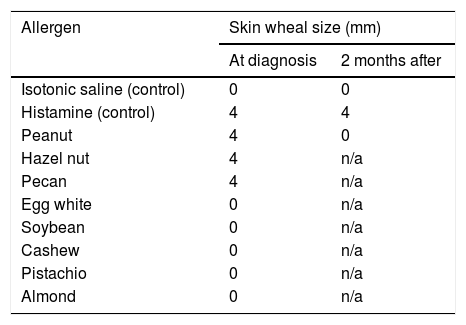
31 years old female with a history of contact dermatitis, eczema, allergic rhinitis, pernicious anemia, alopecia areata and latent tuberculosis was treated concurrently with methotrexate along with isoniazid and pyridoxine. Five months into the therapy she developed acute onset jaundice progressing into fulminant liver failure with altered mentation and worsening liver function tests. Extensive workup including serological and histopathological evaluation revealed drug-induced liver injury as the etiology of her liver failure and she underwent a successful orthotropic liver transplant. On post-transplant follow-up at four months, she was noted to have an allergic reaction consisting of a perioral rash and swelling (without anaphylaxis) after receiving a kiss from her significant other who had just eaten a peanut butter chocolate. She denied any history of allergic reaction to peanuts prior to the transplant. Percutaneous skin testing revealed immediate hypersensitivity to peanut, hazelnut, and pecan believed to be acquired newly post-transplant. Further investigation revealed that the organ donor had a documented history of systemic anaphylaxis from the peanut allergy and a positive peanut-specific IgE level. Also, another parallel solid organ recipient (lung transplant) from the same organ donor experienced a serious anaphylactic reaction after peanut exposure. This is a case of food (peanut) allergy transfer from the donor to the recipient after the liver transplant. This case highlights the importance of incorporating known donor allergies as a part of pre-transplant screening, given the potentially serious consequences from the transfer of allergies to a previously anergic recipient.
31 years old female with a history of shingles, eczema, contact dermatitis, allergic rhinitis, and pernicious anemia (requiring temporary vitamin B12 injections) presented to her primary care physician with patchy hair loss. She was evaluated by dermatology and diagnosed with alopecia areata. After worsening hair loss and failure of localized intradermal Interleukin 10 injections, she was initiated on methotrexate and tapering doses of prednisone. During her pre-methotrexate evaluation, she was found to have latent tuberculosis and concurrently treated with isoniazid/pyridoxine by the infectious disease team. Five months into her combined therapy (methotrexate/isoniazid/prednisone), she presented to her primary care physician with scleral icterus, jaundice, and abdominal discomfort. Laboratory investigation at that time revealed liver function tests with elevated aspartate aminotransferase (AST) of 1665IU/L, alanine aminotransferase (ALT) of 1419IU/L, and total bilirubin (T. bili) of 6.7mg/dl. She was sent home with close lab monitoring but was brought to the emergency room by her family 2 weeks later due to altered mentation and worsening jaundice. Her repeat liver panel tests were notable for AST of 1492IU/L, ALT of 1990IU/L, T. bili of 36.3mg/dl, and her coagulation panel was notable for elevated prothrombin time (PT) of 53.1 (seconds) and international normalized ratio (INR) of 5.7. She was thought to have acute hepatic encephalopathy in the setting of acute fulminant liver failure. Hospital workup and biopsy was consistent with drug induced liver failure and she underwent successful emergent orthotropic liver transplant two days after presentation.
Post-transplant, she tolerated the immunosuppressant therapy well and her liver function tests and coagulation tests normalized. Her initial immunosuppression regimen included tacrolimus, mycophenolate mofetil and prednisone (10mg daily), however mycophenolate was later discontinued due to leucopenia and upper respiratory infection. Four months post-transplant, she was noted to have an immediate allergic reaction consisting of a perioral rash and swelling (without anaphylaxis) after receiving a kiss from her significant other who had just eaten a peanut butter chocolate. She denied any history of allergic reaction to peanuts or other nuts prior to transplant. She was referred to an allergist and was found to have developed immediate hypersensitivity to peanut, hazel nut, and pecan on percutaneous skin testing (Table 1). Serologic testing was negative for total IgE (normal, <1IU/ml), peanut specific allergen and recombinant allergens (<0.1kU/L). Further investigation revealed that the organ donor had a documented history of systemic anaphylaxis from peanut allergy with a peanut specific IgE level of 9.74kU/L and peanut component testing showing positive IgE to Ara h 1, 2, 8 and 9. In addition, previously anergic parallel patient who had received lung transplant from the same donor experienced a serious allergic anaphylactic reaction after exposure to peanuts a few months post-transplant.
Two months after the acquired peanut allergy from liver transplant, the patient followed up in allergy clinic. Skin testing was repeated during that visit and showed negative results for peanut allergies. She subsequently underwent monitored oral food challenge with a peanut butter cup which led to minimal symptoms of lip tingling without developing a rash or an overt hypersensitivity reaction. There was no change in her immunosuppression throughout this period and around the time of repeat allergy testing.
2DiscussionThis is a case of post-transplantation antigen sensitization in a patient with previous anergy to specific antigens like peanuts, hazel nuts, and pecans.
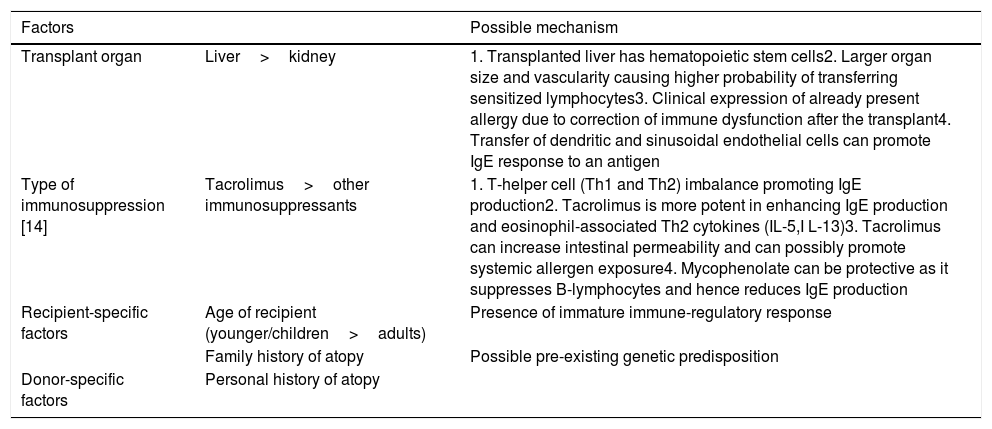
The development of de novo food allergies in transplant recipients has been described after solid organ transplants predominantly in pediatric populations with frequencies ranging from 5% to 38% [1–4]. Furthermore, immunosuppression with tacrolimus was found to be associated with the development of allergies. This effect was also seen primarily in pediatric populations but the type of organ transplanted was also implicated as a risk factor. Interestingly, multiple studies showed that in patients transplanted at a younger age, the development of allergies are much more common in recipients of liver transplants compared to kidney transplants [1,2,5]. Bone marrow, liver and small bowel transplants result in transplantation of hematopoietic lymphoid tissue and are thus affiliated with allergen sensitization [6]. Multiple factors that have been implicated in the process of acquiring new allergies post-transplant are summarized in Table 2[7].
Factors implicated in transplant-acquired food allergy.
| Factors | Possible mechanism | |
|---|---|---|
| Transplant organ | Liver>kidney | 1. Transplanted liver has hematopoietic stem cells2. Larger organ size and vascularity causing higher probability of transferring sensitized lymphocytes3. Clinical expression of already present allergy due to correction of immune dysfunction after the transplant4. Transfer of dendritic and sinusoidal endothelial cells can promote IgE response to an antigen |
| Type of immunosuppression [14] | Tacrolimus>other immunosuppressants | 1. T-helper cell (Th1 and Th2) imbalance promoting IgE production2. Tacrolimus is more potent in enhancing IgE production and eosinophil-associated Th2 cytokines (IL-5,I L-13)3. Tacrolimus can increase intestinal permeability and can possibly promote systemic allergen exposure4. Mycophenolate can be protective as it suppresses B-lymphocytes and hence reduces IgE production |
| Recipient-specific factors | Age of recipient (younger/children>adults) | Presence of immature immune-regulatory response |
| Family history of atopy | Possible pre-existing genetic predisposition | |
| Donor-specific factors | Personal history of atopy | |
Although exact or defining central pathways are yet to be elucidated, various suggested mechanisms and associated factors that lead to post transplantation presentation of food allergy have been postulated as below (5) [7]:
- 1.
Passive transfer of donor IgE. IgE has a short half-life ranging from a few days in serum to a few weeks on the surface of tissue resident mast cells [8].
- 2.
Transfer of allergen specific T- and/or B-lymphocytes, leading to active production of specific IgE for months after transplantation [9,10]. This results in ongoing cellular and humoral immune response to the allergen.
- 3.
Presence of an immature immune-regulatory response in transplant recipients that fail to suppress the expression of new acquired food allergies [11].
Transfer of allergen-specific IgE mediated hypersensitivity after allogeneic bone marrow transplant has been well recognized [12] and a substantial proportion of patients may maintain hypersensitivity past one year. Liver homes pluripotent hematopoietic stem cells which are capable of generating allergen-specific lymphocytes upon transfer to recipient [13]. These carrier lymphocytes can persist for months in the recipient causing delayed food allergy onset in transplant recipients [3]. To detect donor-origin cells in the recipient, DNA extraction and amplification for HLA genotype micro-chimerism testing has been done in the blood and skin of transplant recipients. However, cases have been reported with both detection [9] as well as absence [8] of such micro-chimerism in the recipient skin or blood, thereby highlighting the multi-pathway mechanisms for such allergy transfer. Another case reported a circulating population of donor-derived CD86+ memory T cells in the recipient that could contribute to maintaining the allergen-specific IgE response [14]. The mechanisms involving the transfer of allergen-specific immunoglobulins and lymphocytes would not explain the development of food allergy in transplant recipients where the donors did not have a confirmed similar food allergy [11].
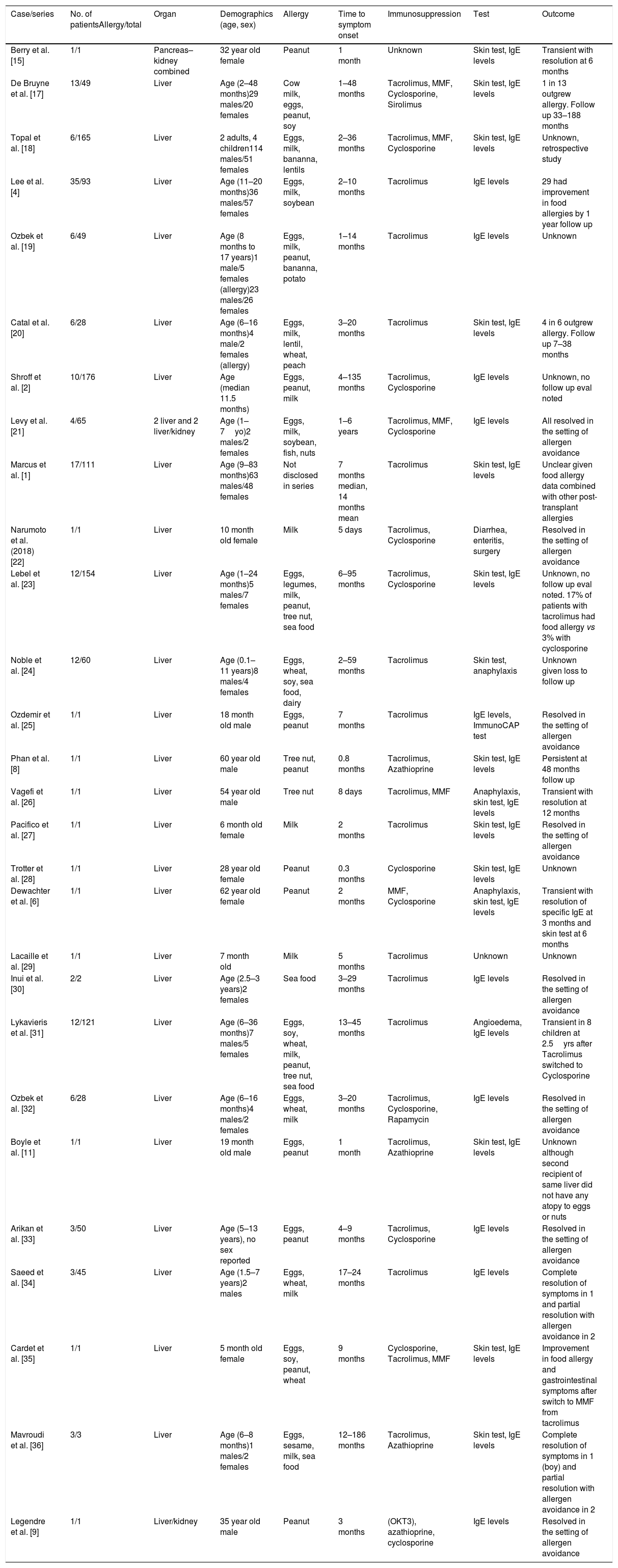
Due to the small number of reported cases of food allergy transfer after solid organ transplant in adults, it is unclear whether the effect is temporary or permanent [6,8,9]. Most of the cases and patient series have been reported in pediatric population and have been summarized in Table 3. In a report by Berry et al. [15], a patient was followed up with serial serum IgE and skin prick testing to show normalization of these biomarkers at 6 months suggesting allergen sensitization in some patients is a transient phenomenon. Similarly, in our patient, the skin patch testing results became negative over 3 months followed by successful oral peanut challenge. For these cases, given the rapid loss of sensitization in these patients, passive transfer of donor IgE is the most likely mechanism of food allergy transfer. Also of note is the possible relation of rash development to mycophenolate discontinuation, which can be protective (Table 2).
Summary of Liver Transplant Acquired Food Allergy Cases.
| Case/series | No. of patientsAllergy/total | Organ | Demographics (age, sex) | Allergy | Time to symptom onset | Immunosuppression | Test | Outcome |
|---|---|---|---|---|---|---|---|---|
| Berry et al. [15] | 1/1 | Pancreas–kidney combined | 32 year old female | Peanut | 1 month | Unknown | Skin test, IgE levels | Transient with resolution at 6 months |
| De Bruyne et al. [17] | 13/49 | Liver | Age (2–48 months)29 males/20 females | Cow milk, eggs, peanut, soy | 1–48 months | Tacrolimus, MMF, Cyclosporine, Sirolimus | Skin test, IgE levels | 1 in 13 outgrew allergy. Follow up 33–188 months |
| Topal et al. [18] | 6/165 | Liver | 2 adults, 4 children114 males/51 females | Eggs, milk, bananna, lentils | 2–36 months | Tacrolimus, MMF, Cyclosporine | Skin test, IgE levels | Unknown, retrospective study |
| Lee et al. [4] | 35/93 | Liver | Age (11–20 months)36 males/57 females | Eggs, milk, soybean | 2–10 months | Tacrolimus | IgE levels | 29 had improvement in food allergies by 1 year follow up |
| Ozbek et al. [19] | 6/49 | Liver | Age (8 months to 17 years)1 male/5 females (allergy)23 males/26 females | Eggs, milk, peanut, bananna, potato | 1–14 months | Tacrolimus | IgE levels | Unknown |
| Catal et al. [20] | 6/28 | Liver | Age (6–16 months)4 male/2 females (allergy) | Eggs, milk, lentil, wheat, peach | 3–20 months | Tacrolimus | Skin test, IgE levels | 4 in 6 outgrew allergy. Follow up 7–38 months |
| Shroff et al. [2] | 10/176 | Liver | Age (median 11.5 months) | Eggs, peanut, milk | 4–135 months | Tacrolimus, Cyclosporine | IgE levels | Unknown, no follow up eval noted |
| Levy et al. [21] | 4/65 | 2 liver and 2 liver/kidney | Age (1–7yo)2 males/2 females | Eggs, milk, soybean, fish, nuts | 1–6 years | Tacrolimus, MMF, Cyclosporine | IgE levels | All resolved in the setting of allergen avoidance |
| Marcus et al. [1] | 17/111 | Liver | Age (9–83 months)63 males/48 females | Not disclosed in series | 7 months median, 14 months mean | Tacrolimus | Skin test, IgE levels | Unclear given food allergy data combined with other post-transplant allergies |
| Narumoto et al. (2018) [22] | 1/1 | Liver | 10 month old female | Milk | 5 days | Tacrolimus, Cyclosporine | Diarrhea, enteritis, surgery | Resolved in the setting of allergen avoidance |
| Lebel et al. [23] | 12/154 | Liver | Age (1–24 months)5 males/7 females | Eggs, legumes, milk, peanut, tree nut, sea food | 6–95 months | Tacrolimus, Cyclosporine | Skin test, IgE levels | Unknown, no follow up eval noted. 17% of patients with tacrolimus had food allergy vs 3% with cyclosporine |
| Noble et al. [24] | 12/60 | Liver | Age (0.1–11 years)8 males/4 females | Eggs, wheat, soy, sea food, dairy | 2–59 months | Tacrolimus | Skin test, anaphylaxis | Unknown given loss to follow up |
| Ozdemir et al. [25] | 1/1 | Liver | 18 month old male | Eggs, peanut | 7 months | Tacrolimus | IgE levels, ImmunoCAP test | Resolved in the setting of allergen avoidance |
| Phan et al. [8] | 1/1 | Liver | 60 year old male | Tree nut, peanut | 0.8 months | Tacrolimus, Azathioprine | Skin test, IgE levels | Persistent at 48 months follow up |
| Vagefi et al. [26] | 1/1 | Liver | 54 year old male | Tree nut | 8 days | Tacrolimus, MMF | Anaphylaxis, skin test, IgE levels | Transient with resolution at 12 months |
| Pacifico et al. [27] | 1/1 | Liver | 6 month old female | Milk | 2 months | Tacrolimus | Skin test, IgE levels | Resolved in the setting of allergen avoidance |
| Trotter et al. [28] | 1/1 | Liver | 28 year old female | Peanut | 0.3 months | Cyclosporine | Skin test, IgE levels | Unknown |
| Dewachter et al. [6] | 1/1 | Liver | 62 year old female | Peanut | 2 months | MMF, Cyclosporine | Anaphylaxis, skin test, IgE levels | Transient with resolution of specific IgE at 3 months and skin test at 6 months |
| Lacaille et al. [29] | 1/1 | Liver | 7 month old | Milk | 5 months | Tacrolimus | Unknown | Unknown |
| Inui et al. [30] | 2/2 | Liver | Age (2.5–3 years)2 females | Sea food | 3–29 months | Tacrolimus | IgE levels | Resolved in the setting of allergen avoidance |
| Lykavieris et al. [31] | 12/121 | Liver | Age (6–36 months)7 males/5 females | Eggs, soy, wheat, milk, peanut, tree nut, sea food | 13–45 months | Tacrolimus | Angioedema, IgE levels | Transient in 8 children at 2.5yrs after Tacrolimus switched to Cyclosporine |
| Ozbek et al. [32] | 6/28 | Liver | Age (6–16 months)4 males/2 females | Eggs, wheat, milk | 3–20 months | Tacrolimus, Cyclosporine, Rapamycin | IgE levels | Resolved in the setting of allergen avoidance |
| Boyle et al. [11] | 1/1 | Liver | 19 month old male | Eggs, peanut | 1 month | Tacrolimus, Azathioprine | Skin test, IgE levels | Unknown although second recipient of same liver did not have any atopy to eggs or nuts |
| Arikan et al. [33] | 3/50 | Liver | Age (5–13 years), no sex reported | Eggs, peanut | 4–9 months | Tacrolimus, Cyclosporine | IgE levels | Resolved in the setting of allergen avoidance |
| Saeed et al. [34] | 3/45 | Liver | Age (1.5–7 years)2 males | Eggs, wheat, milk | 17–24 months | Tacrolimus | IgE levels | Complete resolution of symptoms in 1 and partial resolution with allergen avoidance in 2 |
| Cardet et al. [35] | 1/1 | Liver | 5 month old female | Eggs, soy, peanut, wheat | 9 months | Cyclosporine, Tacrolimus, MMF | Skin test, IgE levels | Improvement in food allergy and gastrointestinal symptoms after switch to MMF from tacrolimus |
| Mavroudi et al. [36] | 3/3 | Liver | Age (6–8 months)1 males/2 females | Eggs, sesame, milk, sea food | 12–186 months | Tacrolimus, Azathioprine | Skin test, IgE levels | Complete resolution of symptoms in 1 (boy) and partial resolution with allergen avoidance in 2 |
| Legendre et al. [9] | 1/1 | Liver/kidney | 35 year old male | Peanut | 3 months | (OKT3), azathioprine, cyclosporine | IgE levels | Resolved in the setting of allergen avoidance |
In summary, although organ recipients and donors undergo a comprehensive pre-transplantation screening, allergy testing is not incorporated as a standard item. Given the potentially serious consequences of allergy transfer post-transplant, careful screening of the donor to including history of food and non-food allergies, when possible, could help identify transplant recipients at risk of experiencing post-transplant anaphylaxis. The ease of an allergy screen makes it a useful tool as we strive for positive outcomes and good organ stewardship post transplantation.AbbreviationsIgE immunoglobulin E aspartate aminotransferase alanine aminotransferase total bilirubin
No funding source to declare.
Conflict of interestNo conflict of interest exists.
Margaret Wiggins, BSN, RN, CCTC (Transplant Nurse Coordinator).