Introduction. Type-1 hepatorenal syndrome (HRS-1) portends a poor prognosis in patients with cirrhosis. Currently available medical therapies are largely ineffective, save for liver transplantation. We aimed to determine if pentoxifylline (PTX) therapy in addition to the standard of care of volume expansion with albumin and vasoconstriction with midodrine and octreotide (AMO) is safe and efficacious compared to AMO in HRS-1 treatment.
Material and methods. Hospitalized subjects with decompensated cirrhosis and HRS-1 were enrolled. PTX or placebo was administered with AMO therapy for up to 14 days. The primary endpoint was HRS-1 resolution (serum creatinine ≤ 1.5 g/dL for > 24 h). Secondary endpoints were change in creatinine and MELD score, partial treatment response, 30-and 180-day overall and transplant free survival.
Results. Twelve subjects with mean age 58.9 ± 6.2 years were enrolled and randomized. Mean MELD score was 26.5 ± 7.4 and 58.3% were male. Overall cohort 30- and 180-day survival was 58.3% and 33.3% respectively. Two subjects underwent liver transplantation. HRS-1 resolution (16.7% vs. 16.7%, p = 1.000), partial treatment response (33.3% vs. 16.7%, p = 0.505), change in creatinine (+0.48 g/dL, 95% CI -0.49-1.46 vs. +0.03 g/dL, 95% CI -0.640.70, p = 0.427), 30-day survival (66.6% vs. 50.0%, p = 0.558) and 180-day survival (50.0% vs. 16.7%, p = 0.221) were similar between the two groups. Serious adverse events necessitating treatment discontinuation were rare (n = 1, PTX).
Discussion. The addition of PTX to AMO in the treatment of HRS-1 is safe when compared to the current standard of care. Future large-scale prospective study to validate the efficacy of this treatment seems warranted.
Hepatorenal syndrome (HRS) is a feared complication of chronic liver disease and cirrhosis presenting a unique challenge to physicians. The incidence of Type 1 hepatorenal syndrome (HRS-1) in patients with cirrhosis and ascites is 18 and 39 % at one and five years, respectively.1 HRS-1 occurs in the absence of underlying kidney disease and leads to renal failure secondary to compensatory renal vasoconstriction due to splanchnic arterial vasodilation and an ineffective interarteriolar blood volume.2,3 The prognosis of HRS-1 is poor with median life expectancy around two weeks.4 Patients often decompensate and die prior to liver transplantation. The current standard of care to treat HRS-1 in North America is the combination of albumin, octreotide and midodrine (AMO).5 Terlipressin in combination with albumin is also effective, however, this is not approved by the Food and Drug Administration or available currently in the United States.6 Noradrenalin and albumin may also be used, however this requires Intensive Care Unit monitoring which is not applicable for all patients or all hospitals.7 Patients who develop HRS-1 and survive are more likely to have HRS recurrence. After the withdrawal of treatment for HRS-1, recurrence can occur in up to 20% of cases.5
Pentoxifylline (PTX) is a phosphodiesterase inhibitor with anti-inflammatory capabilities that lowers blood viscosity and improves erythrocyte flexibility. Pro-inflammatory cytokines, including tumor necrosis factor alpha (TNFa) and interleukin-6 are elevated in patients with cirrhosis in response to circulating endotoxemia.8,9 These cytokines lead to a pro-inflammatory, hyperdynamic state. PTX has been shown to not only decrease levels of TNFa,10 but also to increase systemic vascular resistance directly opposing splanchnic vasodilation without precipitating an increase in the pressure in the portal venous system.11 Patients with HRS have even greater levels of TNFα when compared to cirrhosis patients without HRS.12
Clinically, PTX has been shown to be effective in preventing HRS in patients both with acute alcoholic hepatitis13 and cirrhosis;12,14 however, despite this, no direct survival benefit has been observed. To our knowledge, no study to date has investigated adding PTX to the algorithm for the treatment of HRS-1 despite the benefit of PTX when used as primary prophylaxis. We hypothesize that the addition of PTX to AMO is safe and efficacious when compared to AMO plus placebo in the treatment of HRS1 in hospitalized patients with decompensated cirrhosis.
Material And MethodsPatients at the University of Virginia Health System hospitalized with decompensated cirrhosis and acute renal failure were screened for enrollment from July 2014 through June 2016. HRS-1 was defined according to the criteria put forth by the American Association for the Study of Liver Disease as:
- •
Cirrhosis with ascites;
- •
Serum creatinine greater than 1.5 mg/dL;
- •
No improvement of serum creatinine (decrease to a level of 1.5 mg/dL or less) after at least two days with diuretic withdrawal and volume expansion with albumin;
- •
Absence of shock;
- •
No current or recent treatment with nephrotoxic drugs; and
- •
Absence of parenchymal kidney disease as indicated by proteinuria > 500 mg/day, microhematuria (> 50 red blood cells per high power field), and/or abnormal renal ultrasonography.15
All participants were older than or equal to 18 years of age. Patients with labeled contraindications to PTX were excluded [allergy or hypersensitivity to PTX or intolerance to methylxanthines (e.g. caffeine, theophylline), recent cerebral or retinal hemorrhage or pregnancy, or if they had concurrent use of nephrotoxic drugs, uncontrolled bacterial infection, renal parenchymal disease (e.g. acute tubular necrosis, glomerular disease, interstitial nephritis, urinary obstruction), shock, TNFα antagonist use, severe or poorly controlled comorbid disease as determined by the principal investigator to hinder the ability of the subject to adhere to study protocols. No institutionalized subjects or prisoners were enrolled. No surrogate consent was obtained in subjects with uncontrolled hepatic encephalopathy.
Following enrollment, each subject underwent a predosing medical history, physical exam, and safety assessment, which included a review of laboratories, imaging tests and procedural results performed during their hospitalization. Subjects were blinded to the study investigators and medical care team and were randomized in 1:1 ratio to either a 14-day course of PTX 400 mg three times a day or the equivalent dose adjusted for renal impairment [400 mg twice a day for estimated glomerular filtration rate (eFFR) 10-50 mg/dL and 400 mg once a day for eGFR < 10 mg/ dL] or to placebo. On day three, seven, 10 and 14, an indepth assessment for treatment response and review of side effect profile with medical history and physical exam was completed. All adverse events, including those that were serious, were graded according to the National Cancer CTCAE version 4.0. If a patient was determined to be a “partial responder,” as defined as serum creatinine level decreased by > 50% from baseline but not < 1.5 mg/dL, without dialysis or HRS recurrence, the treating physician was allowed to continue therapy in a blinded fashion for an additional 14 days at their discretion. If a patient was unable to swallow a pill for six consecutive doses, they were withdrawn from the study as PTX cannot be administered through a gastric feeding tube due to its pharmacologic properties.
The primary endpoint was the incidence of HRS-1 resolution, which was defined as a decrease in serum creatinine level to < 1.5 mg/dL for > 24 h without dialysis or death.4,16–18 Secondary endpoints assessed were change in serum creatinine from baseline to day 14, change in Model for End Stage Liver Disease (MELD) score from baseline to day 14, incidence of treatment failure on day 14 (defined as creatinine level above baseline value after day seven, renal replacement therapy or death), partial response without dialysis or HRS recurrence, transplant free survival at day 30 and 180 and overall survival at day 30 and 180. MELD score was calculated using the standard formula (Figure 1), with a lower limit of 1.0 for all variables.19 Survival was assessed by telephone interview and a review of the medical record.
All analyses were conducted based on the intention to treat methodology. Results were expressed as means with standard deviations. Chi-squared and Fisher s exact test were used to analyze categorical endpoints. Student s t test and Mann-Whitney Rank Sum test were used for secondary analysis of differences in continuous lab data. KaplanMeier curves were constructed for overall and transplant-free 180-day survival and compared by a logrank test to assess our secondary endpoints of survival. Subjects were right-censored if they died, were transplanted, progressed to the need for renal replacement therapy or were lost to follow-up. A p value of < 0.05 was considered significant. Data analysis and graph generation were performed using SAS Version 9.4 (Cary, North Carolina, USA) and GraphPad Prism version 7.03 for Windows, GraphPad Software (La Jolla, California, USA). Study approval was obtained from the University of Virginia Institutional Review Board for Health Sciences Research.
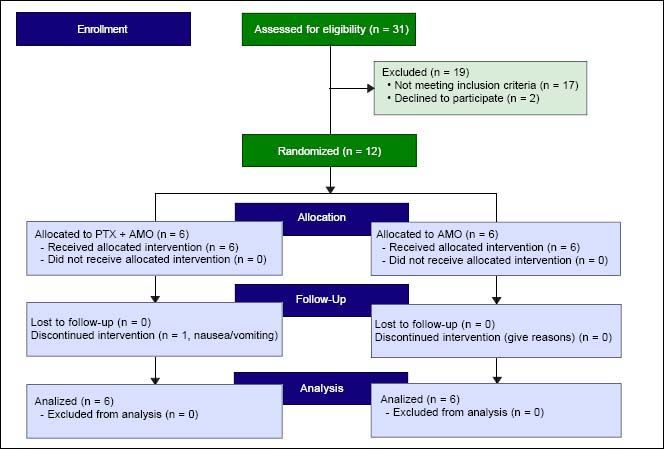
ResultsTwelve subjects with mean age 58.9 ± 6.2 years were enrolled and randomized, six in the PTX + AMO arm and six in the placebo + AMO arm (Figure 2). Overall cohort mean MELD score was 26.5 ± 7.4 and all subjects had Child-Pugh-Turcotte Class B (n = 2) or C (n = 10) liver disease. 58.3% were male. The leading etiologies of liver disease included alcoholic cirrhosis (50.0%) and nonalcoholic steatohepatitis (25.0%). One subject each with chronic hepatitis C, hereditary hemochromatosis and autoimmune hepatitis was enrolled. Overall cohort 30- and 180-day survival was 58.3% and 33.3% respectively. Two subjects underwent liver transplantation, one in the PTX group and one in the control group. There were no clinically significant differences in baseline characteristics (demographics, liver disease etiology, baseline laboratories, medications, HRS risk factors, or manifestations of portal hypertension) between the intervention and the control group, although two PTX subjects had been prescribed AMO treatment in the previous week prior to transfer to our tertiary care institution for further medical care (Table 1). There was a trend towards significance in the use of acid suppressing medications in the standard of care group (33.3% PTX vs.83.3%, p = 0.079).
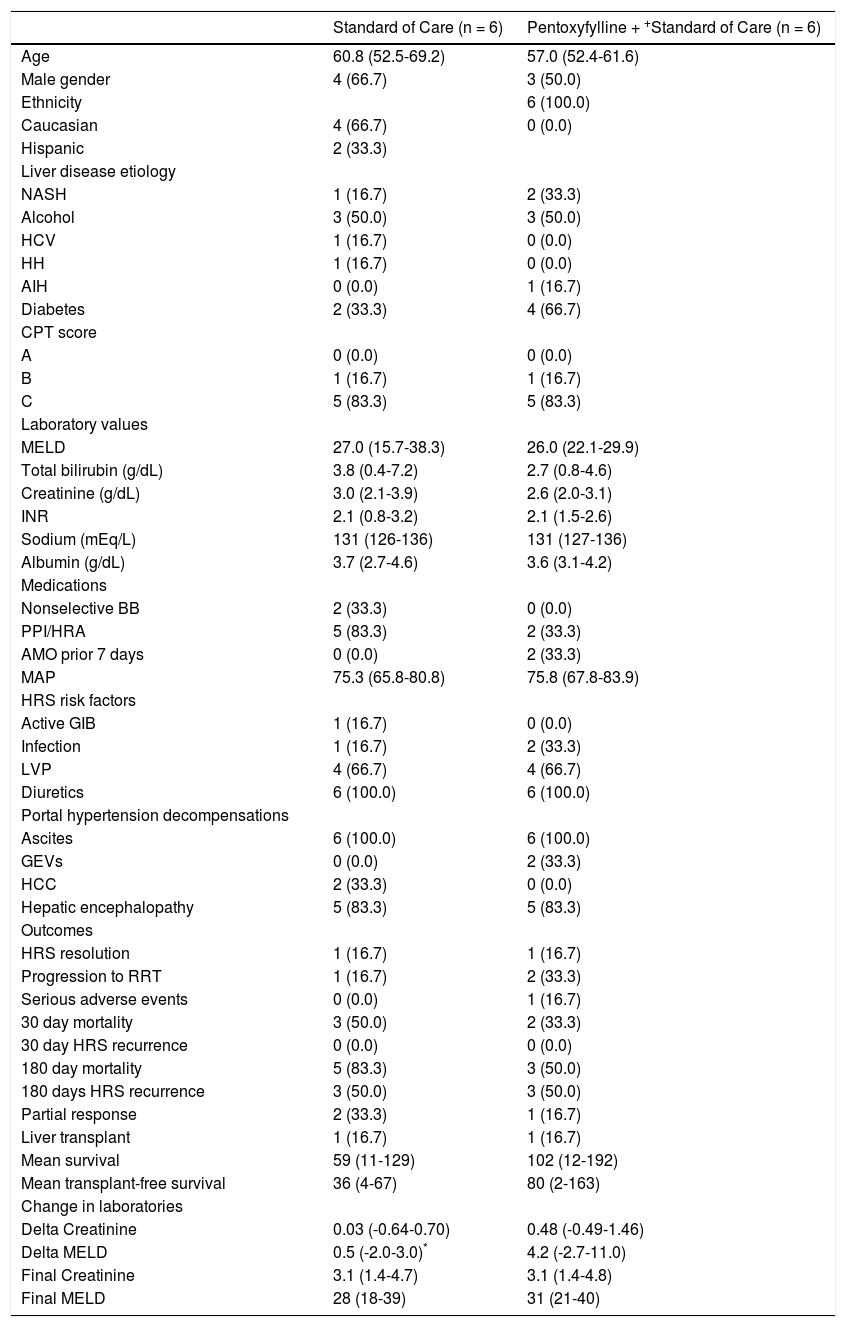
Univariate comparison looking at Standard of Care (albumin, midodrine and octretotide) compared to Pentoxyfylline + Standard of Care.
| Standard of Care (n = 6) | Pentoxyfylline + +Standard of Care (n = 6) | |
|---|---|---|
| Age | 60.8 (52.5-69.2) | 57.0 (52.4-61.6) |
| Male gender | 4 (66.7) | 3 (50.0) |
| Ethnicity | 6 (100.0) | |
| Caucasian | 4 (66.7) | 0 (0.0) |
| Hispanic | 2 (33.3) | |
| Liver disease etiology | ||
| NASH | 1 (16.7) | 2 (33.3) |
| Alcohol | 3 (50.0) | 3 (50.0) |
| HCV | 1 (16.7) | 0 (0.0) |
| HH | 1 (16.7) | 0 (0.0) |
| AIH | 0 (0.0) | 1 (16.7) |
| Diabetes | 2 (33.3) | 4 (66.7) |
| CPT score | ||
| A | 0 (0.0) | 0 (0.0) |
| B | 1 (16.7) | 1 (16.7) |
| C | 5 (83.3) | 5 (83.3) |
| Laboratory values | ||
| MELD | 27.0 (15.7-38.3) | 26.0 (22.1-29.9) |
| Total bilirubin (g/dL) | 3.8 (0.4-7.2) | 2.7 (0.8-4.6) |
| Creatinine (g/dL) | 3.0 (2.1-3.9) | 2.6 (2.0-3.1) |
| INR | 2.1 (0.8-3.2) | 2.1 (1.5-2.6) |
| Sodium (mEq/L) | 131 (126-136) | 131 (127-136) |
| Albumin (g/dL) | 3.7 (2.7-4.6) | 3.6 (3.1-4.2) |
| Medications | ||
| Nonselective BB | 2 (33.3) | 0 (0.0) |
| PPI/HRA | 5 (83.3) | 2 (33.3) |
| AMO prior 7 days | 0 (0.0) | 2 (33.3) |
| MAP | 75.3 (65.8-80.8) | 75.8 (67.8-83.9) |
| HRS risk factors | ||
| Active GIB | 1 (16.7) | 0 (0.0) |
| Infection | 1 (16.7) | 2 (33.3) |
| LVP | 4 (66.7) | 4 (66.7) |
| Diuretics | 6 (100.0) | 6 (100.0) |
| Portal hypertension decompensations | ||
| Ascites | 6 (100.0) | 6 (100.0) |
| GEVs | 0 (0.0) | 2 (33.3) |
| HCC | 2 (33.3) | 0 (0.0) |
| Hepatic encephalopathy | 5 (83.3) | 5 (83.3) |
| Outcomes | ||
| HRS resolution | 1 (16.7) | 1 (16.7) |
| Progression to RRT | 1 (16.7) | 2 (33.3) |
| Serious adverse events | 0 (0.0) | 1 (16.7) |
| 30 day mortality | 3 (50.0) | 2 (33.3) |
| 30 day HRS recurrence | 0 (0.0) | 0 (0.0) |
| 180 day mortality | 5 (83.3) | 3 (50.0) |
| 180 days HRS recurrence | 3 (50.0) | 3 (50.0) |
| Partial response | 2 (33.3) | 1 (16.7) |
| Liver transplant | 1 (16.7) | 1 (16.7) |
| Mean survival | 59 (11-129) | 102 (12-192) |
| Mean transplant-free survival | 36 (4-67) | 80 (2-163) |
| Change in laboratories | ||
| Delta Creatinine | 0.03 (-0.64-0.70) | 0.48 (-0.49-1.46) |
| Delta MELD | 0.5 (-2.0-3.0)* | 4.2 (-2.7-11.0) |
| Final Creatinine | 3.1 (1.4-4.7) | 3.1 (1.4-4.8) |
| Final MELD | 28 (18-39) | 31 (21-40) |
In general, the two different study arms were similar in baseline characteristics, safety and efficacy.
octretotide. BB: beta blocker. CPT: Child-Pugh-Turcotte. GEV: gastroesophageal varices. GIB: gastrointestinal bleeding. HCC: hepatocellular carcinoma. HCV: hepatitis C: HH; hereditary hemochromatoss. HRA: histamine receptor antagonist. HRS: hepatorenal syndrome. INR: international normalized ratio. MAP: mean aterial pressure. MELD: Model for Endstage Liver Disease. NASH: Nonalcoholic Steatohepatitis. PPI: proton pump inhibitor. RRT: renal replacement therapy.
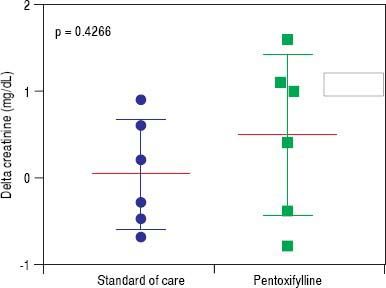
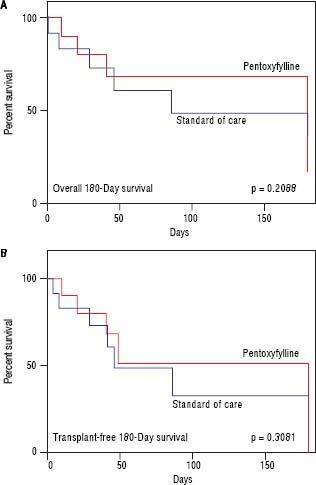
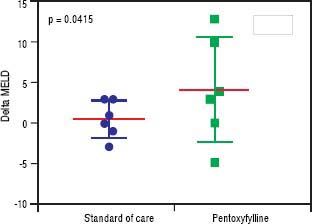
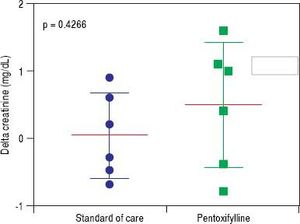
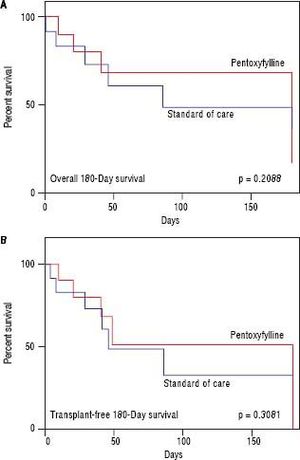
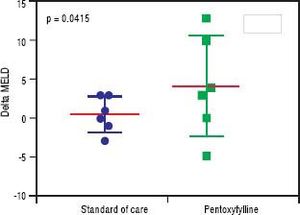
HRS-1 resolution was identical between the two groups with one subject in each arm (16.7%). No differences were seen in partial treatment response [n = 2 (33.3%) vs. n = 1 (16.7%), p = 0.505], change in creatinine (+0.48 g/dL, 95% CI -0.49-1.46 vs. +0.03 g/dL, 95% CI 0.64-0.70, p = 0.427) (Figure 3), 30-day survival [n = 4 (66.6%) vs. n = 3 (50.0%), p = 0.558] and 180-day survival [n = 3 (50.0%) vs. n = 1 (16.7%), p = 0.221] (Figure 4) when comparing PTX to the control group. Mean survival (102.0 days, 95% CI 11.7-192.3 vs. 58.8 days, 95% CI 10.9128.5, p = 0.357) and mean transplant-free survival (80.2 days, 95% CI 2.3-162.7 vs. 35.8 days, 95% CI 4.3-67.3, p = 0.226) were also similar for PTX compared to control. A significant difference was seen when looking at the change in MELD over the course of the entire study as the PTX group had a significantly greater increase in MELD score (+4.2, 95% CI -2.7 to + 11.0) vs. the AMO group (+0.5, 95% CI -2.0 to +3.0) driven largely by the above mentioned change in creatinine, p = 0.042 (Figure 5), however both initial (26.0, 95% CI 22.1-29.9 PTX vs. 27.0, 95% CI 15.7-38.3, p = 0.835) and final MELD scores (30.5, 95% CI 21.4-39.6 PTX vs. 28.0, 95% CI 17.5-38.5, p = 0.654) were statistically similar. No subjects were lost to follow-up. Serious adverse events necessitating treatment discontinuation were rare. One subject in the PTX group discontinued treatment due to significant nausea and vomiting which was later attributed to another concomitant medication. No adverse events ≥ grade 3 were documented in the AMO group.
In a randomized, placebo-controlled, triple blinded pilot study, we found that PTX, when added to standard of care with volume expansion and vasoconstriction, is a safe alternative to the standard of care alone. This potential novel therapy is important to consider in light of the abysmal prognosis for cirrhosis patients who develop HRS-1, especially those who are not eligible for liver transplantation where progression of disease more often than not leads to a palliative, endof-life approach.
PTX has been shown previously to be effective in preventing HRS in patients with either acute alcoholic hepatitis or cirrhosis.12-14 In their study of 101 patients, Akriviadis, et al.,13 demonstrated that compared to placebo, patients randomized to 400 mg PTX orally three times a day had a 71% lower risk of developing HRS as a cause of death (RR 0.29; 95% CI 0.13-0.65, p = 0.009). More importantly, the authors found improved survival (24.5% vs. 46.1% mortality, p = 0.037; RR 0.59, 95% CI 0.35-0.97). Our cohort was comprised of 50% alcoholic cirrhosis subjects. PTX is also effective in preventing HRS in patients with cirrhosis albeit with an attenuated effect. In their randomized controlled trial of 335 patients with advanced cirrhosis, Lebrec, et al.15 demonstrated that the proportions of patients without a composite of complications, including HRS, were higher in the PTX group than the placebo group at two (78.6% vs. 63.4%, p = 0.006) and six months (66.8% vs. 49.7%, p = 0.002) respectively. Furthermore, the probability of being free of renal failure was statistically significant at 6 months in the PTX group (90.9%, 95% CI 86.1-95.6) vs. the placebo group (79.4%, 95% CI 72.686.1%), p = 0.02. However, there was no appreciable survival difference at 2 and 6 months between these two groups. Similarly, Tyagi, et al.12 demonstrated that PTX is effective in preventing HRS in cirrhotic patients with ascites. In their randomized placebo controlled trial of 70 patients, 35 were given PTX with improvement in serum sodium, mean arterial pressure and preservation of creatinine. Of the 12 patients who developed HRS, only two patients were in the PTX arm (p = 0.01).
In general, treatment related serious adverse events were rare. The one patient who suffered a serious adverse event attributable to PTX had significant nausea and vomiting; however, this was in the setting of co-administration of tramadol, a medication the subject remembered they had a similar reaction to when given prior but did not disclose as an allergy/intolerance. Thus, the exact role of PTX in precipitating significant gastrointestinal side effects necessitating drug discontinuation remains unclear, although nausea and vomiting have been reported in upwards of 28% of patients taking PTX. Other common side effects including dizziness, headache, dyspepsia and diarrhea13 were not observed.
While we did not find a survival benefit in this study due to a small sample size (we prematurely terminated study enrollment due to low numbers of recruitment over the two year study period), our study was not powered to detect survival rate and there was a trend towards a survival benefit in the PTX group. Overall cohort survival rate was higher than expected, largely due to the frequency of liver transplantation in the setting of the relatively small sample size. Future large-scale, well-powered multicenter study may be considered to corroborate these findings. Other limitations of our study are the inherent limitations to PTX as this medication cannot be administered through a gastric feeding tube owing to its pharmacologic properties. This is problematic as oftentimes patients with advanced cirrhosis and HRS have significant hepatic encephalopathy which can impair their ability to take oral medications. Additionally, we excluded patients in the intensive care unit, a setting where HRS patients often receive aggressive medical care. This decision limits the generalizability of our findings somewhat and prevented a third comparison to patients who were prescribed the alternative HRS treatment regimen of norepinephrine and albumin.
In conclusion, the addition of PTX to AMO in the treatment of HRS-1 is safe and may offer a well-tolerated novel treatment to the limited armamentarium for this highly morbid condition. Future large-scale prospective study to validate the efficacy of this treatment seems warranted.
Guarantor of ArticleJonathan G. Stine, M.D. MSc, FACP
Disclosure StatementThe authors have nothing to disclose.
Financial SupportThis work was supported in part by grant funding from the National Institutes of Health (Grant 5T32DK007769-15).
This work was also supported by a Transplant Hepatology Fellowship Award from the American Association for the Study of Liver Diseases (AASLD).
Sources of SupportThe sources of financial and material support had no role in the design or conduct of the study; collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
Author ContributionsStine JG, Henry Z, Caldwell SH and Northup PG designed research; Stine JG, Wang J, Cornella SL, Behm BW, Shah NL, Northup PG performed research; Stine JG and Northup PG analyzed data; Stine JG, Wang J, Cornella SL, Behm BW, Henry Z, Caldwell SH, and Northup PG wrote and approved the final version of the paper.
Potential Competing InterestsNone