Background and objectives: The COVID-19 pandemic imperiled the global health system. We aimed to determine the impact of COVID-19 on the care continuum of HCV-infected patients.
Material and Methods: Two hundred and fifty-six patients who were prescribed a course of DAA therapy at three tertiary medical centers in the US and China between January 1, 2019 to June 30, 2020 were included. We assessed the proportions of patients who completed DAA therapy and had HCV RNA testing during and after the end of therapy. We also assessed the impact of utilization of telemedicine.
Results: The proportion of patients undergoing HCV RNA testing during DAA treatment decreased from >81.7% before pandemic to 67.8% during the pandemic (P=0.006), with a more prominent decrease in the US. There were significant decreases in HCV RNA testing >12 (P<0.001) and >20 weeks (P<0.001) post-treatment during COVID-19 era. Compared to pre-COVID period, post-treatment clinic encounters during COVID-19 era decreased significantly in China (Xi'an: 13.6% to 7.4%; Nanjing: 16.7% to 12.5%) but increased in the US (12.5% to 16.7%), mainly due to the use of telemedicine. There was a 4-fold increase in utilization of telemedicine in the US.
Conclusions: COVID-19 pandemic carried profound impact on care for HCV patients in both the US and China. HCV cure rate assessment decreased by half during COVID era but the proportion of patients finishing DAA therapy was not significantly affected. Increased utilization of telemedicine led to increased compliance with DAA therapy but did not encourage patients to have their laboratory assessment for HCV cure.
Since the outbreak of coronavirus disease 2019 (COVID-19) from Wuhan, China to the globe, the pandemic has dramatically imperiled the healthcare system worldwide [1,2]. The fear of being exposed to COVID-19 in a clinic or hospital setting, disruption in mobility and access to care by local policy, and the global attention on COVID-19 pandemic have affected the long-term combat against various diseases [3,4]. For example, this pandemic will impact the World Health Organization (WHO) goal of eliminating viral hepatitis worldwide in 2030 [5].
Currently, the United States (US)’s progress in viral elimination lags far behind other countries (e.g., Australia, France, Iceland, Italy, Japan, South Korea, Spain, Switzerland and the United Kingdom). A recent study indicated that the US will not be able to achieve the goal of viral elimination in 2050 if the current progress is not scaled up [6]. To exacerbate this problem, the cascade of care of hepatitis C virus (HCV) infection might be delayed during COVID-19 pandemic period as recent reports showed a significant decrease in hepatology follow-up visits and hepatocellular carcinoma (HCC) surveillance in the US, Japan, and Singapore [7]. Hospital admissions due to liver-related complications was also reduced during the outbreak [8]. Given the differences in the severity of pandemic and the response of countries, the impact of pandemic on testing and treatment of HCV may vary across countries. Understanding the magnitude of this impact is crucial so that key stakeholders can find remedies to improve the cascade of care. No true solutions have been found so far as updated data are lacking and the problem is still evolving.
The direct-acting antiviral (DAA) therapy is associated with high sustained virological response (SVR), lower risk of advanced liver diseases, and significant increased survival rate of HCC patients [9–13]. Therefore, the emphasis of viral elimination has shifted from pursuing curative armamentarium to establishing a coordinated public health approach to effective screening and treating individuals with HCV infection [14]. However, a recent modeling study showed that the COVID-19 impact on cascade of care of HCV patients will result in substantial increase in HCC and death worldwide by 2030 [15].
Therefore, we aimed to determine the impact of COVID-19 on the care of patients with HCV infection treated with DAAs and assessed the utility of telemedicine before and during COVID-19 pandemic. We conducted a multinational, multicenter cross-sectional study to compare the frequency of HCV RNA testing, follow-up visits, and the completion rates of DAA therapy among study sites in Los Angeles (southwest US), Xi'an (West China), and Nanjing (East China).
MethodsThis is a cross-sectional study of 256 patients with HCV infection who received DAAs at three tertiary medical centers in Los Angeles, USA (Cedars-Sinai Medical Center), Xi'an, China (The Second Affiliated Hospital of Xi'an Jiaotong University), and Nanjing, China (Nanjing Drum Tower Hospital) between January 1, 2019 to June 30, 2020 and followed until November 30, 2020. We identified patients through electronic health medical record at each site and manually reviewed the medical charts for outpatient visit encounter notes, clinical, laboratory, and pharmacy data of adult HCV patients. Patients were excluded if they were treated with DAA before 2019 or after June 2020, under 21 years of age, or died during DAA treatment. The study protocol was approved by the Institutional Review Board at each study site.
Study design and definitionsThe study period was divided into pre-COVID-19 and COVID-19 era. The COVID-19 era was defined as January 1, 2020 to June 30, 2020 while pre-COVID era was defined as two control periods to account for variation in months (January 1, 2019 to June 30, 2019) and to assess the continual trend in targeted outcomes (July 1, 2019 to December 31, 2019). Patients included during COVID-19 era had a minimum of 21 weeks of follow-up.
We included all FDA- and China FDA (cFDA)-approved DAAs in this study. Patients were considered to have completed a full course of DAA therapy if they were noted to have finished 8 or 12 weeks of DAA therapy according to the DAA used. HCV PCR testing was done in each study site. The limits of detection for HCV RNA were 15 IU/mL (The COBAS® Ampliprep/COBAS® Taqman HCV Test, v2.0, Roche Molecular Systems, Inc.) at Cedars-Sinai Medical Center, 20 IU/ml (Roche COBAS AmpliPrep/COBAS TaqMan HCV Quantitative Test, V 2.0) at Nanjing Drum Tower Hospital, 25 IU/ml (Roche) and 100 or 25 IU/ml (Hunan Shengxiang or Roche) at the Second Affiliated Hospital of Xi'an Jiaotong University. To assess the impact of COVID on delaying the assessment for HCV cure or SVR, we analyzed the proportions of patients who had HCV PCR testing between weeks 12-24, 15-20 and 24-26. To account for multi-provider groups, we defined follow-up visits as visits with the prescribing physician or the department which prescribed the DAA. We defined telemedicine visits as encounters for follow-up visits during/after DAA treatment performed using video or phone call platforms.
Statistical analysisFor descriptive statistics, we summarized categorical variables using proportion (%) and continuous variable using mean ± standard deviation (SD). We compared categorical variables using Chi-squared test. The proportion of HCV patients who completed DAA therapy was computed. We also estimated the proportion of patients with HCV RNA testing and follow-up visit during and after DAA treatment. We used three time periods (12-14, 15-20, and 24-26 weeks after end of DAA therapy) to account for the real-life postponement of SVR 12 testing due to physicians’ or patients’ preference. The trend was assessed by using Cochran-Armitage test. We also categorized patients who utilized telemedicine by socioeconomical factors. The study was analysed by R software (version 4.0.4). We defined significance as having a two-sided p-value below 0.05.
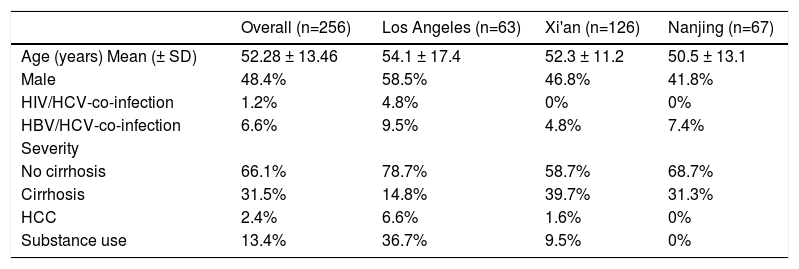
ResultsThe demographic and clinical characteristics of included patients are depicted in Table 1. The mean age was 52.28 ± 13.46 years, with 48.4% of the cohort being males. The proportion of HIV- or HBV-co-infected patients were less than 8% while those with substance use disorder constituted 13.4% of the cohort. A third of the cohort had cirrhosis before receiving DAAs. When categorizing patients by study sites, patients from Los Angeles cohort had a higher proportion of male, HIV patients, HBV-co-infected patients, and individuals with substance use than Nanjing and Xi'an cohorts. The proportion of cirrhotic patients was the highest in Xi'an cohort (39.7%), followed by Nanjing (31.3%) and Los Angeles cohort (14.8%). Educational background information was derived from the China's cohorts, with approximately three quarters of HCV patients having middle or high school education (Supplemental Table 1). The medical insurance system is different between the two countries. In the US, a third of the patients had government-sponsored insurance (Medicare), another one third insured under private insurance plan (Preferred Provider Organization (PPO)) and a third had managed care plans or other forms of plans. In China, 13.5% and 88.1% of patients had governmental insurance in Xi'an and Nanjing cohorts respectively.
Demographic and clinical characteristics of 256 included patients
HCC: Hepatocellular carcinoma; SD: standard deviation
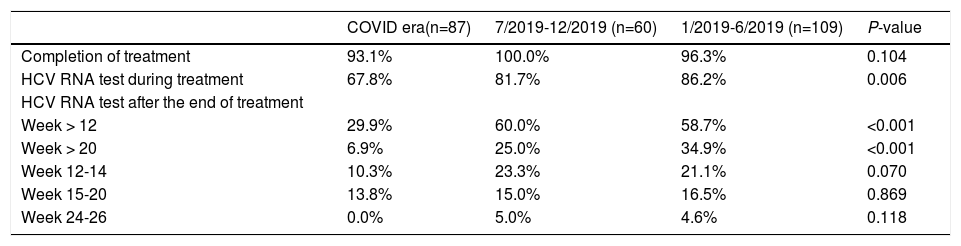
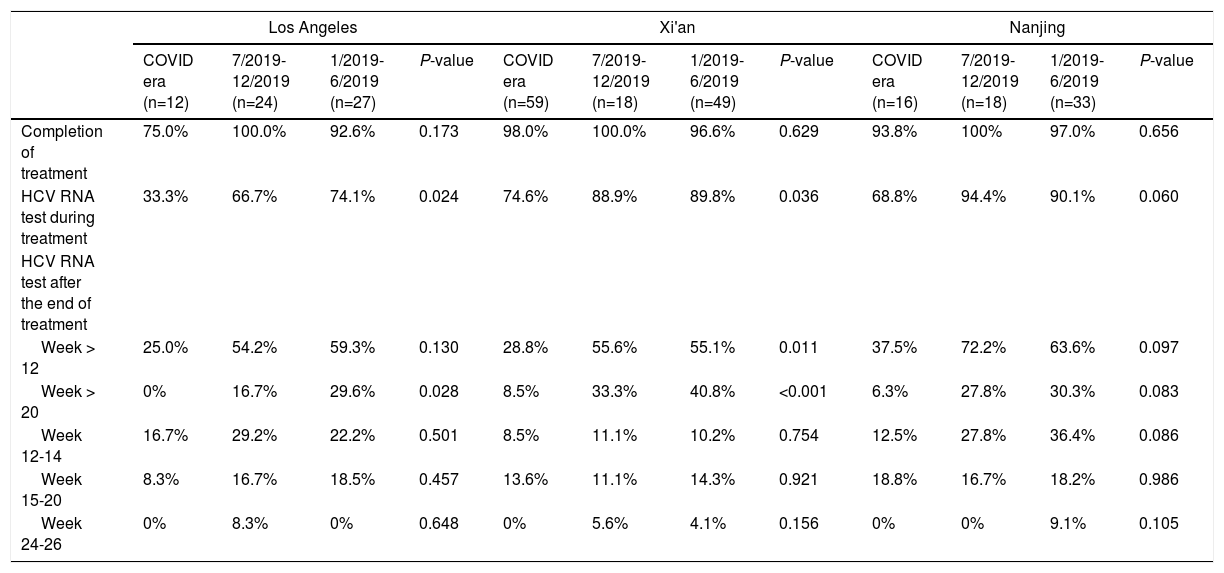
There was no significant difference in the proportion of patients who completed DAAs during or before the COVID-19 pandemic (Table 2). However, the proportion of patients undergoing HCV RNA testing during DAA treatment significantly decreased during the COVID-19 pandemic, with only 67.8% of patients had the testing during and 81.7%-86.2% before pandemic (P = 0.006). The proportion of patients undergoing HCV RNA testing during DAA treatment decreased during pandemic period in all three cohorts (Table 3). In the Los Angeles cohort, the proportion of tested patients during COVID era (33.3%) was about half of that during 7/2019-12/2019 (66.7%) and 1/2019-6/2019 (74.1%) (P = 0.024). In Xi'an, the proportions of tested patients decreased markedly from about 89% in 2019 to 74.6% during COVID era (P = 0.036) while they were above 90% in 2019 and 68.8% during COVID era (P = 0.060) in the Nanjing cohort.
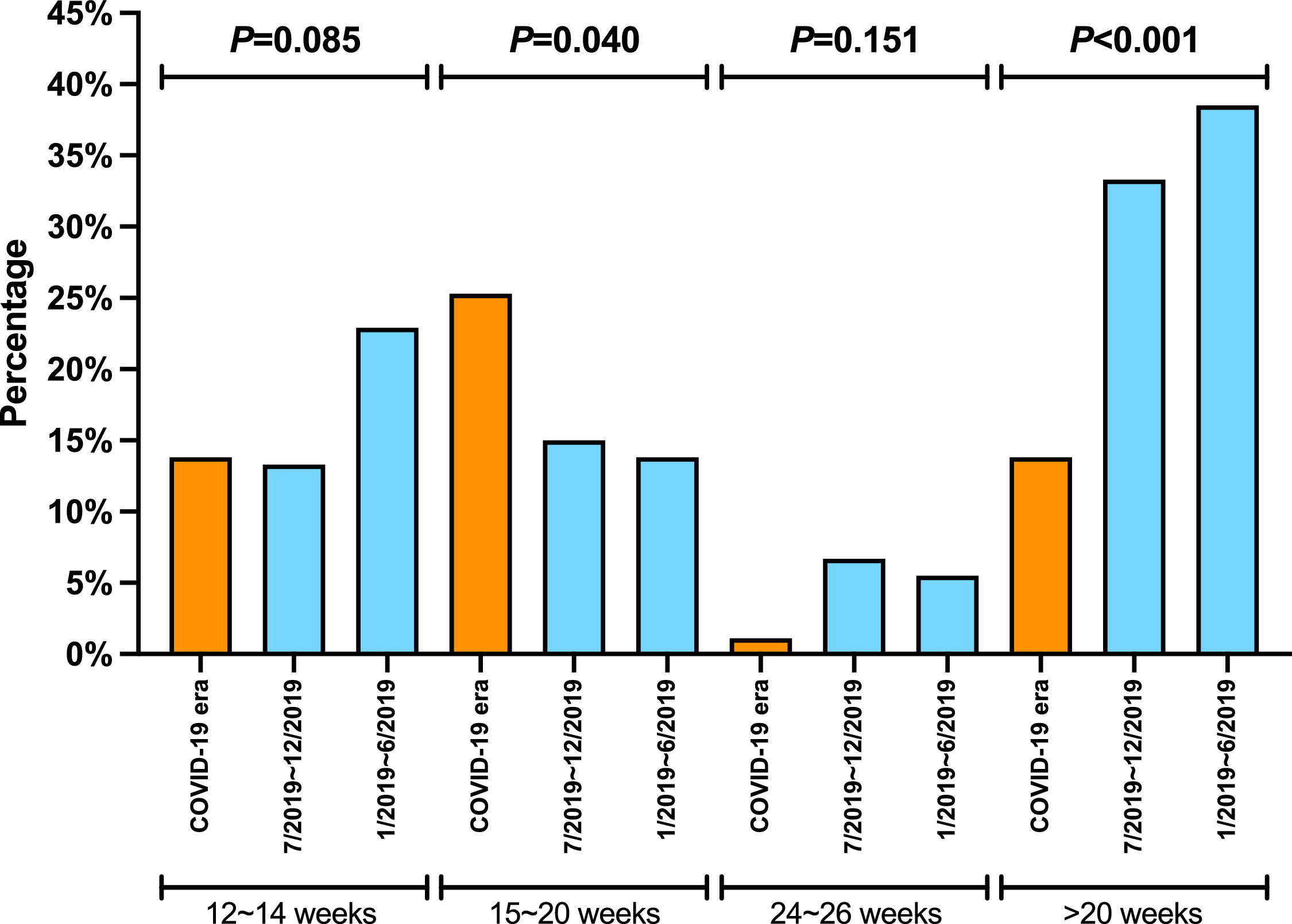
Assessment of the response to the DAA therapy
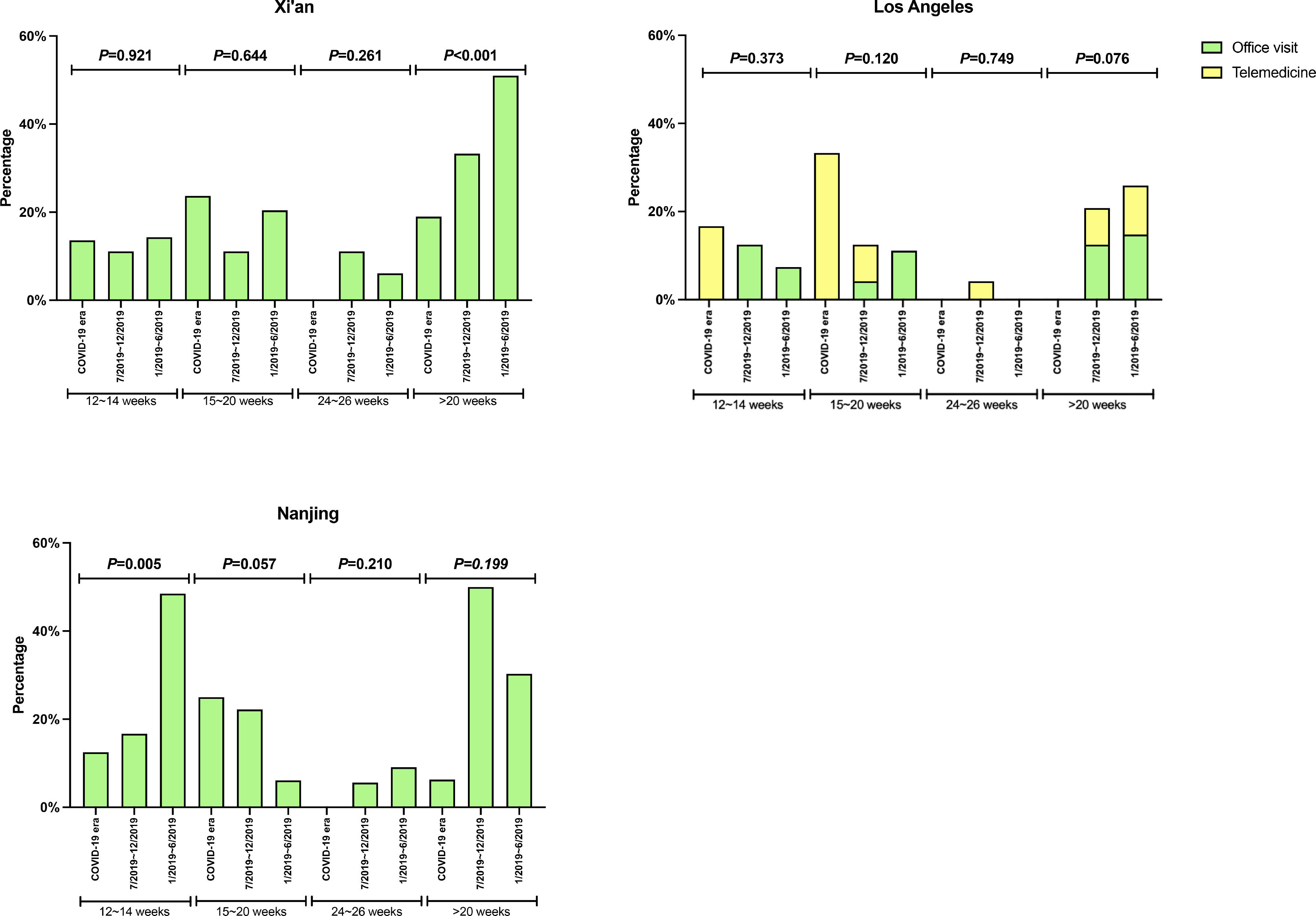
Assessment of the response to the DAA therapy according to study sites
The proportion of patients undergoing HCV RNA testing during DAA treatment period plummeted from 86.2% in 1/2019-6/2019 and 81.7% in 7/2019-12/2019 to 67.8% during COVID era (P = 0.006) (Table 2). Consistently, the proportion of post-treatment HCV RNA testing decreased significantly. The proportions of testing 12 weeks after treatment decreased from about 60% in 2019 to 29.9% during COVID era (P < 0.001). The decrease in HCV RNA testing 20 weeks after treatment decreased from 34.9% during 1/2019-6/2019 and 25% during 7/2019-12/2019 to 6.9% during COVID era (P < 0.001). When subgrouped by post-treatment weeks, there were decreasing trends in post-treatment week 12-14, 15-20, and 24-26. The trends were not significant, probably due to small sample size. Furthermore, the proportions at week 24-26 were very low (5% or less) in both 2019 and 2020. While none of the Los Angeles cohort patients had their HCV cure rate assessment done at 20 weeks post-treatment during COVID-19 pandemic, 8.5% and 6.3% of the Xi'an and Nanjing cohorts had HCV RNA assessed more than 20 weeks after treatment, respectively (Table 3).
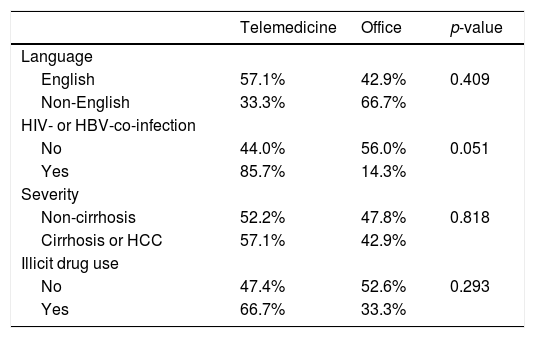
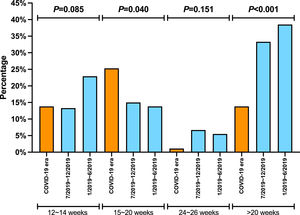
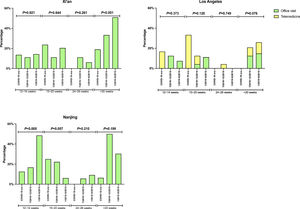
There was no significant difference in the proportion of patients receiving follow-up visits at 12-14 and 24-26 weeks after DAA treatment (Figure 1). Of note, the proportion of follow-up visit at 15-20 weeks post treatment increased during COVID-19 pandemic. Further categorization by study site revealed that patients from Los Angeles had significantly increased 15-20 weeks post treatment visit from 8.3% to 33.3% during COVID-19 pandemic period due to the utilization of telemedicine (Figure 2). Telemedicine also increased the number of follow-up visits 24-26-weeks and >20-weeks post treatment for those who received DAAs in 2019 (Table 4). Categorization of the use of telemedicine by socioeconomic and clinical characteristics showed that the patients with HIV- or HBV-co-infection had a higher proportion of telemedicine use during COVID-19 pandemic.
The proportion of patients who had clinic encounter after the completion of DAA treatment, categorized by type of visit. (A) Los Angeles cohort (B) Xi'an cohort (C) Nanjing cohort
Y axis denotes the proportion of patients who had clinic visit after the completion of DAA therapy. X axis denotes the time period (weeks) after the completion of DAA therapy. Blue bar denotes office visit while orange bar denotes telemedicine (video or audio). There were no visits via telemedicine in Nanjing cohort and Xi'an cohort.
In this study, we illustrated the cascade of care for patients treated by DAA before and during COVID-19 pandemic. We collected data from the US as well as the east and west sides of China and found a significant decrease in the proportion of treated HCV patients undergoing HCV RNA test during and after treatment. We also showed a reduction in follow-up visits during the pandemic in both sites of China but not in the US. Further analysis revealed that it was the utilization of telemedicine that boosted the number of clinic encounters during the COVID-19 pandemic.
Poor medication compliance may lead to treatment failure, [16] and re-treatment will increase the cost and burden of the disease. Completion rate of HCV therapy in China was close to 100% throughout the study period. However, this proportion was reduced from >92% to 75% in the US during the pandemic period. Although the decrease did not meet the definition of significance, this might reflect the severity of disease outbreak in the US (Los Angeles area) and medication non-compliance under the stress of pandemic. Medication non-compliance could have been worse if it was not for the encouragement offered by telemedicine.
Timely HCV RNA testing is essential to determine the efficacy of DAAs. The US showed a lower proportion of testing during the pandemic than in China. This could be due to the fact that routine HCV RNA testing during therapy is not recommended in the AASLD HCV guidance but is a standard procedure in China [17,18]. HCV RNA testing after completion of treatment for SVR 12 or cure is the standard of care. In real world, the schedule of HCV PCR testing may be affected by patient or physician factors. The fear for COVID-19 exposure at the testing site, the lockdown/stay at home policy and the patients’ confidence in achieving a cure rate of >90% with DAA therapy led to significantly lower SVR12 assessment during the pandemic period.
The cost of DAA therapy plays an important factor in the cascade of care of patients with HCV. Since 2017, there has been increasing number of DAAs being included in the China National Formulary reimbursement list leading to offering wider DAA coverage by the local governments. Sofosbuvir/Ledipasvir, Elbasvir/Grazoprevir and Sofosbuvir/Velpatasvir were added to the National Formulary in 2020. After their addition to the national formulary, the cost of Sofosbuvir/Ledipasvir therapy plunged from RMB 21660 (USD 3327) to RMB 2188 (USD 336), Elbasvir/Grazoprevir therapy from RMB 13000 (USD 1997) to RMB 2193 (USD 337), and Sofosbuvir/Velpatasvir from RMB 23200 (USD 3564) to RMB 4368 (USD 671). In anticipation of adding more DAA to the formulary of the Shaanxi province in 2020 (Xi'an is the capital city of Shaanxi), several physicians decided to postpone treating HCV patients in 2019. This explains the low proportion of patients being covered by governmental insurance in the Xi'an cohort. Moreover, “warehousing” of patients till the DAA became reimbursable in 2020 explains the smaller sample size of patients being treated from July to December 2019. Cost reduction is important to enhance the accessibility of the DAAs, especially to patients with socioeconomic disadvantage [19].
Of note, the proportions of patients with substance use disorder as well as HIV and HBV co-infected patients were higher in the US cohort. This is the result of the US HCV elimination project that has been launched since early 2014. In addition, most of the baby boomers with insurance coverage being followed at Cedars-Sinai Medical Center were treated between 2014 and 2018. Therefore, the HCV patients in the US cohort in 2019 and 2020 were more likely patients with newly infected (> 6 months) or re-infected with HCV. Infection through sexual activity or injection drug use are common pathways for acquiring HCV in recent years [20]. Furthermore, the majority of HCV treated patients in China have middle or high school education. One can hypothesizes that this group of patients had financial means to afford the DAA therapy prior to the therapy becoming sponsored by the government.
The pandemic has also necessitated changes in the practice of HCV treatment. The number of in-person outpatient services plummeted, while the utilization of telemedicine in health care surged during COVID-19 pandemic period in the US [21–23]. The increase use of telemedicine has helped patients to avoid exposure to COVID-19, preserved supplies of personal protection equipment, reduced the cost of travel and parking, and cut down patient's and physician's waiting time. Altogether, it enhances the adherence to medication and post-treatment follow-up during the COVID-19 pandemic. In our study, we showed that the use of telemedicine increased the number of follow-up visits when compared to 2019 (from 8.5% to 33.3%). In contrast, two sites of China had lower proportion of follow-up visits during the pandemic. However, access to telemedicine is influenced by the patients’ access to enabling devices such as a smartphone or a computer. Patients of low socioeconomic status have limited access to such devices. The discordance between the increase in the number of telemedicine visits and the low number of HCV PCR testing may be explained by the patients refusing to have their labs drawn during the pandemic.
There are several limitations in our study. First, the data on educational background is not available in the US cohort as the electronic medical records did not capture this information. Second, the dates of the DAA prescription may not reflect the start and end date of therapy. To overcome this issue, the investigators manually reviewed the charts and/or contacted the patients to determine the exact start and end date of treatment. Third, the duration of COVID-19 outbreak was different in China than in the US. The outbreak started in January 2020 in China, but it became more prevalent in the US in March 2020. In this study, we defined the COVID-19 pandemic on both sites as the same period to have better comparison. Finally, patients who received DAA in June of 2020 did not have follow-up greater than 20 weeks as we followed the patients until November 30, 2020. The proportion of both HCV RNA testing and clinic encounters may be affected. However, there were less than 10 patients receiving DAA during this period. Therefore, this will not change the main results.
ConclusionThe profound impact of COVID-19 pandemic on the cascade of care for HCV in both the US and China halted the global HCV elimination momentum. In our study, the assessment for a HCV cure in patients who completed their DAA therapy has decreased significantly during the pandemic era. Patients had less laboratory visits as reflected by the decreased number of HCV PCR tests. The utilization of telemedicine emerged as an important tool to enhance the provision of health during pandemic. In the midst of the COVID-19 pandemic, physicians and patients must remain vigilant to the cascade of care of HCV infection. Stakeholders should develop feasible interventions to retain HCV patients in care, even after the completion of DAAs while withstanding the pandemic.
None.