Hepatitis C signifies a highly prevalent infection in patients with end stage renal disease. Most frequently it is associated to glomerulonephritis, patients on hemodyalisis and renal transplants. The prevalence of HCV antibodies in hemodialysis patients varies between 5-70% depending on the geographical location of the patients. Factors associated with the prevalence of anti HCV in patients with hemodialysis include: age, blood transfusions, tattoos, use of illegal drugs, time in hemodialysis, more than two hospitalizations, treatment in multiple hemodialysis units or a kidney transplant. In some of the reported outbreaks of hepatitis in hemodialysis units, the phylogenetic analysis indicate that the transmission of HCV could relate to failures or breaches in general precautions in the management of these type of patients resulting in nosocomial transmission owed to sharing equipment or instruments employed in the hemodialysis or by transmission from professional members of the hemodialysis units. Antiviral treatment may be affected by a number of co-factors and co-morbidities, it consist mainly of non pegylated interferon or pegylated inter-feron. The treatment with interferon after a renal transplant is associated with an increase in the number of rejections; reason enough to recommended that treatment should be administered before the transplant.
Patients with chronic renal failure are at greater risk to acquire hepatitis C because of their frequent exposure to blood products or to hepatitis C virus (HCV) contaminated medical equipment.
The most frequent renal diseases associated to hepatitis C infection include: glomerulonephritis, patients on hemodyalisis and renal transplants.
Hcv Related GlomerulonephritisGlomerulonephritis develops many years often decades, after the initial infection with hepatitis C. The most common type of nephropathy is the membranous glomerulopathy commonly in the context of cryoglobulinemia. The triad of purpura, asthenia and arthralgia occurs in approximately 30% of cases. Renal signs of cryoglobulinemia include proteinuria, microscopic hematuria with mild to moderate renal insufficiency. The majority of patients go on to develop severe hypertension often difficult to con-trol.1
The treatment of these patients entails the administration of blood pressure lowering and antiprotei-nuric agents, diuretics, inhibitors of the renin-angiotensin system (inhibitors of the converting enzyme of angiotensin or blockers of the receptors II of angiotensin), hypolipidemic agents, antivirals and immunosuppressive drugs.
Antiviral treatment may be affected by a number of co-factors and co-morbidities, it consist mainly of non pegylated interferon or pegylated interferon for 6 months. Pegylated interferon is available in two different presentations, Peg-Interferon alpha 2a which is metabolized in liver and kidneys and Peg-In-terferon alpha 2 b that is eliminated only in kidneys. This results in pharmacokinetic differences with a slow onset of secondary effects by Peg-Inter-feron alpha 2a, however at the end the adverse effects in both groups are similar.2
Patients with mixed cryoglobulinemia and renal involvement have also been treated with plasma exchange administration with the purpose of removing circulating cryoglobulins or by the administration of immunosuppressive drugs (steroids or cyclophosphamide) to suppress antibody or cryoglobulin production. The immunosupprsessive agents increase the risk of viral replication.3
For patients unresponsive to the treatment with steroids or other immunosuppressant, Rituximab (human-mouse chimeric monoclonal antibody, which selectively depletes B cells by binding to the CD20 cells cell surface antigen) has been proposed either alone or combined with Peg-Interferon.4
Hepatitis C in Dialysis PatientsOverall the prevalence of HCV antibodies in he-modialysis patients varies between 5-10% in developed countries and 10-70% in developing countries.
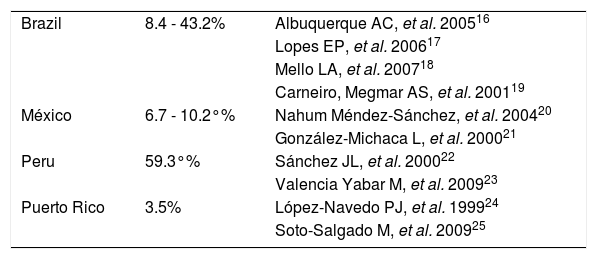
In Latin-America the prevalence of hepatitis C in patients with hemodialysis varies greatly according to its geographical location (Table 1).
Prevalence of hepatitis C in hemodialysis patients in Latin America.
| Brazil | 8.4 - 43.2% | Albuquerque AC, et al. 200516 |
| Lopes EP, et al. 200617 | ||
| Mello LA, et al. 200718 | ||
| Carneiro, Megmar AS, et al. 200119 | ||
| México | 6.7 - 10.2°% | Nahum Méndez-Sánchez, et al. 200420 |
| González-Michaca L, et al. 200021 | ||
| Peru | 59.3°% | Sánchez JL, et al. 200022 |
| Valencia Yabar M, et al. 200923 | ||
| Puerto Rico | 3.5% | López-Navedo PJ, et al. 199924 |
| Soto-Salgado M, et al. 200925 |
Patients on hemodialysis are at greater risk to become infected with hepatitis C with a delay in se-roconversion, often in the initial stages they present normal aminotransferases. This has led to the recommendation of requesting anti HCV every three months. Accordingly this represents an important hurdle in their diagnosis as it weakens precisely the diagnostic value of the aminotransferases in the diagnosis of hepatitis C in these patients. The fact that frequently the levels of aminotransferases are normal especially at the beginning of the renal damage is an inconsistency that has been associated to deficiency of vitamin B 6, presence of uremic toxins or blood components that interfere with UV absorption. Evenmore aminotransferases do not relate with the viral load or the magnitude of tissular damage.1
Factors associated with the prevalence of anti HCV in patients with hemodialysis include: age, blood transfusions, tattoos, use of illegal drugs, time in hemodialysis, more than two hospitalizatio-ns, treatment in multiple hemodialysis units or a kidney transplant.
In some of the reported outbreaks of hepatitis in hemodialysis units, the phylogenetic analysis indicate that the transmission of HCV could relate to failures or breaches in general precautions in the management of these type of patients resulting in nosocomial transmission owed to sharing equipment or instruments employed in the hemodialysis or by transmission from professional members of the he-modialysis units.
It has been demonstrated that some of the more useful regulations in order to lessen the frequency of HCV contamination in the hemodialysis units include frequent hand washing by the personnel, the use of disposable globes at each particular procedure, the circulation of the personnel and the adequate washing of the dialysis machines. These recommendations have been validated and recommended by the Centers of Disease Control and prevention (CDC) in the USA.5-6
Isolation of patients in hemodialysis infected with hepatitis C is controversial and is not recommended by the CDC, an alternative is that patients infected with hepatitis C access to their connection and disconnection after the non-infected patients.7
Clinical trials linked to the treatment of patients with hepatitis C in hemodialysis are incomplete because of the small number of patients included in the protocols, frequently the studies are no randomized and hardly any of them include cases and controls.
Regarding the use of interferon in patients with hepatitis C on hemodialysis, in 2008 two meta-analysis were published, one analyzing 645 patients and another that included 459 patients (19 studies were duplicates). The overall sustained viral response rate (SVR) was 40%, in genotype 1, 33%. Overall pegylated interferon offered few additional benefits than conventional interferon. Adverse effects were reported in < 50% of cases. A significant number of patients did however had anemia that was not reported but that required the administration of erythropoietin, intravenous iron or blood transfusions. Flu like symptoms occured in 41%. Administration of the antiviral treatment had to be stopped in 11% of the cases.8
In 2009 another metanalysis details information with regard to factors associated to SVR in patients with hepatitis C on hemodialysis treated with interferon. The authors performed a search in Medline from 1966 to Feb 2009. They revise 2795 articles of which 119 were likely relevant for the theme. At the end only 21 works were included that satisfied the inclusion criteria for the analysis. The results show that treatment should consist on the administration of at least three millions of units of interferon, three times a week for a minimum of six months. When they compared the 21 studies, the SVR responsde in patients in hemodyalisis treated with interferon varied between 41 and 45%. It was found that those factors associated with a greater SVR include a larger dose of interferon, a more prolonged time of treatment, female gender, lower basal levels of HCV-RNA and a rapid virological response.
Discontinuation of interferon due to adverse effects in the hemodialysis patients was greater among patients with a previous renal transplant, presence of liver cirrhosis or the use of more than 3 millions units of interferon.9-10
Most of the studies with interferon in patients with hepatitis C on hemodialysis by including small samples often lead to non reliable studies and very seldom are set to compare the risk-benefit between standard and pegylated interferon. We can take for granted that further well designed studies are needed with larger cohorts.
Patients with end stage renal diseases have a decrease capacity of clearing ribavirin, a drug whose metabolism is mainly renal, therefore increased levels of ribavirin suppose a higher risk of hemolytic anemia, circumstance that leads to the recommendation to avoid its use in patients in hemodialysis.
In a study that included 78 patients in hemodialy-sis with hepatitis C treated with Peg-Interferon alpha 2a at a dose of 135 μg/week, it was found that 48/78, 61,5% had a rapid viral response at week 12. The response at end of treatment was evaluated in 21 patients (26.9%) that were treated for 48 weeks. Only 15-patients (19.2% of the initially enrolled) had undetectable levels of HCV-RNA. In these 15 patients, SVR was achieved in 11 (14.1% of the initial population on intention to treatment). A high prevalence of non compliance 32% was documented and adverse effects were reported in 83%, which were mainly minor. The incidence of severe adverse effects was 0.19/patient/year (median 20.5 weeks) and the incidence of deaths was 0-11/patient/ year.11
Another study included 16 patients treated with Peg - Interferon alpha 2b at a dose of 1.0 μg/kg (9 patients) or 0.5 μg/kg (7 patients). In the group of patients receiving pegylated interferon at the dose of 1.0 μg/kg severe adverse effects leading to interruption of treatment occurred in 5 patients (56%) and in 2 (28%) of the patients in the group receiving 0.5 μg/kg. The most common adverse effects were arterial hypertension and infections not related to neu-tropenia. Of the patients treated with Peg-Interferon alpha 2b at a dose of 1.0 μg/kg, 22% achieved SVR while none of the patients in the group on 0.5 μg/kg achieved SVR, five patients in the group of 1.0 μg/kg completed 24 weeks or more of treatment and of them 2 (40%) had a SVR.12
In another study that included 12 patients treated with Peg-Interferon alpha 2a at a dose of 13 μg/ week for 48 weeks, 75% of the patients achieved a SVR, 75% had anemia, 58% fatigue, 33% thrombo-cytopenia and 33% leucopenia.13
Hepatitis C and Kidney TransplantCommonly the objectives of the treatment of a patient infected with hepatitis C on hemodialysis are similar to those of patients who are not on hemo-dialysis, however in these patients the pre-trans-plant period offers greater opportunities of response in particular in absence of an optimal scheme in the post-transplant treatment. The infection with HCV before the transplant is as high as 40%. These kinds of patients have a greater risk of diabetes, glomeru-lonephritis and sepsis.
The treatment with interferon after a renal transplant is associated with an increase in the number of rejections; reason enough to recommended that treatment should be administered before the transplant. The administration of Interferon post-transplant one or three times per week in patients with stable renal function, improves liver function and in 25% of the cases HCV-RNA becomes negative.14
In a meta-analysis of 12 clinical studies (only one controlled) which included 102 patients that evaluate the efficacy and security of the antiviral therapy with Interferon (Interferon or Interferon + ribavirin) on patients with a renal transplant and hepatitis C, SVR was achieved in 18%. Patients had to abandon treatment in 35%. The most frequent cause of the interruption of the treatment was the dysfunction of the renal implant (n = 28, 71.7%). Vascular rejection or acute cellular rejection was present in 15 - 60% and the loss of the implant, often irreversible and resistant to steroids in 20%. This study suggests that the therapy based on Interferon is poorly tolerated and of low security if administered after the renal transplant.15
Hepatitis C infection remains highly prevalent in patients with end stage renal disease, the guidelines for its prevention, diagnosis and treatment are under regular assessment in order to incorporate newer evidence as it becomes available.