-8%27 and 4-7%,109 respectively. Such discordant but mainly discouraging findings probably originate from very different (and sometimes inappropriate) experimental set-ups. Possible factors influencing liver repopulation with BM-derived hepatic parenchymal cells include the model and timing of liver injury, the route of stem cells administration (systemic vs intraportal) and the selection of the stem/precursor cell population as well as its activation before infusion.
What is the role of stem cells in hepatic fibrogenesis?Myofibroblasts play a key role in the inflammatory response and in the process of hepatic fibrogenesis, due to their capacity to produce extracellular matrix.115 Working in collaboration with Malcolm Alison and Stuart Forbes and using the Y chromosome as a marker, we were able to identify recipient-derived myofibroblasts in liver grafts following sex-mismatched transplantation.116 These preliminary data were later confirmed in a larger series, suggesting a contribution of the BM to the fibrogenetic process leading to liver cirrhosis and pointing to a possible negative effect of BM-derived cell transplantation in liver disease.117 However, experimental work in rodents indicates that a specific cell population in the BM may actually prevent the fibrotic process in the liver. Systemic infusion of a subpopulation of BM-derived nonhematopoietic cells, separated using an anti-Liv8 antibody, resulted in the resolution of liver fibrosis induced by CCl4 treatment and normalized the synthetic function of the liver.118 These reports suggest that the BM may be actively involved in hepatic fibrogenesis, but its role in promoting or preventing the scarring process has yet to be defined.
Can the regenerative potential of stem cells be exploited for the treatment of liver diseases?The treatment of liver disease with BM-derived hepatic stem cells might have considerable advantages over the use of hepatocytes. BM can be obtained from millions of potential living donors with simple procedures, in contrast to obtaining hepatocytes from the few cadaveric livers rejected for whole organ transplantation but still suitable for cell isolation. It was postulated that a population of stem cells resident in the BM can be released into the circulation, in response to stimuli derived from injured tissue, migrate to injured site and participate in regeneration.119-123 We hypothesize that such cells represent the vestiges of a very ancient body repair system present in more primitive life forms. During evolution, with the development of more complex organisms, such a system probably became obsolete and was mostly replaced by more efficient, specific tissue/organ stem/precursor cells. In the light of this hypothesis, it is not surprising that the participation of BM-derived cells to non-hematopoietic tissue has often been described as insignificant. However, we could probably take advantage of the peculiar characteristics of such cells by concentrating them in the injured tissue and providing the optimal conditions to promote their participation in the regenerative process.
With respect to the cell population, about twenty different phenotypes of BM-or UCB-derived cells with potential for hepatic differentiation have been identified using a variety of surface markers.20 It is reasonable to assume that some degree of overlap exists among the different cell populations, and there is much need for a joined effort in order to select a single phenotype including the most significant markers and demonstrating the most convincing and reproducible potential for hepatic differentiation. Clearly, a similar approach should also be applied to isolate intra-hepatic «resident» stem/precursor cells.
Following the pioneering work of Nancy Rolando124,125 as well as similar applications in the fields of Cardiology,126 several investigators have approached the use of G-CSF as a method to mobilize from the BM stem/precursor cells with the aim to induce hepatic colonization improving parenchymal regeneration both in acute and in chronic liver disease. However, no convincing data have been published so far, suggesting that a significant therapeutic effect has yet to be demonstrated. Meanwhile, studies in laboratory animals have shown that the improvement in hepatic regeneration associated with G-CSF administration was not associated with increased liver repopulation by BM-derived parenchymal cells, but rather to a more efficient repair process mediated by resident hepatic parenchymal cells.127 Probably the most convincing clinical evidence for a possible therapeutic application of liver stem cells was published recently by am Esch II et al.128 These Authors infused autologous CD 133+ BM-derived cells into the portal vein following partial portal embolization, and they observed a significant improvement in hepatic regeneration with respect to the control group, which did not receive cell infusion.
Recent work, performed in collaboration between our laboratory and the Laboratory of Surgical Research of the Cedars-Sinai Medical Center in Los Angeles, led to successful isolation and characterization of a putative subpopulation of β2-microglubulin-/Thy1+ hepatic stem cells both from the liver and from the BM.129,130 Selective intraportal infusion of syngeneic β2-microglubulin-/Thy1+ BM-derived cells, following allogeneic liver transplantation in rats with subtherapeutic immunosuppression, resulted in up to 62 ± 5.0% repopulation of the transplanted lobes with syngeneic hepatocytes and cholangiocytes. Moreover, the survival of the animals which received cell infusion was doubled with respect to control group, suggesting that graft repopulation with BM-derived cells can rescue liver grafts undergoing rejection. We then used reversible ischemia/reperfusion liver injury to induce engraftment and hepatic parenchymal differentiation of exogenous β2-microglubulin-/Thy1+ BM-derived cells.131 Transplantation of BM-derived cells obtained from GFP-transgenic rats into Lewis rats resulted in the presence of up to 20% of GFP(+) hepatocytes in ischemia/reperfusion-injured liver lobes after one month. Infusion of wild-type BM-derived cells into GFP-transgenic rats resulted in the appearance of GFP(-) hepatocytes, suggesting that the main mechanism underlying parenchymal repopulation was differentiation rather than cell fusion. Transplantation of wild-type BM-derived cells into hyperbilirubinemic Gunn rats with deficient bilirubin conjugation after ischemia/reperfusion damage resulted in 30% decrease of serum bilirubin, in the appearance of bilirubin conjugates in bile and in the expression of normal UDP-glucuronyltransferase enzyme, evaluated by PCR analysis. Thus, reversible ischemia/reperfusion injury induced hepatic parenchymal engraftment and differentiation into mature hepatocytes of BM-derived cells, and transplantation of BM-derived cells from non affected animals resulted in the partial correction of hyperbilirubinemia in the Gunn rat, suggesting that this procedure could potentially be used for the treatment of inherited metabolic liver diseases.
ConclusionsThe use of isolated viable cells to restore the function of organs and tissues is emerging as a promising therapeutic tool. In the field of Hepatology, multiple sources of hepatic stem/precursor cells have been identified, and preliminary evidence of therapeutic effectiveness has been provided in animal models. However, we have to learn more on the mechanisms of liver regeneration, including the role of stem/precursor cells. Definite (and possibly joined) protocols for the selection of a specific cell population and for in vitro expansion/differentiation should be developed, as well as protocols for clinical liver repopulation. The long-term fate of the transplanted cells should also be assessed in animal models, with respect to function, possible extra-hepatic localization, genetic/epigenetic stability and especially tumorigenesis. The risk is that superficial planning, without adequate consideration and knowledge of the underlying pathophysiology, will result in poorly focused clinical trials and possible complications, which could in turn originate skepticism on the development of cell therapy in Hepatology. Even if the biological characteristics of hepatic stem cells still justify the hope for successful future clinical applications, only a more cautious and systematic «bench to bedside» approach will guarantee consistent results.
AcknowledgmentWe thank Professor Giuseppe Realdi and Professor Claudio Tiribelli for helpful discussion.